Intrinsic Hippocampal Activity as a Biomarker for Cognition and Symptoms in Schizophrenia
Abstract
Objective
Identification of biomarkers for cognitive dysfunction in schizophrenia is a priority for psychiatry research. Functional imaging studies suggest that intrinsic “resting state” hippocampal hyperactivity is a characteristic feature of schizophrenia. The relationships between this phenotype and symptoms of the illness, however, are largely unexplored. The authors examined resting hippocampal activity in schizophrenia patients and healthy comparison subjects and analyzed the relationship between intrinsic hippocampal activity and cognitive function in patients as measured by the MATRICS Consensus Cognitive Battery (MCCB).
Method
Twenty-eight schizophrenia patients and 28 age-matched healthy comparison subjects underwent functional “resting state” 3-T MR scanning. Hippocampal activity was extracted by group independent component analysis. Correlation analyses were used to examine the relationship between hippocampal activity and MCCB composite and domain scores in patients, as well as between hippocampal activity and positive and negative symptoms.
Results
Greater activity of the right hippocampus at rest was observed in patients relative to comparison subjects. In patients, a significant negative correlation was observed between right hippocampal activity and composite MCCB T-score. The correlation was driven by the MCCB domains of attention/vigilance, working memory, and visual learning. Hippocampal activity was positively correlated with negative symptoms. MCCB scores were inversely correlated with negative symptoms.
Conclusions
These findings suggest that greater intrinsic hippocampal activity is a characteristic feature of schizophrenia that is broadly associated with cognitive dysfunction, and they support hippocampal activity as a candidate biomarker for therapeutic development.
Despite being the best predictor of functional outcome and quality of life in schizophrenia (1), cognitive dysfunction remains the most poorly treated symptom. The illness is associated with deficits in multiple cognitive domains, including selective and sustained attention, working memory, episodic memory, processing speed, executive function, and social cognition (2). Because of the lack of effective treatments for these deficits, schizophrenia often continues to exact a devastating toll, with 80% of patients remaining unemployed and less than 30% living independently (3).
In order for therapeutic development for cognitive deficits in schizophrenia to move forward, biomarkers must be identified that can be used to determine whether therapeutic candidates elicit the targeted biological effects. In neuropsychiatric research, a biomarker is an indicator of neuronal function, hypothesized to be related to disease mechanisms, that can serve as an immediate and objective measure of the biological effects of therapeutic candidates. An effective biomarker should also be related to clinical symptoms of the illness, such that it can be used to track illness severity as a new treatment is evaluated.
Hippocampal pathology in schizophrenia has emerged as a potential biomarker for therapeutic development. Structural imaging meta-analyses have shown that hippocampal volume is lower in schizophrenia and is correlated with positive, negative, and cognitive symptoms (4–8). Greater hippocampal resting metabolism is also observed in schizophrenia, as evidenced by findings of abnormally high resting blood flow (9–11) and blood volume (12, 13) in the region.
Functional MRI (fMRI) and positron emission tomography studies have demonstrated abnormalities in hippocampal response in patients compared with healthy subjects. A prevailing theme of these findings is hyperactivity during tasks that require minimal or no cognitive load, such as fixation on a point (14), smooth-pursuit eye movements (15), passive viewing of fearful faces (16), and passive listening to noise (17, 18). Intrinsic hyperactivity may contribute to the inability of the region to be recruited during cognitive tasks in which it is thought to be required, such as image encoding (19) and verbal encoding (20). Thus, it is possible that intrinsic hyperactivity contributes to cognitive dysfunction by decreasing the dynamic range over which activity in the region can be modified according to task demands.
Despite the hypothesized role of hippocampal activity in cognitive dysfunction, no study has yet examined the relationship between intrinsic hippocampal activity and cognition in schizophrenia using a comprehensive cognitive battery. The MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery (MCCB) was created under an initiative from the National Institute of Mental Health (NIMH) to “support the development of pharmacological agents for improving the neurocognitive impairments that are a core feature of schizophrenia” by providing a “reliable and valid assessment of cognition at the level of all cognitive domains” (2, 21). The MCCB includes seven cognitive domains: speed of processing; attention and vigilance; working memory; verbal learning; visual learning; reasoning and problem solving; and social cognition. In addition to domain index scores, a composite score is calculated based on the index scores for all domains. Despite the battery’s comprehensive nature, high test-retest reliability, predictive value, relationship to functional outcome, and utility in clinical trials (2, 21, 22), to our knowledge no study has reported an association between MCCB scores and brain function in schizophrenia. Our goals in the present study were to test the hypotheses that 1) relative to healthy subjects, patients with schizophrenia have greater intrinsic “resting state” hippocampal activity, and 2) this activity is inversely related to cognitive ability as assessed by the MCCB. Findings in support of these hypotheses would suggest that hippocampal activity may be useful as a biomarker for therapeutic development.
Method
The study was approved by the Colorado Multiple Institutional Review Board. Among schizophrenia patients, only decisionally capable individuals were eligible for study participation.
Participants
Demographic data for the study participants are provided in Table S1 in the data supplement that accompanies the online edition of this article. Patients and comparison subjects did not significantly differ in age or gender distribution. Two additional subjects were excluded because of excessive movement (>1.5 mm) during scanning. Patients were recruited by referral from a psychiatrist involved in the study (A.O.) and by other local clinicians and mental health professionals. Exclusion criteria were a current diagnosis of substance abuse, neurological disorders, or head trauma, as well as MRI exclusion factors (claustrophobia, weight >300 lb, metal in the body). Clinical assessment was performed at the time of screening, using the Brief Psychiatric Rating Scale (BPRS) (23) and a modified Scale for the Assessment of Negative Symptoms (SANS) (24). The modified SANS used the four domains most closely associated with core negative symptoms: affective flatness, alogia, anhedonia, and apathy (25). The mean total BPRS score was 33.7 (SD=7.5), and the mean total SANS score (sum of the global ratings for each domain) was 4.2 (SD=3.1). All patients were being treated with antipsychotics at the time of the study. All participants provided written informed consent after receiving a complete description of the study, and they received compensation for their participation.
fMRI Parameters
Images were acquired on a 3-T scanner (General Electric, Milwaukee) using a standard quadrature head coil. An inversion-recovery echo planar image (IR-EPI; TI=505 ms) was collected to improve coregistration of functional images. Functional scans were acquired with the following parameters: TR=2,000 ms, TE=30 ms, field of view=240 mm2, matrix=64×64, voxel size=3.75×3.75 mm2, slice thickness=2.6 mm, gap=1.4 mm, interleaved, flip angle=70°. These parameters were used to minimize signal loss in the medial temporal lobes associated with magnetic susceptibility artifact. Resting fMRI scan duration was 10 minutes. Participants were instructed to rest with their eyes open.
fMRI Data Analysis
MRI data were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) in MATLAB, release 2012a (http://www.mathworks.com/products/matlab/). The first four images were excluded for saturation effects. Echo planar images from each subject were realigned to the first volume. The realigned images were then normalized to the Montreal Neurological Institute template using the unified segmentation algorithm (26) on the IR-EPI image and applying the estimated warp parameters to the coregistered EPI data. During normalization, data were resliced to a 3-mm3 voxel size. Finally, functional images were smoothed with an 8-mm full width at half maximum Gaussian kernel.
Group spatial independent component analysis was performed using GIFT, version 1.3g (http://icatb.sourceforge.net). Independent component analysis is a data-driven technique that separates a set of signals into statistically independent components. The advantages of this technique are that it separates signals due to noise (e.g., CSF perturbations) and does not require prespecification of anatomically based seeds of unknown reliability and validity (27). Data were intensity-normalized to improve the accuracy and test-retest reliability of the independent component analysis output (28). The dimensionality of the data from each subject was reduced using principal-component analysis and concatenated into an aggregate data set. Twenty independent sources were estimated in each group with an independent component analysis using the Infomax algorithm (29). Recent methodological studies have reported good reproducibility and correspondence with other analytical methods with this component number (30). Individual subject data sets were then back-reconstructed. Given hippocampal involvement in the default network (31), the component containing hippocampal activity was identified by selecting the component from each group with the highest spatial correlation to a default network mask. The mask contained the posterior parietal cortex, frontal pole, occipitoparietal junction, posterior cingulate, precuneus, and hippocampus, as defined by the Wake Forest University PickAtlas (32, 33). The selected component, averaged across all subjects, is shown in Figure S1 in the online data supplement. Other components were not examined. The hippocampal signal (Figure 1A,B) was extracted as the first eigenvariate from an anatomically defined region of interest (Figure 1C) generated from the PickAtlas. Both right and left hippocampi were examined. The term “activity” as used here reflects the average amplitude of signal intensity fluctuations identified by independent component analysis (34) and spatial template matching. In the context of the present study, these signals are likely dominated by the low-frequency spontaneous oscillations that characterize neuronal activity in the functionally connected default network (35).
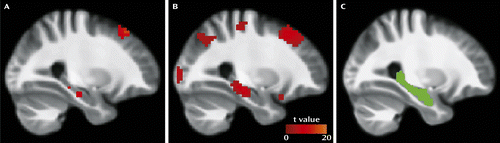
a Intrinsic resting hippocampal activity is shown in healthy subjects (panel A) and in patients with schizophrenia (panel B), with the statistical parametric map thresholded at p<0.001. Panel C shows the hippocampal region of interest used. Activity and region of interest are overlaid on a group average anatomical scan for visualization.
The MCCB
The MCCB has been described in detail elsewhere (21). A brief description of each measure is provided in the online data supplement. The individual tests were administered in the following order: Trail Making Test, part A; Brief Assessment of Cognition in Schizophrenia, symbol coding subtest; Hopkins Verbal Learning Test–Revised; Wechsler Memory Scale–III, spatial span and letter-number span subtests; Neuropsychological Assessment Battery, mazes subtest; Brief Visuospatial Memory Test–Revised; category fluency test; Mayer-Salovey-Caruso Emotional Intelligence Test, managing emotions subtest; and Continuous Performance Test–Identical Pairs Version. Raw scores were converted to normalized T-scores, and seven domain T-scores (speed of processing; attention and vigilance; working memory; verbal learning; visual learning; reasoning and problem solving; social cognition) as well as a composite T-score were derived using the MCCB scoring program. The MCCB and fMRI scans were administered on the same day.
Correlation Analyses
Correlation analyses tested for significant associations between intrinsic hippocampal activity and MCCB T-scores (overall and specific domains) and BPRS and SANS scores. T-scores are normalized scores that have been adjusted for the effects of age, gender, and education.
Results
Hippocampal Activity
Intrinsic activity was greater in the right hippocampus in schizophrenia patients relative to age- and gender-matched healthy comparison subjects (t=4.81, p<0.001) (Figure 2). No group differences were observed in the left hippocampus.
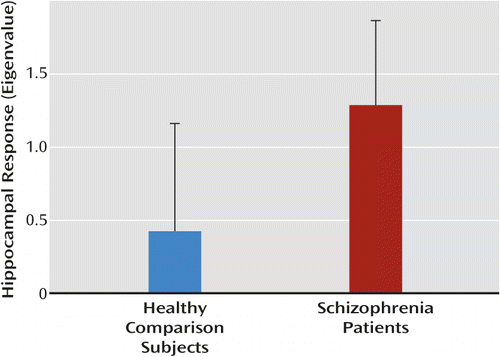
a Error bars indicate standard deviation.
MCCB Assessment
MCCB summary results are presented in Table S2 in the online data supplement. Overall, patients had average T-scores within one standard deviation of the average T-score for a healthy population (a score of 50, with a standard deviation of 10) for the verbal learning, working memory, visual learning, and reasoning/problem solving cognitive domains. Patients scored more than one standard deviation below the healthy population mean for overall composite score, processing speed, attention/vigilance, and social cognition.
MCCB Associations With Hippocampal Activity
A significant negative correlation was observed between MCCB composite score and right hippocampal activity (R=−0.53, p=0.004) (Figure 3). Exploratory correlation analyses revealed that the effect was driven by negative associations between activity and the MCCB domains attention/vigilance, working memory, and visual learning (Table 1). No associations were observed with speed of processing, verbal learning, reasoning/problem solving, and social cognition. No significant associations were observed with the left hippocampus. No significant difference between right and left hippocampal activity was observed. Because no significant associations were observed between right hippocampal activity and age or gender, values were not adjusted for these factors.
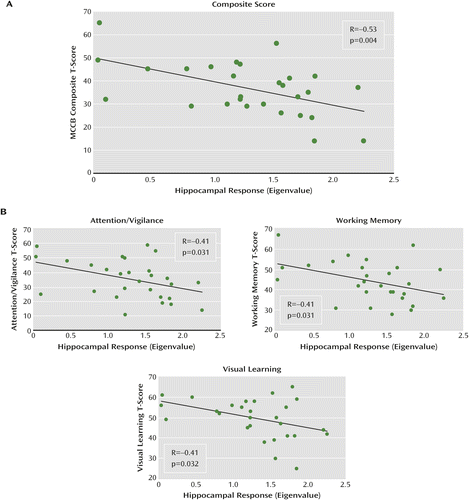
a Panel A shows a significant association between cognition, as measured by the composite score on the MATRICS Consensus Cognitive Battery (MCCB) and intrinsic hippocampal activity in patients. Panel B shows results of an exploratory analysis of the relationships between MCCB domains and hippocampal activity.
| Measure | R | p |
|---|---|---|
| MCCB | ||
| Composite score | –0.53 | 0.004 |
| Domains | ||
| Processing speed | –0.26 | 0.181 |
| Attention/vigilance | –0.41 | 0.031 |
| Working memory | –0.41 | 0.031 |
| Verbal learning | –0.34 | 0.078 |
| Visual learning | –0.41 | 0.032 |
| Reasoning/problem solving | –0.25 | 0.190 |
| Social cognition | –0.37 | 0.054 |
| SANS | 0.42 | 0.028 |
| BPRS | 0.02 | 0.910 |
Clinical Correlates
A significant positive correlation was observed between total SANS score and right hippocampal response (R=0.42, p=0.028) (Figure 4). This relationship was driven by a significant positive correlation with SANS avolition subscore (R=0.46, p=0.013). No significant associations with BPRS scores were observed. Left hippocampal response was not significantly associated with SANS or BPRS scores.
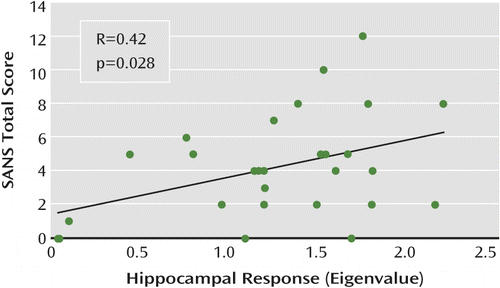
a Negative symptoms are assessed with the Scale for the Assessment of Negative Symptoms (SANS).
Significant negative correlations were observed between total SANS score and the MCCB working memory subscale (R=−0.45, p=0.016) and verbal learning subscale (R=−0.45, p=0.017). A negative correlation with composite MCCB score fell short of statistical significance (R=−0.37, p=0.053).
To ensure that the results were not affected by motion artifacts, the data were reanalyzed using a stringent movement scrubbing routine (36) whereby all volumes that shifted 0.5 mm or more from the previous volume were removed prior to independent component analysis. After this strict criterion was applied, the main findings persisted, with patients showing greater hippocampal response relative to comparison subjects (T=4.51, p<0.001). The inverse correlation between hippocampal activity and composite MCCB score remained significant (R=−0.46, p=0.014), as did the positive correlation between activity and total SANS score (R=0.42, p=0.028).
Discussion
Both study hypotheses—that patients would show greater intrinsic hippocampal activity than healthy subjects and that this activity would be inversely related to cognitive function—were supported. The finding of greater resting hippocampal response is consistent with results of previous studies that have used different but conceptually related techniques, including resting cerebral blood flow and volume (9–13). The present findings may also be consistent with studies showing elevated hippocampal responses in patients during simple or entirely passive sensory processing (14–18). The observed hyperactivity both supports models of hippocampal dysfunction in schizophrenia (37) and increases the appeal of this measure as a potential biomarker.
To our knowledge, this is the first study to report a significant association between intrinsic hippocampal activity and a broad-spectrum measure of cognition in schizophrenia. Beginning with postmortem studies in the 1970s (e.g., reference 38), investigators have repeatedly observed abnormalities in hippocampal structure and function in schizophrenia, with the most recent studies reporting increased general metabolism or activity in the region (9, 12, 13, 39). The cognitive implications of hippocampal dysfunction have largely remained unknown, however, likely because of the lack of standardized methods for examining cognitive function in schizophrenia.
The MCCB was developed in accordance with an NIMH initiative to provide a standardized comprehensive battery of cognitive tests for use in therapeutic development. However, few studies have found associations between neuroimaging-based measures of hippocampal function and global cognition in schizophrenia, although studies have reported correlations with specific cognitive tests (e.g., reference 40). Perhaps the most comparable recent study was by Toulopoulou et al. (41), who reported an association between IQ and right hippocampal volume across a large sample of schizophrenia patients and first-degree relatives. The results of the present study suggest that a therapeutic treatment that reduces intrinsic hippocampal activity may improve cognition in schizophrenia. Indeed, the α7 receptor partial agonists tropisetron and DMXB-A (3-[2,4-dimethoxybenzylidine] anabaseine), which have shown promising effects on cognition in early trials (42, 43), are proposed to modulate inhibitory neurotransmission through activation of nicotinic receptors on GABA-ergic interneurons in the hippocampus (44).
The relationship we observed between high activity and low composite MCCB score was driven by negative correlations between activity and visual learning, attention/vigilance, and working memory, which suggests that these cognitive processes may be particularly affected by hippocampal dysfunction. Although the mechanisms involved are not clear, it is possible that increased activity at “rest” reduces the region’s ability to be recruited according to task demands, resulting in impaired performance.
Multiple lines of evidence suggest that the hippocampus plays an important role in the MCCB domains driving the observed effect. For visual learning, studies have demonstrated recruitment of the right hippocampus in spatial and visual learning tasks (e.g., reference 45). In the context of attention/vigilance, the cholinergic projection from the septal area to the hippocampus is essential for selective attention (46). Furthermore, the hippocampus exhibits sensory information processing properties, in which it is involved in the suppression of responses to repetitive stimulation (47). As a result, it may contribute to selective attention by filtering out irrelevant information. This process is altered in schizophrenia, leading to hyperactivity in the region during the presence of distracting or unimportant noise (17). This hippocampal hyperresponsiveness to distracting noise has been shown to be related to patients’ inability to appropriately engage cortical networks subserving selective attention (48). In regard to working memory, hippocampal recruitment has been proposed as a secondary mechanism by which information can be actively maintained in the brain, specifically when working memory capacity in other areas (e.g., the prefrontal cortex) is exceeded (49). A previous working memory study (50) found increased hippocampal activity in patients relative to controls under conditions of moderate load as well as impaired performance, suggesting that functional pathology of the area may contribute to impairment of working memory in schizophrenia.
Both intrinsic hippocampal activity and MCCB scores were associated with negative symptoms. The observed relationship between cognitive dysfunction and negative symptoms is conceptually in agreement with numerous studies demonstrating stronger relationships between cognition and negative symptoms compared with cognition and positive symptoms (see reference 51). Relative to positive symptoms, cognitive and negative symptoms are more stable over time, are a better predictor of functional outcomes, and are less responsive to medication (1, 51). Given that hippocampal dysfunction, cognitive impairment, and negative symptoms are often present before the first episode of psychosis (12, 52), it is possible to speculate that these phenotypes may constitute core traits of the disorder that may reflect neuropathological status more accurately than do positive symptoms.
Our findings in this study were lateralized to the right. The neurobiological basis for this finding is unclear, as there is no strong evidence to suggest that hippocampal pathology is specific to either hemisphere in schizophrenia. It is unlikely that the lateralized effects were due to a floor or ceiling effect only in the left hippocampus, as no significant difference in activity was observed between the left and right hippocampi. It is possible that the lateralization we observed is a reflection of the type of MCCB tasks with which significant associations were demonstrated. For example, previous studies have found preferential involvement of the right hippocampus in visual learning and memory tasks (45, 53). Patients with schizophrenia also demonstrate a loss of functional connectivity between the right hippocampus and the left prefrontal cortex during working memory (54). Given that factor analyses suggest that the processing speed, attention/vigilance, working memory, and visual learning domains most strongly predict composite MCCB score (55), the relationships between lateralized hippocampal activity and cognitive function in these domains in schizophrenia require further investigation.
Our findings should be considered in the context of the study’s limitations. In order to fully evaluate intrinsic hippocampal activity as a biomarker, its reliability must be established by studying subjects at additional time points, which ideally would be spaced sufficiently far apart for the tracking of changes in symptoms during disease progression. Another limitation is that the sample patient population in this study exhibited less impairment on the MCCB than did samples in other studies (e.g., reference 56). Additional research is needed to determine whether the observed differences in hippocampal activity and associations with MCCB scores hold true for populations with greater cognitive impairment. Finally, it is unclear whether hippocampal hyperactivity and its association with cognitive function are specific to schizophrenia. Studies examining MCCB performance and hippocampal activity in healthy subjects and other patient populations (e.g., patients with bipolar disorder) are needed to examine this possibility. Indeed, previous work has demonstrated that patients with poor working memory performance exhibit reduced right hippocampal gray matter volume compared with healthy subjects, whereas intact performers do not (57), which is suggestive of a complex interaction between cognitive performance, hippocampal pathology, and disease state in schizophrenia.
Conclusions
This study is the first to observe significant inverse correlations between intrinsic hippocampal activity and a broad-based measure of cognition in schizophrenia. Associations were also observed between activity and negative symptoms. Our results suggest that intrinsic hippocampal activity may be a useful biomarker for therapeutic development for cognitive dysfunction in schizophrenia.
1 : What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Link, Google Scholar
2 : Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry 2004; 56:301–307Crossref, Medline, Google Scholar
3 Torrey EF: Surviving Schizophrenia: A Manual for Families, Patients, and Providers, 5th ed. New York, HarperCollins, 2006Google Scholar
4 : Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 2001; 11:520–528Crossref, Medline, Google Scholar
5 : A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1–52Crossref, Medline, Google Scholar
6 : The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res 2004; 70:117–145Crossref, Medline, Google Scholar
7 : An MRI study of temporal lobe abnormalities and negative symptoms in chronic schizophrenia. Schizophr Res 2002; 58:123–134Crossref, Medline, Google Scholar
8 : Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry 2000; 57:769–775Crossref, Medline, Google Scholar
9 : Resting neural activity distinguishes subgroups of schizophrenia patients. Biol Psychiatry 2004; 56:931–937Crossref, Medline, Google Scholar
10 : Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus 2001; 11:543–550Crossref, Medline, Google Scholar
11 : Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology 2010; 256:253–260Crossref, Medline, Google Scholar
12 : Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 2009; 66:938–946Crossref, Medline, Google Scholar
13 : Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013; 78:81–93Crossref, Medline, Google Scholar
14 : SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry 1999; 46:89–93Crossref, Medline, Google Scholar
15 : Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry 2004; 161:315–321Link, Google Scholar
16 : Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry 2005; 57:1011–1019Crossref, Medline, Google Scholar
17 : Increased hippocampal, thalamic, and prefrontal hemodynamic response to an urban noise stimulus in schizophrenia. Am J Psychiatry 2009; 166:354–360Link, Google Scholar
18 : Increased hemodynamic response in the hippocampus, thalamus, and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res 2007; 92:262–272Crossref, Medline, Google Scholar
19 : Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch Gen Psychiatry 2007; 64:999–1014Crossref, Medline, Google Scholar
20 : Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1998; 1:318–323Crossref, Medline, Google Scholar
21 : The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008; 165:203–213Link, Google Scholar
22 : The MATRICS Consensus Cognitive Battery (MCCB): clinical and cognitive correlates. Schizophr Res 2012; 134:76–82Crossref, Medline, Google Scholar
23 : The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
24 : The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl 1989; 7:49–58Crossref, Medline, Google Scholar
25 : Clozapine’s effect on negative symptoms in treatment-refractory schizophrenics. Compr Psychiatry 1994; 35:8–15Crossref, Medline, Google Scholar
26 : Unified segmentation. Neuroimage 2005; 26:839–851Crossref, Medline, Google Scholar
27 : Centenary of Brodmann’s map: conception and fate. Nat Rev Neurosci 2010; 11:139–145Crossref, Medline, Google Scholar
28 Allen E, Erhardt E, Eichele T, Mayer AR, Calhoun V: Comparison of pre-normalization methods on the accuracy and reliability of group ICA results. Presented at the 16th Annual Meeting of the Organization for Human Brain Mapping, June 6–10, 2010, Barcelona (http://mialab.mrn.org/software/gift/publications/2010_OHBM_Elena_ICAPrenormalization_submitted.pdf)Google Scholar
29 : An information-maximization approach to blind separation and blind deconvolution. Neural Comput 1995; 7:1129–1159Crossref, Medline, Google Scholar
30 : Functional connectivity during resting-state functional MR imaging: study of the correspondence between independent component analysis and region-of-interest-based methods. AJNR Am J Neuroradiol 2012; 33:180–187Crossref, Medline, Google Scholar
31 : The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008; 1124:1–38Crossref, Medline, Google Scholar
32 : An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239Crossref, Medline, Google Scholar
33 : Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 2007; 164:450–457Link, Google Scholar
34 : Independent component analysis of fMRI data: examining the assumptions. Hum Brain Mapp 1998; 6:368–372Crossref, Medline, Google Scholar
35 : Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007; 8:700–711Crossref, Medline, Google Scholar
36 : Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59:2142–2154Crossref, Medline, Google Scholar
37 : Neuroimaging biomarkers for early drug development in schizophrenia. Biol Psychiatry (Epub ahead of print, Oct 3, 2013)Google Scholar
38 : Possible changes in striatal and limbic cholinergic systems in schizophrenia. Arch Gen Psychiatry 1977; 34:1319–1323Crossref, Medline, Google Scholar
39 : Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar
40 : Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J Psychiatry 1993; 150:1849–1855Link, Google Scholar
41 : The relationship between volumetric brain changes and cognitive function: a family study on schizophrenia. Biol Psychiatry 2004; 56:447–453Crossref, Medline, Google Scholar
42 : Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry 2008; 165:1040–1047Link, Google Scholar
43 : Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry 2012; 169:974–981Link, Google Scholar
44 : Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron 2011; 70:20–33Crossref, Medline, Google Scholar
45 : Right hippocampal contribution to visual memory: a presurgical and postsurgical study in patients with temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 1998; 65:665–669Crossref, Medline, Google Scholar
46 : What is remembered? Role of attention on the encoding and retrieval of hippocampal representations. J Physiol 2009; 587:2837–2854Crossref, Medline, Google Scholar
47 : Neuronal substrates of sensory gating within the human brain. Biol Psychiatry 2003; 53:511–519Crossref, Medline, Google Scholar
48 : The effect of distracting noise on the neuronal mechanisms of attention in schizophrenia. Schizophr Res 2012; 142:230–236Crossref, Medline, Google Scholar
49 : The role of the hippocampus in retaining relational information across short delays: the importance of memory load. Learn Mem 2011; 18:301–305Crossref, Medline, Google Scholar
50 : A systematic fMRI investigation of the brain systems subserving different working memory components in schizophrenia. Eur J Neurosci 2009; 30:693–702Crossref, Medline, Google Scholar
51 : Cognitive impairment and negative symptoms in geriatric chronic schizophrenic patients: a follow-up study. Schizophr Res 1996; 22:223–231Crossref, Medline, Google Scholar
52 : Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 1994; 344:1398–1402Crossref, Medline, Google Scholar
53 : The hippocampus and memory of verbal and pictorial material. Learn Mem 2002; 9:99–104Crossref, Medline, Google Scholar
54 : Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. PLoS ONE 2009; 4:e7911Crossref, Medline, Google Scholar
55 : Factor structure of the MATRICS Consensus Cognitive Battery (MCCB) in schizophrenia. Schizophr Res 2013; 146:244–248Crossref, Medline, Google Scholar
56 : Feasibility and pilot efficacy results from the multisite Cognitive Remediation in the Schizophrenia Trials Network (CRSTN) randomized controlled trial. J Clin Psychiatry 2012; 73:1016–1022Crossref, Medline, Google Scholar
57 : Neuropsychological near normality and brain structure abnormality in schizophrenia. Am J Psychiatry 2009; 166:189–195Link, Google Scholar



