A Selective Review of Cerebral Abnormalities in Patients With First-Episode Schizophrenia Before and After Treatment
Abstract
The question of whether there are significant changes in brain anatomy and function at illness onset and over the early course of schizophrenia is a crucial issue with broad implications for prognosis, patient care, and models of illness pathophysiology. Prefrontal hypoactivity and hippocampal and subcortical hyperactivity at the onset of illness may represent a core illness pathophysiology. Gray matter changes have been most robust within thalamo-cortical networks, whereas altered brain activity has been most pronounced in fronto-parietal and default-mode networks. These findings indicate that regional anatomical and functional brain abnormalities are significantly dissociated during the early course of schizophrenia prior to antipsychotic treatment. One possible explanation is that functional MRI may reflect physiological alterations related to acute psychosis or consequences of distal anatomic changes, while changes in brain anatomy reflect more stable and long-standing alterations. The relative stability of brain measures in the early years after illness onset stands in contrast to indirect evidence for moderate progressive changes provided by our recent study of chronic never-treated patients. Our recent cross-sectional study of never-treated patients with chronic schizophrenia found an accelerated age-related decline in cortical thickness, relative to healthy controls, that could not be attributed to medication effects. Studies must consider both the clinical heterogeneity that affects findings of brain changes within schizophrenia and the low specificity of the brain changes, which can occur in other early-onset mental disorders as well.
Are there brain changes that cause schizophrenia, and if so, do they progress over the course of illness? These questions were raised more than a century ago by Kraepelin, but only now are answers being provided by systematic brain imaging research (1). Studies of first-episode schizophrenia have been especially important in this regard, providing an opportunity to study brain systems without the potential confounding effects of antipsychotic medication and to study changes over the early course of illness. Importantly, they also provide a strategy for studying short-term brain changes that occur after initiation of treatment with antipsychotic medication.
Available evidence indicates that 1) regionally dissociated functional and structural brain changes are already present at the onset of schizophrenia and can predict clinical outcome (2); 2) starting antipsychotic treatment leads to acute changes in brain anatomy and function; and 3) alterations seen in first-episode patients are different from those observed in chronic patients, and there is preliminary evidence for progressive brain changes in longitudinal studies (3). For example, using meta-analysis, Radua et al. (3) identified conjoint structural and functional differences in the insula/superior temporal gyrus and the medial frontal/anterior cingulate cortex bilaterally, with some changes progressing over the course of illness. While current longitudinal studies of first-episode schizophrenia patients are far from characterizing changes over the full course of the disorder, these studies are providing a new understanding of dynamic changes in neural networks related to acute episodes of illness and illness progression effects in a dynamic manner. In time, a systematic understanding of these issues may provide objective methods for improved differential diagnosis based on biologically defined subgroups of patients with psychotic disorders.
Most early MRI studies of schizophrenia examined chronically ill patients, for whom findings are potentially influenced by both illness duration and prolonged exposure to antipsychotic medication (4, 5). These studies left some important questions unanswered: What and how much pathology is evident at illness onset, especially before treatment? How do cerebral deficits manifest themselves dynamically over the early illness course? How do illness-related brain alterations relate to clinical manifestations and cognitive impairments? Do brain changes predict or track therapeutic outcome? Answers to these questions are critical for elucidating the core pathophysiology of schizophrenia and could contribute greatly toward early detection and more effective intervention for this illness (6). To address these issues, several schizophrenia research programs began to study patients at illness onset and follow them longitudinally with MRI studies.
The increasing sophistication of MRI studies of the brain over the past two decades has greatly accelerated progress in understanding the neural basis of schizophrenia. For example, in structural imaging of gray matter, studies of cortical thickness and gyrification now complement knowledge provided by studies of regional brain volume (7). Similarly, studies of regional white matter volume are now supplemented by diffusion tensor imaging and tractography, which provide detailed quantitative information about the integrity and myelination of major fiber tracts (8). Tasked-based fMRI studies along with resting-state fMRI have been widely used to investigate changes in regional brain activity. Mapping activity in brain regions is now complemented by evaluations of functional connectivity to examine how brain regions work together in a circuit fashion to support sensory, cognitive, motor, and affective processes (9). These advances in multimodal imaging and quantitative image analysis tools have been especially crucial for studies of psychiatric disorders in which brain alterations are subtle relative to those seen in degenerative diseases, stroke, and other neurological disorders.
China and other emerging countries have advantages for conducting this kind of work, because of the continuing presence of large numbers of never-treated patients seeking treatment for the first time in centralized hospital systems. For example, our program is in West China Hospital, which, with over 5,000 beds, houses one of the four main mental health centers in China. The large majority of first-episode psychiatric patients come to our hospital for care before having previously received any psychiatric medications. This circumstance is largely due to the current health care framework in China, in which only now are primary health care systems providing psychiatric care being established (10). Therefore in the past 10 years, our Huaxi MR Research Center group has been able to carry out large prospective studies using high-field MR imaging with never-treated first-episode patients. In addition to pretreatment characterization, we have followed patients longitudinally, compared disorders, and worked to predict clinical outcomes, monitor treatment effects on brain, and identify subgroups of patients based on MRI findings. Although there are potential limitations regarding the generalizability of findings from particular geographic regions with particular ethnic profiles, our recent study (11) indicated that schizophrenia patients from four ethnically distinct cohorts (white Caucasians, African-Caribbeans, Japanese, and Chinese) shared similar brain anatomical deficits, suggesting that schizophrenia across cultures shares a common pattern of brain abnormalities. This is consistent with ethnicity analyses in imaging data from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study in the United States. Similar MRI programs investigating first-episode psychosis are active at other medical centers around the world.
Our aim in the present study was to selectively review relevant findings and discuss their clinical and research implications. For this purpose, we included 40 cross-sectional MRI studies of treatment-naive first-episode schizophrenia patients and 12 longitudinal studies of first-episode schizophrenia patients before and after treatment (see Figure S1, studies listed and described in Tables S1 and S2 in the data supplement that accompanies the online edition of this article). In doing so, our aim is not to present a meta-analytic review, but to express a viewpoint about what are the most compelling findings in this field based on our evaluation. In addition to our overview of anatomical and functional changes before and after treatment in first-episode schizophrenia, we discuss their relationship and clinical relevance. We also discuss the inconsistency of findings across studies and the potential to leverage illness heterogeneity by using MRI findings to identify neurobiologically distinct patient subgroups.
Structural Findings in Treatment-Naive Patients
Recent studies with high-resolution MRI have begun to characterize gray matter deficits in antipsychotic-naive first-episode schizophrenia patients. These studies have found widespread volumetric abnormalities mainly in fronto-temporal, thalamo-cortical, and subcortical-limbic circuits (see Table S1), although no findings are consistent. For example, the established enlarged third/lateral ventricle volume in treated chronic patients has been reported in some but not all studies. Multiple studies have demonstrated reduced gray matter volume in the dorsolateral prefrontal cortex, thalamus, superior temporal gyrus, insula, and anterior cingulate cortex (12, 13), consistent with hypotheses of “hypofrontality” and abnormal fronto-temporal networks in schizophrenia. Other studies have reported increased gray matter volume bilaterally in the thalamus, insula, anterior cingulate cortex, and orbital frontal gyrus (12, 14). This increased volume at illness onset in some of these brain regions may result from neurodevelopmental alterations such as neuronal overgrowth, a deficit in normal pruning during neurogenesis, or early illness pathophysiology effects. Interestingly, we have also seen such atypical increases in regional cortical volumes in depression at illness onset (15). This highlights the importance of early-course studies and investigations of the differences in brain abnormalities seen at illness onset compared with chronically ill individuals across disorders to identify shared and illness-specific factors. This finding in first-episode schizophrenia patients also suggests a complex pathophysiological process that may involve neuroinflammatory and other mechanisms that affect brain anatomy and function at the onset of illness. For example, acute neuroinflammation may cause swelling and enlargement of brain structures. Some of these changes appear to be related to clinical manifestations. For example, patients with prominent negative symptoms have been reported to exhibit greater reductions of gray matter volume in the temporal lobe (13), while bilaterally decreased gray matter volumes have been associated with hallucination severity (16, 17). While there no doubt are methodological reasons for the inconsistency of findings across studies, these clinical associations suggest that heterogeneity in the schizophrenia syndrome itself could contribute to variability in MRI findings across studies; if so, then resolving this heterogeneity using neurobiological parameters from MRI studies may be an especially promising strategy for future studies. Little is yet known about the linkage between morphometric measures and neurochemical ones, although the regional distribution of effects does not suggest that MRI abnormalities are linked selectively to alteration in particular monoamine systems that are the target of current therapies. For example, while brain regions such as the medial prefrontal cortex, the striatum, and the thalamus are within the “dopamine pathway,” which is both a treatment target and a system implicated in the pathogenesis of schizophrenia, there are many other regions where illness effects have been seen, including parietal and occipital regions that do not receive prominent dopaminergic innervation (Figure 1).
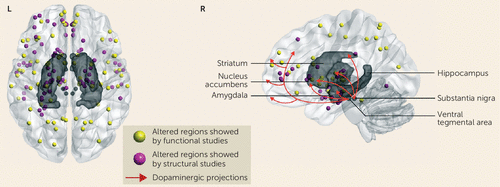
FIGURE 1. Altered Brain Regions Demonstrated by Structural and Functional MRI Studies of First-Episode Schizophrenia Before Treatment and Innervation of Brain Regions by Dopaminergic Projectionsa
a The color-coded nodes represent the coordinates of the altered gray matter volume reported by structural studies and of the altered function reported by functional studies (the studies are listed in Table S1 in the online data supplement).
Similarly, studies of white matter in first-episode schizophrenia indicate widespread abnormalities across white matter tracts (18), with evidence for reductions in fractional anisotropy in the uncinate (19), cingulum (20), fornix (21), corpus callosum (22), and inferior longitudinal fasciculus (23), although negative results have also been reported (24). The inconsistent localization of findings is reflected in a recent meta-analysis of fractional anisotropy findings in schizophrenia, as reported in 23 published papers, where nonoverlapping and scattered findings were seen throughout white matter tracts (25). As with morphometric studies, this likely results in part from factors such as differences in image acquisition and analysis, small sample sizes, variable prior duration of illness, and illness heterogeneity.
In all areas of MRI research, methodological advances have provided the tools needed to generate important new insights into the underlying neuropathology of schizophrenia. In diffusion tensor imaging studies, the use of tract-based spatial statistics, which is a quantitative approach for studying white matter tracts individually and along their length, has shown localized abnormalities within the superior longitudinal fasciculus (26) and widespread white matter abnormalities throughout the brain (8), potentially with different groups of patients showing these two different patterns of abnormality, as observed in our recent study using a data-driven patient clustering method (27). Neurodevelopmental alterations or neuroinflammatory changes may account for the widespread deficits (28), while neurotoxic effects related to acute psychosis or changes predicted by macro-circuit theory (that is, specific white matter tracts are disrupted either as a cause or a consequence of a disorder in the gray matter regions they connect) may contribute to heterogeneity in localization of findings (29).
Another important observation has been that many alterations in brain anatomy and function are not diagnostically specific, even if they are perhaps more prominent in some disorders than others. For example, several gray and white matter findings seen in first-episode schizophrenia are also seen in depression, bipolar disorder, and obsessive-compulsive disorder. In particular, patients with schizophrenia and with bipolar disorder (especially bipolar disorder with psychotic features) appear to share similar functional and anatomical deficits within thalamo-cortical circuitry (30, 31). Unmedicated depressed patients and schizophrenia patients show volumetric decreases in the amygdala (32, 33), and corpus callosum disruptions have been found in obsessive-compulsive disorder (34) and schizophrenia (22). This overlap across disorders in MRI findings, paralleling overlap in symptom presentation (35), raises broader questions about whether there are overlapping pathophysiologies across disorders and whether our current diagnostic nosology, which focuses primarily on behavioral and psychological features, fails to differentiate conditions based on consistent patterns and types of neurobiological abnormalities.
Functional Findings in Treatment-Naive Patients
Compared with anatomical MRI, fewer functional studies have examined drug-naive first-episode schizophrenia patients, and the results are also variable across different patient samples. Reduced activity of the medial/dorsolateral prefrontal cortex, superior temporal gyrus, thalamus, and parietal lobe has been reported in treatment-naive first-episode schizophrenia. Notably, decreased activity in the medial prefrontal cortex and orbital frontal cortex have been reported in both task-based and resting-state functional MRI studies (13, 36–39), including the two resting-state fMRI studies with large samples of first-episode schizophrenia patients (13, 36) (N=100 and N=104). Positron emission tomography and task-based fMRI studies have reported reduced activation of the medial prefrontal cortex (40, 41), which may recover with clinical improvement, suggesting that the hypoactivity may be related to the presence or severity of psychosis (42). A similar pattern of state-related changes has also been reported in dorsal fronto-parietal circuitry (43–45). Several regions within the thalamus, temporo-parietal cortex, and frontal lobe have shown increased or decreased activity during task-based fMRI studies (46–48) (see Table S1). However, these findings varied among different samples, tasks, scanners, and image analysis methods. For example, hypoactivity of the medial prefrontal cortex was not reported in many studies, which may be due partly to variable signal loss caused by the magnetic susceptibility artifacts above the anterior cranial base. Besides hypoactivity, increased intrinsic regional activity has also been identified in first-episode schizophrenia, especially in the hippocampus bilaterally (49, 50) and in the putamen (13). Greater hippocampal blood flow and blood volume have been observed in schizophrenia (50); it has been associated with cognitive dysfunction (49, 50) and sensory processing deficits (51), and it is believed to reflect medial temporal lobe pathology that is core to the pathogenesis of schizophrenia (52). These findings generally support a model of prefrontal hypoactivity and hippocampal and subcortical hyperactivity at the onset of illness, which may represent a core illness pathophysiology. These regional changes represent potential candidate biomarkers for use in delineating subgroups of patients and as outcome measures for therapeutic development.
Relations to Clinical Symptoms and Course of Illness
Several anatomical and functional MRI studies have reported correlations between regional volume of gray/white matter or neural activity and the severity of positive symptoms in drug-naive first-episode schizophrenia patients (13, 53–55). Negative symptoms have less frequently been correlated significantly with neuroimaging findings at illness onset. This is consistent with the view that brain changes associated with acute psychosis may have particularly powerful influences on brain imaging parameters at illness onset. However, structural deficits of regional gray matter have been observed in patients with prominent negative symptoms (2, 13). Perhaps most notably, the correlations between measures of brain pathology and clinical symptoms have been modest, suggesting that brain changes may not track current symptom levels well. While MRI changes may reflect illness processes that evolve more gradually than the clinical onset of acute psychosis, they potentially have greater validity for predicting longer-term patterns of symptoms and illness course.
The initial expectation of many investigators was that brain regions with gray matter volume changes would be the sites where regional functional activity would be most altered. This has not proven to be the case in medication-naive first-episode schizophrenia, as regional anatomical and functional changes have often been found in different brain areas (56). Gray matter changes have been most robust within thalamo-cortical networks, whereas altered brain activity has been most pronounced in fronto-parietal and default mode networks (Figure 1). These findings indicate that regional anatomical and functional brain abnormalities are significantly dissociated during the early course of schizophrenia, prior to antipsychotic treatment. One possible explanation is that functional MRI may reflect physiological alterations related to acute psychosis, while changes in brain anatomy reflect more stable and long-standing alterations. This possibility is supported by findings showing that functional changes in fronto-parietal and default mode networks were normalized after 6 weeks of antipsychotic treatment and clinical remission (42, 44), while anatomical deficits in thalamo-cortical networks are more stable, showing some slow and modest progressive change over the course of illness (57).
One study found that regionally reduced gray matter volume could affect clinical symptoms by disrupting functional connectivity (58). Subsequent studies verified this finding (54, 59). Guo et al. (54) established that the causal connectivity within altered prefrontal-thalamic (limbic)-cerebellar (sensorimotor) circuit was related to structural deficits. Thus, the regional changes of gray matter anatomy may affect function not only at the site of gray matter change, but also in widely distributed functional networks. To the extent that this is the case, it could account for the dissociation of regional anatomical and functional changes.
Regarding the relationship between cerebral changes and illness duration, no robust associations between functional or anatomical abnormalities and illness duration have been found in drug-naive patients with an illness duration less than 5 years (13, 55), and little progressive change has been found after patients are stabilized on antipsychotic treatment. The observation of static rather than progressive MRI findings early in the illness course (13, 55, 60) parallels findings from our neuropsychological studies in an independent sample (61).
The observation of a relative stability of brain measures in the early years after illness onset stands in contrast to indirect evidence for moderate progressive changes provided by our recent study of never-treated patients with chronic schizophrenia. In chronic never-treated patients, we found an accelerated age-related decline in cortical thickness, relative to healthy subjects, that could not be attributed to medication effects using nonlinear modeling of age-related effects (62). The progressive changes were restricted to specific brain regions, including the medial and lateral prefrontal cortex, the temporal cortex, and the hippocampus (62–65). Hypertrophic changes were observed in the striatum, and notably they could not be attributed to effects of antipsychotic treatment (57).
These findings suggest that changes in brain anatomy and function evident in patients with first-episode psychosis may predate or be early-evolving features of the illness that remain relatively stable in the early years after first psychosis, and only become progressive during the later phase of illness. Evidence for progressive cerebral atrophy in MRI studies may result from neuropathological mechanisms revealed by postmortem studies including shrinkage of neuropil, reduced neuron size, and apoptotic cell loss (66, 67).
What causes these neuroimaging abnormalities? The neurodevelopmental hypothesis proposes that schizophrenia is related to an interaction of genetic and environmental factors leading to abnormal brain development during the pre- or postnatal period (68). Consistent with this view, reduced gray and white matter anatomical integrity has been reported in subjects at ultra-high risk for schizophrenia (69–71). Altered regional brain function (72) and abnormal network connectivity have also been identified in these individuals (73). Most findings suggest that high-risk individuals, and first-degree relatives generally, show qualitatively similar but less severe abnormalities than those observed in patients with first-episode schizophrenia. Genetic mechanisms may account for these effects, and a few studies have demonstrated an association of candidate genes for schizophrenia with neurophysiological and MRI findings (74–77). Other studies have shown a relationship between brain imaging findings and minor changes in endocrine (78) and immune systems (79).
The contribution of environmental factors to brain imaging findings in first-episode schizophrenia, such as antenatal maternal virus infections, and obstetric complications such as hypoxia and nicotine exposure, may also affect brain development during the perinatal period, possibly contributing to smaller gray matter volumes in patients with schizophrenia (6, 49). However, research is needed to directly link imaging findings with environmentally induced changes at the cellular or molecular level to support such possibilities on a mechanistic basis.
Structural Findings After Treatment
Studies of nonhuman primates have demonstrated that antipsychotic medication can significantly reduce brain volume with a lower glial cell number and increased neuronal cell packing density (80, 81). Imaging studies also provide evidence suggesting that antipsychotics can reduce regional brain volumes and increase ventricular fluid volume (82, 83). However, central questions remain to be resolved, including which brain regions or networks change most significantly after treatment, which changes are beneficial and which are adverse, and how these changes are associated with symptoms and cognitive alterations. These are each important clinical questions. Adverse cognitive effects of antipsychotics have been described in clinical studies and parallel studies of nonhuman primates, with some evidence suggesting genetic prediction of these effects (84, 85). One would expect significant reductions in regional brain volume to have adverse behavioral consequences, so identifying them and understanding their causes may help guide the development of new treatments with less risk for such effects.
Structural MRI studies demonstrate neuroanatomic changes in multiple brain regions after 6 weeks of acute treatment (86), even with lower antipsychotic doses (87). The most robust changes have been seen in the fronto-temporal cortex and basal ganglia (88–90). The dorsolateral prefrontal cortex and superior temporal gyrus are most frequently reported to exhibit gray matter loss (82, 91, 92) with associated cognitive impairment (56, 63) after initiation of antipsychotic medication. An increase in the thickness of the prefrontal and temporal cortex over time has also been reported, sometimes accompanied by a significant improvement in symptoms (93, 94). Gray matter reductions have been observed in the cingulate gyrus (95) and hippocampus (96). Higher baseline positive symptom severity has been associated with increased striatal and hippocampal volume loss over time (96). A greater cognitive reserve or a lower level of psychopathology at baseline may confer an increased vulnerability to developing gray matter volume reductions during acute treatment with antipsychotic medication (63, 83, 97, 98). However, despite some positive findings and limited prior research in this area, at present, the relationship of structural changes to cognitive and psychopathological ratings appears to be modest and inconsistent.
Besides volume loss in the cortex, increases in gray matter volume after treatment have been reported within basal ganglia regions, including the caudate nuclei (99) and the putamen (83), which notably are dopamine receptor-rich brain regions (Figure 1) (100, 101). In particular, increased gray matter volume in the putamen after treatment has been shown to correlate with symptom improvement (86). Although conventional and atypical antipsychotics may exert neurotrophic, neurogenetic, and neuroprotective effects differently (102), the mechanisms of the effects of these antipsychotics on brain structure remain unclear. Atypical antipsychotics may reduce pathophysiological effects or neuronal degeneration in schizophrenia through agonism on N-methyl-d-aspartate receptors (103, 104), increased expression of neurotrophic factors (105, 106), and stimulation of neurogenesis (107). There clearly is great clinical importance to clarifying these mechanisms of treatment-related effects.
White matter changes after treatment initiation have not been investigated as frequently as have gray matter changes; however, changes in white matter after medication treatment appear to exhibit a regional pattern similar to that of cortical gray matter changes (63), involving primarily the frontal and temporal lobes (108, 109). Importantly, any treatment-related reductions in white matter integrity may contribute to treatment-related functional connectivity changes across brain regions and thereby account for some adverse changes in higher cognitive functions and secondary negative symptoms of schizophrenia (110).
Antipsychotic medication exhibits acute effects on cerebral function after only a few days of treatment (111). We have reported increased synchronous neural activity mainly in frontal and parietal regions after short-term (6 weeks) antipsychotic therapy (42), and similar changes in parallel with clinical improvement have been reported with long-term treatment (112) (Figure 2). One important finding from the functional studies was that abnormal activity in some regions, notably the medial prefrontal cortex, recovered after therapy (113). This normalization of dysfunction evident before treatment did not occur in all brain regions or networks in first-episode patients and was stable or increased in the dorsolateral prefrontal cortex (114). These studies suggest that there are specific abnormalities of brain function that are relatively stable over time, whereas others may be predominantly state-related phenomena, more readily corrected by current treatment approaches. Available evidence suggests that in addition to apparently beneficial changes in brain function after treatment initiation, there may some adverse effects of treatment on some brain functions. For example, reduced activity of the dorsomedial thalamus and decreased functional connectivity of the mediodorsal thalamus and cerebellum have been reported despite their being normal before treatment (44, 115). These findings provide evidence for a complex pattern of effects by antipsychotics on functional brain systems in schizophrenia and illustrate the potential use of neuroimaging biomarkers for defining and advancing our understanding of both beneficial and adverse antipsychotic drug effects on brain function.
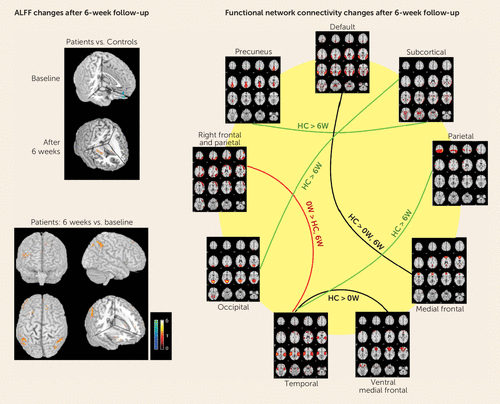
FIGURE 2. Significantly Increased Synchronous Neural Activity After 6 Weeks of Antipsychotic Treatmenta
a ALFF=amplitude of low-frequency fluctuations; HC=healthy controls; 0W=patients at antipsychotic-naive state; 6W=patients after 6 weeks of antipsychotic treatment. The warm colors in the brain images represent regions of increased amplitude of low-frequency fluctuations or functional connectivity. The red line indicates connectivity that was abnormal at baseline and normalized after treatment; the green lines indicate connectivity that was normal at baseline and significantly decreased after treatment; the black lines indicate connectivity that did not change after treatment. The regions mainly involved after 6 weeks of antipsychotic therapy are the medial prefrontal cortex, the left and right basal ganglia, and parietal regions. (Adapted from reference 42. Copyright 2010 American Medical Association. All rights reserved.)
None of these clinical studies of first-episode patients can definitively resolve the question of whether these brain changes after treatment initiation are related to drug treatment or to the treatment-related reduction in illness pathophysiology and other secondary factors. However, their rapid onset relative to what typically are much longer periods of illness before first treatment, their similarity to drug-related changes seen in nonhuman primates, and the fact that they remain relatively stable over the course of illness are most consistent with their being drug effects (13).
Relationship Between Anatomical and Functional Changes After Treatment
One important finding from parallel structural and functional MRI studies of first-episode schizophrenia patients before and after acute treatment has been that anatomical changes are quite different from functional changes with regard to their regional distribution (see Table S2 in the online data supplement). Most studies observed decreased gray matter volume in the frontal cortex after treatment, whereas functional changes were more widely distributed, involving the prefrontal, temporal, and parietal cortices as well as the thalamus, basal ganglia, and cerebellum (42, 114, 116, 117). Even within brain regions, some surprising patterns of changes in anatomy and function have been seen. For example, a recent cross-sectional study of treated patients showed that short-term treatment with antipsychotics was associated with prefrontal cortical thinning, as well as increased prefrontal functional activity that was accompanied by enhanced cognitive control (56). As a second example, our previous studies identified an acute reduction in white matter integrity around the anterior cingulate gyrus and the anterior corona radiata of the frontal lobe (118), together with increased regional synchronous neural activity in the prefrontal and parietal cortices in the same patients after treatment (42). A normalization of fronto-parietal attentional system abnormalities and the emergence of reduced activity in the dorsolateral prefrontal cortex in task-based fMRI studies has also been reported and replicated (43).
The reasons for these dissociations and their mechanisms require further study in patients and in animal models. Given the surprising nature of these patterns of change, developing a mechanistic understanding of these outcomes might provide novel insights into the causes of psychosis and into how antipsychotic treatments alter systems-level brain biology in beneficial and adverse ways. It also is important to note that antipsychotics may not all have similar effects on brain anatomy and function. In particular, the conventional and atypical antipsychotics appear to have different effects (119). Lieberman et al. (5) reported that haloperidol was associated with significant reductions in gray matter volume, whereas olanzapine was not. These results are consistent with those of van Haren et al. (65), who studied both first-episode and chronic patients and also found that a higher cumulative intake of conventional antipsychotics was associated with more pronounced cortical thinning, whereas atypical antipsychotics did not show such an association. There is also evidence for differences between effects of conventional and atypical antipsychotics on brain function (120).
Few studies have investigated the predictive value of MRI findings in relation to treatment response in first-episode schizophrenia, but some results have been promising. Larger temporal gray matter (97) and smaller hippocampal, parahippocampal, and striato-thalamic volumes have been related to lower rates of symptom remission (121, 122). A recent systematic review by Dazzan et al. (2) showed that gray matter alterations in medial temporal and prefrontal cortical areas and in the networks that connect them with subcortical structures are promising neuroanatomical biomarkers of poor symptomatic and functional outcomes. Although they also acknowledged the inconsistent findings from different studies, the value of using baseline anatomical MRI studies to predict the clinical outcome of first-episode schizophrenia appears promising. White matter integrity of the uncinate, cingulum, and corpus callosum also have been found to be lower in treatment nonresponders (123). Functional studies have shown that fronto-parietal components and default mode network measures predicted negative symptom improvement (112).
While psychiatric imaging has been useful for research purposes, its clinical application has been limited to investigating suspected neurological disorders. As understanding of brain alterations in schizophrenia evolves, research will gradually turn to determining whether imaging results can be helpful in treatment development and as a tool to guide precision medicine. There are now regular talks and discussion groups at radiology meetings beginning to think about and prepare for this challenge. If successful, and the field of psychiatric imaging evolves, applications of MRI studies for differential diagnosis and for individualizing and tracking treatment effects may significantly advance patient care, although much work needs to be done before the MRI research findings can be translated into clinical practice. Such efforts may be especially useful in first-episode patients, for whom optimizing initial treatment might reduce the functional and social disability that often follow from recurrent episodes of psychosis.
For this purpose, it will be important to first resolve neurobiological heterogeneity using anatomical and functional MRI measures. This can be accomplished using pattern recognition approaches to identify biologically similar patient groups, such as cluster analysis and machine learning approaches with support vector machines (124). Using support vector machine techniques in schizophrenia and healthy controls, Davatzikos et al. (125) found that while fronto-temporal measures contributed to subgroup discrimination, parts of the occipital cortex did as well, which is an area not traditionally implicated in schizophrenia. Other studies have also used support vector machines, either with multimodal imaging data or combined with cognitive and genetic data, to discriminate schizophrenia patients and controls. Some achieved encouraging accuracy based on the pattern of abnormalities distributed across the brain (126–128). Moreover, using MRI data obtained at illness onset, some data suggest that it may be possible to predict whether a specific individual is likely to experience a remission or a poorer course of illness (129). The use of multivariate modeling of multimodal imaging data holds promise in providing a path forward for refining diagnostic practice by integrating multiple neurobiological dimensions of illness. There have been some preliminary successes in this regard in first-episode (27) and in chronic patients (130), which raises hope that work along these lines may, in the longer term, be successful and become an important strategy for improving patient care.
As noted above, reports from MRI studies of first-episode schizophrenia have not been highly consistent. Potential reasons for inconsistent findings across studies include the inhomogeneity of patient samples, differences in MRI methodology, small sample sizes, different control groups or lack thereof, patient history of drug abuse, prior psychiatric treatment, and other clinical and demographic factors. Given the inhomogeneity of patient samples, the small sample sizes especially may lead to varied results. The ongoing development of MR acquisition and analysis methods may also be important reasons for inconsistent findings among previous studies. Differences in genetic factors, duration of untreated illness, and variations in symptom levels, and the short-term focus of most symptom assessments used in previous work (severity over the past week) may be important factors affecting studies of relationships between neurobiological alterations and clinical manifestations (131). Clarifying the impact of such factors will be crucial for future research and potential clinical application. Recently, using a fully data-driven method, we identified two different patterns of white matter deficits in 113 drug-naive first-episode schizophrenia patients (Figure 3), suggesting the qualitatively distinct genetic influences or neurodevelopmental alterations (27). This is one step forward in the use of MRI measures to identify and classify patient subgroups based on neurobiological parameters rather than symptoms and clinical history. If successful, MRI-based methods may not only advance psychiatric research, but eventually help in diagnosis and treatment planning for patients with psychotic disorders, as they do in other fields of medicine. This aim is in accordance with the broad aims of the National Institute of Mental Health’s Research Domain Criteria project and of the B-SNIP, with which we have collaborated.
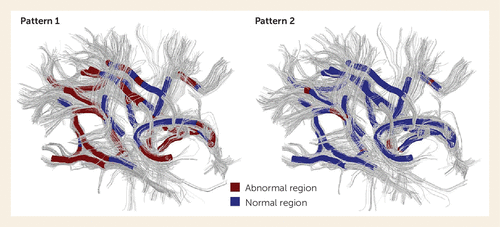
FIGURE 3. Two Different Patterns of White Matter Deficits in 113 Drug-Naive First-Episode Schizophrenia Patients, Identified by Diffusion Tensor Imaging With a Fully Data-Driven Methoda
a The red regions are tracts with significantly decreased fractional anisotropy and increased mean diffusivity, and the blue regions indicate normal tracts. The existence of the two different patterns of white matter deficits in first-episode schizophrenia suggests qualitatively distinct genetic influences or neurodevelopmental alterations and may explain the inconsistent findings among studies. This is one step forward in the use of such measures to identify and classify patient subgroups based on neurobiological parameters rather than symptoms and clinical history. (Adapted from reference 27. Copyright 2015 American Medical Association. All rights reserved.)
Summary
In summary, anatomical and functional MRI studies of treatment-naive first-episode schizophrenia patients have revealed brain deficits at the onset of illness, which have modest correlations with clinical symptoms. Findings from longitudinal studies of first-episode schizophrenia and comparison of findings from first-episode and chronic patients suggest considerable variability in the anatomical changes in the early phase of illness and differences in the magnitude of deficits between first-episode and chronic schizophrenia. Some data suggest regional progression of brain changes over the longer-term course of illness.
Findings also suggest that functional changes occur primarily in regions different from those in which anatomical findings are identified even in the same patients. Hypofunction of the medial prefrontal cortex and hyperactivity of the hippocampus and striatum appear to be consistent findings across studies before treatment and may in time provide biomarkers for the disorder and targets for treatment. While both beneficial and adverse changes in anatomy and function have been seen after treatment in some brain regions, many changes in anatomy and function appear to remain relatively stable early in the course of illness, paralleling the pattern of cognitive deficits (132). While progressive changes do not appear to be robust in the early course of illness, progressive changes may occur in some brain regions during the later phase of illness. Recent evidence is beginning to support relationships between imaging findings and genetic and environmental factors, and clarifying these associations is an important direction for future research in this area.
MRI studies have shown that antipsychotic drugs can cause gray matter loss in the neocortex, although potential compensatory effects involving increased striatal volumes have also been observed. Changes in local resting-state function and functional connectivity across the brain have also been reported after treatment initiation. The potential effects of antipsychotic drugs on brain anatomy and function are not well established, and these effects, while very important to understand, will likely take some time to clarify. Using MRI in this population to understand treatment effects and to define neurobiologically homogeneous subgroups of patients appears to be an especially promising direction for future research. As findings are confirmed in different laboratories, and as molecular mechanisms and clinical significance are clarified, MRI studies may provide an objective assessment of brain alterations that can inform diagnosis and treatment planning, as in other fields of medicine.
1 : Paul Eugen Bleuler and the birth of schizophrenia (1908). Am J Psychiatry 2008; 165:1407Link, Google Scholar
2 : Magnetic resonance imaging and the prediction of outcome in first-episode schizophrenia: a review of current evidence and directions for future research. Schizophr Bull 2015; 41:574–583Crossref, Medline, Google Scholar
3 : Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev 2012; 36:2325–2333Crossref, Medline, Google Scholar
4 : Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull 2013; 39:1129–1138Crossref, Medline, Google Scholar
5 : Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry 2005; 62:361–370Crossref, Medline, Google Scholar
6 : The impact of environmental factors in severe psychiatric disorders. Front Neurosci 2014; 8:19Crossref, Medline, Google Scholar
7 : Differential effects of surface area, gyrification, and cortical thickness on voxel based morphometric deficits in schizophrenia. Neuroimage 2012; 60:693–699Crossref, Medline, Google Scholar
8 : Extensive white matter abnormalities in patients with first-episode schizophrenia: a diffusion tensor imaging (DTI) study. Schizophr Res 2013; 143:231–238Crossref, Medline, Google Scholar
9 : Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev 2011; 35:1110–1124Crossref, Medline, Google Scholar
10 : Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001–05: an epidemiological survey. Lancet 2009; 373:2041–2053Crossref, Medline, Google Scholar
11 : A neuroanatomical signature for schizophrenia across different ethnic groups. Schizophr Bull 2015; 41:1266–1275Crossref, Medline, Google Scholar
12 : The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry 2008; 165:1015–1023Link, Google Scholar
13 : Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry 2013; 170:1308–1316Link, Google Scholar
14 : Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res 1999; 90:1–15Crossref, Medline, Google Scholar
15 : Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl Psychiatry 2014; 4:e378Crossref, Medline, Google Scholar
16 : A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cereb Cortex 2002; 12:1331–1341Crossref, Medline, Google Scholar
17 : Structural covariance in the hallucinating brain: a voxel-based morphometry study. J Psychiatry Neurosci 2009; 34:465–469Medline, Google Scholar
18 : White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry 2005; 162:602–605Link, Google Scholar
19 : Altered white matter connectivity in never-medicated patients with schizophrenia. Hum Brain Mapp 2013; 34:2353–2365Crossref, Medline, Google Scholar
20 : Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry 2003; 54:1171–1180Crossref, Medline, Google Scholar
21 : Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry 2006; 60:22–31Crossref, Medline, Google Scholar
22 : The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage 2008; 39:1522–1532Crossref, Medline, Google Scholar
23 : Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry 2007; 64:1270–1280Crossref, Medline, Google Scholar
24 : The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 2005; 76:585–587Crossref, Medline, Google Scholar
25 : Voxel-based morphometry (VBM) studies in schizophrenia: can white matter changes be reliably detected with VBM? Psychiatry Res 2011; 193:65–70Crossref, Medline, Google Scholar
26 : Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry 2008; 63:512–518Crossref, Medline, Google Scholar
27 : Two patterns of white matter abnormalities in medication-naive patients with first-episode schizophrenia revealed by diffusion tensor imaging and cluster analysis. JAMA Psychiatry 2015; 72:678–686Crossref, Medline, Google Scholar
28 : Is there a flame in the brain in psychosis? Biol Psychiatry 2014; 75:258–259Crossref, Medline, Google Scholar
29 : Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr Bull 2008; 34:72–92Crossref, Medline, Google Scholar
30 : Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol Med 2015; 45:97–108Crossref, Medline, Google Scholar
31 : White matter microstructure in untreated first episode bipolar disorder with psychosis: comparison with schizophrenia. Bipolar Disord 2011; 13:604–613Crossref, Medline, Google Scholar
32 : Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry 2008; 13:993–1000Crossref, Medline, Google Scholar
33 : The amygdala and schizophrenia: a volumetric magnetic resonance imaging study in first-episode, neuroleptic-naive patients. Biol Psychiatry 2003; 54:1302–1304Crossref, Medline, Google Scholar
34 : Diffusion tensor imaging (DTI) studies in patients with obsessive-compulsive disorder (OCD): a review. J Psychiatr Res 2014; 54:26–35Crossref, Medline, Google Scholar
35 : Phenomenology of first-episode psychosis in schizophrenia, bipolar disorder, and unipolar depression: a comparative analysis. Clin Schizophr Relat Psychoses 2012; 6:145–151Crossref, Medline, Google Scholar
36 : Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychol Med 2013; 43:769–780Crossref, Medline, Google Scholar
37 : Dysconnectivity within the default mode in first-episode schizophrenia: a stochastic dynamic causal modeling study with functional magnetic resonance imaging. Schizophr Bull 2015; 41:144–153Crossref, Medline, Google Scholar
38 : Abnormal default-mode network homogeneity in first-episode, drug-naive schizophrenia at rest. Prog Neuropsychopharmacol Biol Psychiatry 2014; 49:16–20Crossref, Medline, Google Scholar
39 : Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage 2010; 49:2901–2906Crossref, Medline, Google Scholar
40 : The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev 2010; 34:468–486Crossref, Medline, Google Scholar
41 : Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry 2009; 166:863–874Link, Google Scholar
42 : Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry 2010; 67:783–792Crossref, Medline, Google Scholar
43 : Impact of antipsychotic treatment on attention and motor learning systems in first-episode schizophrenia. Schizophr Bull 2015; 41:355–365Crossref, Medline, Google Scholar
44 : An fMRI study of visual attention and sensorimotor function before and after antipsychotic treatment in first-episode schizophrenia. Psychiatry Res 2009; 172:16–23Crossref, Medline, Google Scholar
45 : Task-evoked substantia nigra hyperactivity associated with prefrontal hypofunction, prefrontonigral disconnectivity and nigrostriatal connectivity predicting psychosis severity in medication naive first episode schizophrenia. Schizophr Res 2014; 159:521–526Crossref, Medline, Google Scholar
46 : Cerebral changes and cognitive dysfunctions in medication-free schizophrenia: an fMRI study. J Psychiatr Res 2008; 42:469–476Crossref, Medline, Google Scholar
47 : Impaired temporoparietal deactivation with working memory load in antipsychotic-naive patients with first-episode schizophrenia. World J Biol Psychiatry 2011; 12:271–281Crossref, Medline, Google Scholar
48 : Left dorsolateral prefrontal cortex dysfunction in medication-naive schizophrenia. Schizophr Res 2010; 123:22–29Crossref, Medline, Google Scholar
49 : Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology 2010; 35:938–942Crossref, Medline, Google Scholar
50 : Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry 2014; 171:549–556Link, Google Scholar
51 : Increased hemodynamic response in the hippocampus, thalamus, and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res 2007; 92:262–272Crossref, Medline, Google Scholar
52 : Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry 2015; 172:17–31Link, Google Scholar
53 : Extensive brain structural network abnormality in first-episode treatment-naive patients with schizophrenia: morphometrical and covariation study. Psychol Med 2014; 44:2489–2501Crossref, Medline, Google Scholar
54 : Abnormal causal connectivity by structural deficits in first-episode, drug-naive schizophrenia at rest. Schizophr Bull 2015; 41:57–65Crossref, Medline, Google Scholar
55 : Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia. Schizophr Bull 2015; 41:201–210Crossref, Medline, Google Scholar
56 : A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry 2015; 72:226–234Crossref, Medline, Google Scholar
57 : Brain structural abnormalities in a group of never-medicated patients with long-term schizophrenia. Am J Psychiatry 2015; 172:995–1003Link, Google Scholar
58 : Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry 2009; 166:196–205Link, Google Scholar
59 : Reduced anterior cingulate cognitive activation is associated with prefrontal-temporal cortical thinning in schizophrenia. Biol Psychiatry 2012; 71:146–153Crossref, Medline, Google Scholar
60 : Global and regional cortical thinning in first-episode psychosis patients: relationships with clinical and cognitive features. Psychol Med 2011; 41:1449–1460Crossref, Medline, Google Scholar
61 : Impairment of verbal memory and learning in antipsychotic-naive patients with first-episode schizophrenia. Schizophr Res 2004; 68:127–136Crossref, Medline, Google Scholar
62 : Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry 2006; 188:510–518Crossref, Medline, Google Scholar
63 : Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 2011; 70:672–679Crossref, Medline, Google Scholar
64 : The lifetime trajectory of schizophrenia and the concept of neurodevelopment. Dialogues Clin Neurosci 2010; 12:409–415Medline, Google Scholar
65 : Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry 2011; 68:871–880Crossref, Medline, Google Scholar
66 : Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry 1998; 55:215–224Crossref, Medline, Google Scholar
67 : The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17–25Crossref, Medline, Google Scholar
68 : Brain maturational processes and delayed onset in schizophrenia. Dev Psychopathol 1999; 11:525–543Crossref, Medline, Google Scholar
69 : Hippocampus and amygdala volumes in children and young adults at high-risk of schizophrenia: research synthesis. Schizophr Res 2014; 156:76–86Crossref, Medline, Google Scholar
70 : Anterior cingulate morphology in people at genetic high-risk of schizophrenia. Eur Psychiatry 2012; 27:377–385Crossref, Medline, Google Scholar
71 : Abnormalities in connectivity of white-matter tracts in patients with familial and non-familial schizophrenia. Psychol Med 2011; 41:1691–1700Crossref, Medline, Google Scholar
72 : Neural correlates of visuospatial working memory in the “at-risk mental state”. Psychol Med 2010; 40:1987–1999Crossref, Medline, Google Scholar
73 : Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophr Bull 2014; 40:1105–1116Crossref, Medline, Google Scholar
74 : Association of a risk allele of ANK3 with cognitive performance and cortical thickness in patients with first-episode psychosis. J Psychiatry Neurosci 2014; 39:31–39Crossref, Medline, Google Scholar
75 : Cortical surface area correlates with STON2 gene Ser307Pro polymorphism in first-episode treatment-naive patients with schizophrenia. PLoS One 2013; 8:e64090Crossref, Medline, Google Scholar
76 : Association of variants in DRD2 and GRM3 with motor and cognitive function in first-episode psychosis. Eur Arch Psychiatry Clin Neurosci 2014; 264:345–355Crossref, Medline, Google Scholar
77 : Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci USA 2014; 111:E2066–E2075Crossref, Medline, Google Scholar
78 : Effects of bisphenol-A and other endocrine disruptors compared with abnormalities of schizophrenia: an endocrine-disruption theory of schizophrenia. Schizophr Bull 2009; 35:256–278Crossref, Medline, Google Scholar
79 : Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 2015; 161:102–112Crossref, Medline, Google Scholar
80 : Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology 2007; 32:1216–1223Crossref, Medline, Google Scholar
81 : Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry 2008; 63:759–765Crossref, Medline, Google Scholar
82 : A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med 2010; 40:1409–1422Crossref, Medline, Google Scholar
83 : Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011; 68:128–137Crossref, Medline, Google Scholar
84 : Pharmacogenetic associations of the type-3 metabotropic glutamate receptor (GRM3) gene with working memory and clinical symptom response to antipsychotics in first-episode schizophrenia. Psychopharmacology (Berl) 2015; 232:145–154Crossref, Medline, Google Scholar
85 : Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science 2000; 287:2020–2022Crossref, Medline, Google Scholar
86 : Volume increases in putamen associated with positive symptom reduction in previously drug-naive schizophrenia after 6 weeks antipsychotic treatment. Psychol Med 2012; 42:1475–1483Crossref, Medline, Google Scholar
87 : Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 2003; 60:585–594Crossref, Medline, Google Scholar
88 : Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment: anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull 2011; 37:199–211Crossref, Medline, Google Scholar
89 : The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia? A systematic review. Curr Pharm Des 2009; 15:2535–2549Crossref, Medline, Google Scholar
90 : Changes in caudate volume with neuroleptic treatment. Lancet 1994; 344:1434Crossref, Medline, Google Scholar
91 : Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry 2012; 69:16–26Crossref, Medline, Google Scholar
92 : Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. Neuroimage 2012; 59:986–996Crossref, Medline, Google Scholar
93 : Effects of eight weeks of atypical antipsychotic treatment on middle frontal thickness in drug-naive first-episode psychosis patients. Schizophr Res 2013; 149:149–155Crossref, Medline, Google Scholar
94 : Risperidone in first-episode psychosis: a longitudinal, exploratory voxel-based morphometric study. Schizophr Res 2006; 82:89–94Crossref, Medline, Google Scholar
95 : A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry 2008; 65:746–760Crossref, Medline, Google Scholar
96 : Progressive striatal and hippocampal volume loss in initially antipsychotic-naive, first-episode schizophrenia patients treated with quetiapine: relationship to dose and symptoms. Int J Neuropsychopharmacol 2011; 14:69–82Crossref, Medline, Google Scholar
97 : Magnetic resonance imaging predictors of treatment response in first-episode schizophrenia. Schizophr Bull 2012; 38:569–578Crossref, Medline, Google Scholar
98 : Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev 2013; 37:1680–1691Crossref, Medline, Google Scholar
99 : Volume changes in gray matter in first-episode neuroleptic-naive schizophrenic patients treated with risperidone. J Clin Psychopharmacol 2005; 25:111–117Crossref, Medline, Google Scholar
100 : Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151:1430–1436Link, Google Scholar
101 : Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155:1711–1717Link, Google Scholar
102 : Effect of antipsychotic drugs on brain morphometry: a randomized controlled one-year follow-up study of haloperidol, risperidone, and olanzapine. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1936–1943Crossref, Medline, Google Scholar
103 : Clozapine, ziprasidone, and aripiprazole but not haloperidol protect against kainic acid-induced lesion of the striatum in mice, in vivo: role of 5-HT1A receptor activation. Brain Res 2005; 1043:32–41Crossref, Medline, Google Scholar
104 : Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie 2005; 60:441–460Crossref, Medline, Google Scholar
105 : Effect of chronic olanzapine treatment on nerve growth factor and brain-derived neurotrophic factor in the rat brain. Eur Neuropsychopharmacol 2005; 15:311–317Crossref, Medline, Google Scholar
106 : Quetiapine regulates FGF-2 and BDNF expression in the hippocampus of animals treated with MK-801. Neuroreport 2004; 15:2109–2112Crossref, Medline, Google Scholar
107 : Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology 2004; 29:1063–1069Crossref, Medline, Google Scholar
108 : MRI-morphometric changes in first-episode schizophrenic patients at 14 months follow-up. Schizophr Res 2004; 67:301–303Crossref, Medline, Google Scholar
109 : A volumetric MRI and magnetization transfer imaging follow-up study of patients with first-episode schizophrenia. Schizophr Res 2006; 87:100–108Crossref, Medline, Google Scholar
110 : Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci 2015; 35:267–286Crossref, Medline, Google Scholar
111 : Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology 2014; 39:1020–1030Crossref, Medline, Google Scholar
112 : Neural markers of negative symptom outcomes in distributed working memory brain activity of antipsychotic-naive schizophrenia patients. Int J Neuropsychopharmacol 2013; 16:1195–1204Crossref, Medline, Google Scholar
113 : Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry 2012; 69:1195–1204Crossref, Medline, Google Scholar
114 : Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry 2005; 162:2322–2329Link, Google Scholar
115 : Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by fMRI during a simple motor task. Psychol Med 2001; 31:1065–1078Crossref, Medline, Google Scholar
116 : Functional magnetic resonance imaging correlates of memory encoding in relation to achieving remission in first-episode schizophrenia. Br J Psychiatry 2012; 200:300–307Crossref, Medline, Google Scholar
117 : Stability of emotional dysfunctions? A long-term fMRI study in first-episode schizophrenia. J Psychiatr Res 2007; 41:918–927Crossref, Medline, Google Scholar
118 : White-matter microstructure in previously drug-naive patients with schizophrenia after 6 weeks of treatment. Psychol Med 2013; 43:2301–2309Crossref, Medline, Google Scholar
119 : Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med 2009; 39:1763–1777Crossref, Medline, Google Scholar
120 : Antipsychotic drug effects on motor activation measured by functional magnetic resonance imaging in schizophrenic patients. Schizophr Res 1999; 39:19–29Crossref, Medline, Google Scholar
121 : Neural markers of early remission in first-episode schizophrenia: a volumetric neuroimaging study of the parahippocampus. Psychiatry Res 2012; 201:40–47Crossref, Medline, Google Scholar
122 : MRI predicts remission at 1 year in first-episode schizophrenia in females with larger striato-thalamic volumes. Neuropsychobiology 2014; 69:243–248Crossref, Medline, Google Scholar
123 : White matter integrity as a predictor of response to treatment in first episode psychosis. Brain 2014; 137:172–182Crossref, Medline, Google Scholar
124 : Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev 2012; 36:1140–1152Crossref, Medline, Google Scholar
125 : Classifying spatial patterns of brain activity with machine learning methods: application to lie detection. Neuroimage 2005; 28:663–668Crossref, Medline, Google Scholar
126 : Using genetic, cognitive, and multi-modal neuroimaging data to identify ultra-high-risk and first-episode psychosis at the individual level. Psychol Med 2013; 43:2547–2562Crossref, Medline, Google Scholar
127 : Can structural MRI aid in clinical classification? A machine learning study in two independent samples of patients with schizophrenia, bipolar disorder, and healthy subjects. Neuroimage 2014; 84:299–306Crossref, Medline, Google Scholar
128 : A hybrid machine learning method for fusing fMRI and genetic data: combining both improves classification of schizophrenia. Front Hum Neurosci 2010; 4:192Crossref, Medline, Google Scholar
129 : Neuroimaging biomarkers to predict treatment response in schizophrenia: the end of 30 years of solitude? Dialogues Clin Neurosci 2014; 16:491–503Medline, Google Scholar
130 : Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp 2008; 29:1265–1275Crossref, Medline, Google Scholar
131 : Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 2009; 108:3–10Crossref, Medline, Google Scholar
132 : Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophr Res 2004; 68:49–63Crossref, Medline, Google Scholar



