Brain Structural Abnormalities in a Group of Never-Medicated Patients With Long-Term Schizophrenia
Abstract
Objective:
This study investigated brain morphometry in chronically ill but never-medicated schizophrenia patients and whether the relation of age to morphometric abnormalities differed from that in healthy subjects.
Method:
In a cross-sectional design, high-resolution T1-weighted images were acquired from 25 schizophrenia patients with untreated chronic illness lasting 5 to 47 years and 33 matched healthy comparison subjects. Cortical thickness and gray matter volume were compared in the two groups. In regions with significant group differences, nonlinear modeling of age-related effects was used to test for accelerated decline in the patients.
Results:
Schizophrenia patients had less cortical thickness in the bilateral ventromedial prefrontal cortices, left superior temporal gyrus, and right pars triangularis, relative to comparison subjects, and greater cortical thickness in the left superior parietal lobe. The relation of age to cortical thickness indicated faster age-related cortical thinning in the right ventromedial prefrontal cortex, left superior temporal gyrus, and right pars triangularis in patients than in comparison subjects, but slower thinning in the left superior parietal lobe. Gray matter volume was greater in the putamen bilaterally and smaller in the right middle temporal gyrus and right lingual gyrus of the patients, but age-related effects did not differ from those of the comparison subjects.
Conclusions:
The accelerated age-related decline in prefrontal and temporal cortical thickness in never-medicated schizophrenia patients suggests a neuroprogressive process in some brain regions. Slower age-related cortical thinning of the superior parietal cortex and striatal volumetric abnormalities unrelated to age suggest different pathological processes over time in these regions.
Since the hypothesis of progressive brain changes in schizophrenia was put forward by Kraepelin in his concept of dementia praecox (1), this possibility has been of sustained interest. Meta-analyses provide compelling evidence for structural brain abnormalities in schizophrenia (2, 3), while longitudinal studies further suggest that there may be a progression of these effects early in the illness (4–6).
Across follow-up studies of first-episode patients, a complex pattern of structural alterations has been described over the course of illness, with frontotemporal cortex abnormalities being most consistently observed (4, 7, 8). However, follow-up studies of first-episode patients are restricted to the early illness course for the foreseeable future because of the decades required to track lifespan effects. Further, these longitudinal studies are all conducted with patients treated with antipsychotic medications, which may adversely impact brain anatomy and confound tests for disease effects over the illness course (3). Therefore, questions about how different brain regions may be altered over the longer-term course of schizophrenia and the degree to which identified changes represent treatment or illness effects remain unresolved.
In this circumstance, cross-sectional studies that examine structural abnormalities in relation to age or disease duration in chronically ill patients never treated with antipsychotic medication may provide important insights about longer-term changes in brain anatomy over the illness course (9, 10), without the confounding influences of antipsychotic and other psychiatric medications (11–13) or other secondary factors (14). This could help determine the degree to which progressive effects seen in childhood-onset schizophrenia (15) are also seen following illness onset in more typical patients, with onset in early adulthood, and whether such changes are seen in specific brain regions in patients never exposed to antipsychotic medications.
In developing countries, such samples of never-treated chronically ill individuals can still be identified in both rural and urban areas. Recently, 17 Chinese medication-naive chronically ill patients were found to have less gray matter volume and cortical thickness in the temporal lobe than healthy subjects (16). However, in that study, the potential relationship between morphometric abnormalities and age or illness duration was not reported. In a similar sample from India, McCreadie et al. found that duration of untreated illness was not related to the size of lentiform nuclei or to ventricle-hemisphere ratio abnormalities, but relations to regional neocortical volumes and thickness were not examined (17).
In the present study, we conducted MRI studies of never-medicated chronic schizophrenia patients with illness durations ranging from 5 to 47 years. We sought first to identify brain regions where cortical thickness or gray matter volume differed from that of matched comparison subjects, and we then determined whether within those regions there were group differences in age-related abnormalities in brain anatomy. Primary analyses were performed with the cortical thickness data; key findings from brain volume analyses are summarized in this article, with the full analyses presented in the data supplement that accompanies on the online version of the article.
Method
Participants
The study included 25 chronically ill, never-medicated schizophrenia patients. Most patients were identified from the Mental Health Screening Program supported by West China Hospital, a project aiming to provide psychiatric care to people living in the community who have serious mental illness but who have not had access to or previously sought psychiatric treatment. When a suitable individual was identified from local medical or police records, a psychiatrist from the program visited the person’s home, accompanied by a psychiatrist from our research team. Nearly 90% of identified individuals agreed to participate in this imaging study. All were living with their families, as is common in west China, and family members provided history information. Clinical diagnostic evaluations were conducted by the research team to confirm clinical diagnoses with the Structured Interview for DSM-IV Axis I Disorders (SCID). If the diagnosis of schizophrenia was confirmed, affected individuals were brought to our MRI facility for scans, after which the majority began treatment.
These patients, 18 in their 40s or older, were recruited from within approximately 35 km of downtown Chengdu in west China. The mean duration of illness was 21 years. Patients were all of Han ancestry. Fifteen lived in rural areas, mostly in small villages 10 km away from Chengdu with tight social networks; the others were living in urban or suburban areas. The onsets of symptoms were gradual and insidious for 16 of the patients, while the other nine developed acute psychosis after some significant life trauma, such as the loss of a close relative, parental marital difficulties, or infidelity by the patient’s spouse. Before the study, 14 of them had not been involved in any type of employment and had not contributed any significant physical work in their home or community, and 11 were able to care for themselves in many activities of daily living. The families of 23 patients had regular incomes, even though some were relatively low, and two patients and their families were living primarily with support from the government. The family members worked mainly as manual laborers and farmers, and their education levels were not high. Most of the patients and their family members had 9 years of typical education, including 6 years in primary school and 3 years in junior high school, as is most common in China. All of the patients had been well taken care of by their families since the onset of their illness.
These patients had not received any prior treatment with psychiatric medications for various reasons, primarily because of 1) parental concern about family stigma associated with mental illness (prominent in 11 cases), 2) a lack of understanding or recognition of the severity of mental illness in a child or spouse (noteworthy in seven cases), 3) poor socioeconomic conditions that limited travel and funds for medical care (four cases), and 4) emotional rejection of medical care by the family due to conflicts with physicians when the patient was first brought to medical attention close to the time of illness onset (three cases). As a result of these factors, each patient had been cared for and sheltered in the parents’ home without medical care through the course of the illness. While not common practice now, in the years during and immediately following the Cultural Revolution, many patients with serious mental illness did not receive psychiatric care for similar reasons.
Duration of untreated illness was evaluated by the Nottingham Onset Schedule (18) (with information provided by the patients, other family members, and other sources). Psychopathology ratings were obtained by using the Positive and Negative Syndrome Scale (PANSS) (19), and they included total scores as well as factor scores derived by using the previously published six-factor structure pattern of PANSS items (20).
Healthy comparison subjects (N=33) were recruited through poster advertisements; they were from the same geographical region as the patients and had similar socioeconomic and educational backgrounds. The nonpatient edition of the SCID was used to establish the lifetime absence of psychiatric illness, and there was no known history of major psychiatric illness in their first-degree relatives. The patients and comparison subjects were matched in age, sex, and years of education (Table 1). Age distributions for male and female subjects did not differ across subject groups.
| Characteristic | Schizophrenia Patients (N=25) | Healthy Comparison Subjects (N=33) | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |
| Age (years) | 46.68 | 13.54 | 46.21 | 13.79 | 0.90 |
| Education (years) | 7.88 | 3.83 | 9.56 | 3.70 | 0.10 |
| Duration of illness (years) | 21.04 | 12.00 | |||
| Age at onset (years) | 25.64 | 6.53 | |||
| PANSS scores | |||||
| Total | 88.71 | 15.44 | |||
| Negative symptoms | 22.50 | 6.59 | |||
| Positive symptoms | 24.08 | 7.34 | |||
| General psychopathology symptoms | 41.54 | 7.50 | |||
| Thought disturbance | 13.00 | 3.67 | |||
| Activation | 8.29 | 2.12 | |||
| Paranoid | 8.42 | 3.08 | |||
| Depression | 6.29 | 2.73 | |||
| Anergia | 10.71 | 3.86 | |||
| Impulsive aggression | 8.00 | 2.84 | |||
| N | % | N | % | p | |
| Gender | 0.91 | ||||
| Female | 11 | 44 | 15 | 45 | |
| Male | 14 | 56 | 18 | 54 | |
TABLE 1. Demographic and Clinical Characteristics of Never-Medicated Patients With Long-Term Schizophrenia and Healthy Comparison Subjects
No participants had a history of significant systemic or neurological illness. All were right-handed as assessed with the Annett Handedness Scale (21). The following exclusion criteria applied to both groups: 1) age younger than 18 years or older than 80 years, 2) history of substance abuse, 3) pregnancy, and 4) major physical illness, such as cardiovascular disease or hepatitis, as assessed by clinical evaluations and medical records. The ethics committee of Sichuan University approved the study, and all participants gave written informed consent to their participation.
Data Acquisition
MRI examinations were performed on a 3-T GE Signa EXCITE scanner (General Electric, Milwaukee, Wis.) with an eight-channel phase array head coil. High-resolution T1-weighted images were acquired with a volumetric three-dimensional spoiled gradient recall (SPGR) sequence (repetition time=8.5 ms, echo time=3.4 ms, flip angle=12°). A field of view of 240×240 mm2 was used with an acquisition matrix comprising 256 readings of 128 phase-encoding steps, producing 156 contiguous 1.0-mm coronal slices. The final matrix of T1-weighted images was automatically interpolated in plane to 512×512, yielding an in-plane resolution of 0.47×0.47 mm2. T1- and T2-weighted magnetic resonance (MR) images were inspected by an experienced neuroradiologist; no scan artifacts or gross brain abnormalities were observed in any participant.
Imaging Processing
Cortical modeling and volumetric segmentation of structural MRI data were performed with the FreeSurfer package (version 5.1.0, http://surfer.nmr.mgh.harvard.edu/). Cortical thickness was measured as the difference between equivalent vertices in the pial and gray/white matter surfaces (22), by using both intensity and continuity information from the entire three-dimensional MR volume in segmentation and deformation procedures. The reliability of the method has been validated against histological analysis on postmortem brains (23) and manual measurements (24), and test-retest reliability is high (25). Briefly, the main process included automated registration to Talairach space, segmentation of the subcortical white matter and gray matter volumetric structures, intensity normalization, tessellation of gray matter and white matter boundaries, and automated topology correction and surface deformation following intensity gradients to optimally place the gray/white and gray/CSF borders defined at the location with the greatest shift in signal intensity (22, 26–30). The surface maps, following registration of all subjects’ cortical reconstructions to a common average surface, were analyzed after the interpolation steps, and they are thus capable of detecting submillimeter differences between groups (22). Since the surface-based analysis was restricted to the cortical mantle, we used volumetric analysis to assess deep gray matter by means of diffeomorphic anatomical registration using the exponentiated lie algebra (DARTEL) toolbox (31). Details of the standard analysis procedures are presented in the online data supplement. Postprocessing visual inspection for quality of both imaging processes was conducted without knowledge of subject characteristics.
Statistical Analysis
In the graphical interface of FreeSurfer known as QDEC (Query, Design, Estimate, Contrast), a smoothing step with 10 mm full width at half maximum was initiated to average the cortical thickness data across participants in the common spherical coordinate system. Then vertex-by-vertex cortical maps of the patients and comparison subjects were compared by using a general linear model with age, sex, and total intracranial volume included as covariates. Nonparametric cluster-wise correction for multiple comparisons was performed by means of the FreeSurfer Monte Carlo simulation tool with a corrected cluster-forming threshold of p<0.05 (32).
To identify differential relationships between anatomical measures and age in regions where group differences were detected, we first extracted and averaged values of cortical thickness and gray matter volume in regions with significant group differences for each subject. Aging effects on the human brain are believed to follow a nonlinear trajectory in schizophrenia patients (15, 33), as in healthy individuals (34). Therefore, nonlinear regression analyses using a quadratic model of age effects in relation to anatomic measures were conducted for each group. Age-related trajectories of brain changes have been modeled with this approach as it is straightforward and flexible for accommodating different nonlinear effects (34–36). These models were compared across groups to determine whether there was a significant differential rate of age-related change in patients and comparison subjects. This was achieved by conducting F tests to determine whether the shapes of regression curves were significantly different (37). A “leave-one-out” cross-validation approach was adopted to identify potential high-impact cases in modeling of age effects and group differences in aging effects; in all cases, the significant effects reported below were confirmed. The effect of duration of illness was examined with the same modeling approach in patients. Partial correlations controlling for age and sex were determined for associations between structural brain measurements and clinical symptom severity ratings at the time of the scans, as determined by PANSS total score, subscales, and factor scores.
Results
Group Differences in Cortical Thickness
In relation to the healthy comparison subjects, the patients showed significantly less cortical thickness in the bilateral ventromedial prefrontal cortices extending laterally to the orbitofrontal cortices and in the left superior temporal gyrus and right pars triangularis, while greater cortical thickness was found in the left superior parietal lobe extending to the occipital cortex (p<0.05, Figure 1).
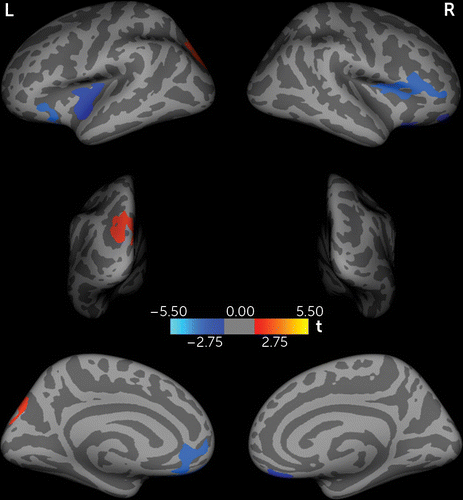
FIGURE 1. Differences in Cortical Thickness Between Never-Medicated Patients With Long-Term Schizophrenia and Healthy Comparison Subjectsa
a Significance was determined by Monte Carlo simulation. Less cortical thickness in patients than in comparison subjects is indicated by blue/cool color, and greater thickness relative to comparison subjects is indicated by red/warm color. L, left hemisphere; R, right hemisphere.
Age-Related Differences in Cortical Thickness
The schizophrenia patients had a significant association between greater age and less cortical thickness in the bilateral ventromedial prefrontal cortices, left superior temporal gyrus, and right pars triangularis, but no significant relationship with age was found in the left superior parietal lobe. In the healthy comparison subjects, measurements in the bilateral ventromedial prefrontal cortices, left superior temporal gyrus, and right pars triangularis showed no significant association with age, whereas left superior parietal lobe cortical thickness decreased with age (Figure 2, Table 2).
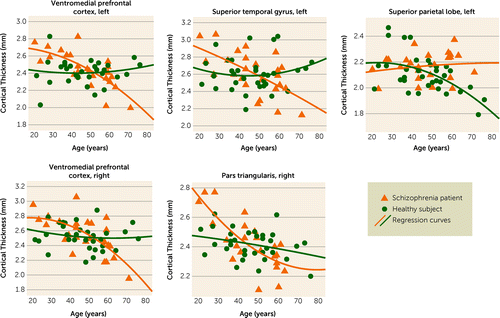
FIGURE 2. Quadratic Models of Cortical Thickness in Relation to Age in Brain Regions With Significant Differences in Cortical Thickness Between Never-Medicated Patients With Long-Term Schizophrenia and Healthy Comparison Subjectsa
a L, left hemisphere; R, right hemisphere. Two outliers (43 and 71 years) were excluded when age effects were modeled in the right pars triangularis, to better track the potential trajectory in later life of individuals with untreated schizophrenia.
| Model for Schizophrenia | Model for Healthy Comparison Subjects | Group Comparison | ||||
|---|---|---|---|---|---|---|
| Region | Mean Rate of Decline/Increase (mm/year)b | p | Mean Rate of Decline/Increase (mm/year)b | p | F | p |
| Ventromedial prefrontal cortex, right | –0.013 | 0.001 | –0.002 | 0.66 | 1.79 | 0.02 |
| Ventromedial prefrontal cortex, left | –0.011 | <0.001 | +0.001 | 0.84 | 1.56 | 0.053 |
| Pars triangularis, right | –0.009 | <0.001 | –0.003 | 0.22 | 1.79 | 0.02 |
| Superior temporal gyrus, left | –0.011 | 0.005 | +0.001 | 0.66 | 1.80 | 0.02 |
| Superior parietal lobe, left | +0.001 | 0.75 | –0.006 | 0.003 | 1.65 | 0.04 |
TABLE 2. Average Rate of Change in Cortical Thickness per Year for Never-Medicated Patients With Long-Term Schizophrenia and Healthy Comparison Subjectsa
As age-related effects were not seen in some brain regions in our healthy subjects where they have been reported in previous studies of healthy but somewhat older individuals, we conducted a similar analysis on a larger sample of healthy subjects (N=102) by adding data from previously reported healthy subjects to help establish the representativeness of our comparison data. All findings were consistent with the effects seen in the matched healthy comparison sample used for group comparison in the present study. Details of this analysis are reported in the online data supplement.
In the direct group comparisons of age-related differences in the areas of interest, we observed differences indicating faster age-related reductions of cortical thickness in the right ventromedial prefrontal cortex, left superior temporal gyrus, and right pars triangularis and significantly slower age-related cortical thinning of the left superior parietal lobe in the patients than in the healthy comparison subjects (p<0.05). A trend toward greater age-related cortical thickness reduction in the patients was observed in the left ventromedial prefrontal cortex (p=0.053, Table 2).
When these effects were examined in relation to disease duration rather than age, we found that greater duration was significantly associated with lower cortical thickness of the bilateral ventromedial prefrontal cortices, left superior temporal gyrus, and right pars triangularis (p<0.05), a relationship similar to the modeled age-related effects (see Figure S2 in the online data supplement). No significant association was observed between the mean cortical thickness of the left superior parietal lobe and the duration of illness in patients (p>0.05). Cortical thickness of the right pars triangularis was negatively correlated with age at onset in patients (p<0.05), while those of other regions showed no significant associations. No correlation was found between cortical thickness measures and current symptom severity as reflected in PANSS scores.
Voxel-Based Morphometry Analysis of Gray Matter Volume
The patients had larger gray matter volumes in the left putamen extending to the insula and in the right putamen, but they had smaller gray matter volume in the right lingual gyrus extending to the cuneus and in the right middle temporal gyrus, relative to the healthy comparison subjects (Figure 3). Gray matter volume of the bilateral putamen was not significantly associated with age in either group, or with illness duration or clinical symptoms in patients. Smaller gray matter volume of the right middle temporal gyrus was significantly associated with greater age in both patients and healthy subjects, but there was no significant group difference in these effects. Volumes of the right lingual gyrus were not related to age or illness duration, but they were negatively associated with scores on the PANSS activation factor.
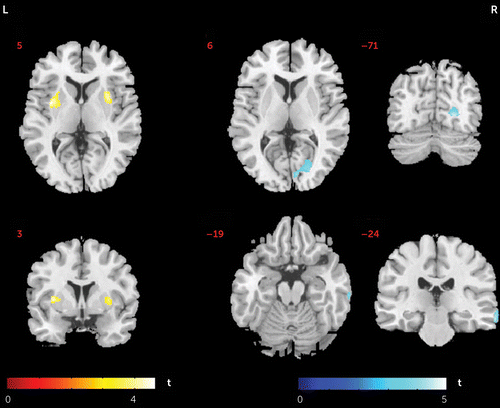
FIGURE 3. Differences in Gray Matter Volume Between Never-Medicated Patients With Long-Term Schizophrenia and Healthy Comparison Subjectsa
a Greater cortical volume in patients than in comparison subjects is indicated by red/warm color, and lower volume is indicated by blue/cold color. L, left hemisphere; R, right hemisphere.
Discussion
By studying this relatively unique sample of chronically ill but never-medicated schizophrenia patients, the current study adds important new evidence about gray matter changes over the course of schizophrenia without the potential confounding influences of antipsychotic treatment. Surface-based analysis demonstrated cortical abnormalities in schizophrenia patients involving prefrontal, temporal, and parietal lobe regions. More important, age-related effects suggest a more rapid rate of cortical thinning in the right ventromedial prefrontal cortex, right pars triangularis, and left superior temporal gyrus in patients than in healthy comparison subjects, whereas age-related changes in the posterior superior parietal lobe appeared to be less rapid than in the healthy subjects. Volumetric analysis demonstrated significant abnormalities in cortical and subcortical gray matter, notably including greater bilateral putamen volume in patients, but these effects did not have differential aging effects in the schizophrenia patients. Thus, the most important finding of our study is that age-related changes in cortical thickness suggestive of a neuroprogressive process in schizophrenia were found in the prefrontal and temporal cortices, while abnormalities in other areas, including the putamen and parietal cortex, did not show accelerated age-related changes, suggesting different pathological or compensatory processes. These results reinforce the notion that schizophrenia may involve neuroprogressive changes in specific brain regions over the course of the disease, potentially resulting from neuropathologic mechanisms, including the previously reported shrinkage of neuropil, reduced neuron size, and cell loss (38–40).
Previous longitudinal studies with 10-year follow-up of treated patients have shown that the lateral ventricles exhibited increased enlargement over the course of illness (41, 42), suggesting atrophic changes over time. The current study of chronically ill but never-treated schizophrenia patients is consistent with this observation and extends it in two important ways. First, our findings suggest that this pattern may continue in some brain regions later in life. In fact, our modeling of age-related changes in cortical thickness suggests that neuroprogressive mechanisms may begin slowly in the early phase of the illness and become more prominent later in the course of illness in specific brain regions. This may account for why we and others have not identified progressive changes early in the illness course (43–45). However, the degree to which these changes represent a direct pathophysiological consequence of the disease or a secondary factor, such as long-term disengagement from social relations and other life activities, remains to be determined. Second, our findings suggest that changes over the course of illness are not secondary to antipsychotic treatment. Whether other regions might also show similar effects in addition to those identified is possible, as we may have lacked statistical power to detect less robust effects.
The accelerated reduction in cortical thickness in the frontal cortex in patients supports the view that frontal cortex abnormalities are an important component in schizophrenia-related morbidity, and it suggests that the identified frontal lobe regions may be more vulnerable to the long-term effects of the illness (3, 4, 46, 47). Previously, medial prefrontal cortex pathology has been identified in multimodal imaging studies (48), and volume loss in the ventromedial prefrontal cortex in schizophrenia has been associated with deficits in the theory of mind skills needed for social relationships (49). Prolonged social isolation might contribute to progressive gray matter loss in this region, as suggested by animal models (50).
Another frontal region exhibited age-related reductions in cortical thickness, the pars triangularis. This lateral-posterior part of the inferior frontal gyrus has been observed to manifest cortical thinning in untreated first-episode patients (45). While volume reduction of this region has been suggested to represent a risk factor for schizophrenia (51), the neuroprogressive changes in this region may represent a neurobiological underpinning of the early clinical changes associated with the prodrome period preceding onset of psychosis. Given the importance of this region for speech and language processing, there may be a broader clinical significance to changes in this region.
The superior temporal gyrus was also found to show progressive changes in our study, consistent with studies of early illness course in which progressive cortical alterations manifested their greatest severity in the frontal and temporal lobes (5, 52). Cortical thinning in the left superior temporal gyrus was observed in a previous study with untreated chronically ill patients (16). Temporal volume change has emerged as a promising predictor of treatment response, as gray matter loss in this region has been found in treatment-resistant schizophrenia (53). Thus, progressive changes in this region might contribute to higher rates of poor outcome reported for patients with chronic schizophrenia than for first-episode patients (5).
In individuals at high risk for psychosis, as in first-episode patients, cortical thinning and gray matter reduction of the superior temporal gyrus have been shown to progressively worsen over time (7, 54, 55). The observed cortical thinning of the superior temporal gyrus appears to be a familial trait linked to schizophrenia (56) that begins at or before illness onset and worsens over the course of illness. It also should be noted that such progressive effects seen in the frontal and temporal cortices have also been observed in childhood-onset schizophrenia, though the progressive volume loss in those patients seems to occur at a faster rate than is suggested by our data (57, 58).
In contrast to findings of accelerated age-related cortical thinning in some brain regions, we observed a lower rate of decline in cortical thickness with age in the left superior parietal lobe in patients. Gray matter reduction and cortical thinning of the superior parietal lobe have been reported in previous MRI studies with first-episode or early-course patients (59, 60). However, in the meta-analysis by Olabi et al. (3), no progressive decline in patients was seen in parietal gray matter. In addition, increased cortical thickness in the parietal cortex of treated patients with chronic schizophrenia was reported previously in another study (5). Without the confounds of medication, our findings suggest that changes over the course of illness in the parietal cortex are different from those seen in the frontal and temporal cortices. This might represent either a compensatory response related to increased use of the parietal cortex to support perceptual or other processes or a different pathological process, such as insufficient synaptic pruning with distinct anatomic and functional consequences (61). As our observation of reduced age-related effects in the parietal cortex is relatively novel, more work is needed to confirm the effect and investigate its causes and clinical implications.
Another finding from our study was the larger bilateral putamen seen in the volumetric analysis. This effect has in the past been attributed to antipsychotic medication (11, 12, 62), so our study adds important new knowledge by showing that illness-related processes can also induce hypertrophic striatal changes independent of effects of antipsychotic treatment. In our previous study of drug-naive first episode patients, we observed increased spontaneous neural activity in the putamen bilaterally by means of resting-state fMRI (44), a finding similar to earlier observations of increased striatal metabolic rates in untreated schizophrenia patients (63). As the dorsal striatum is rich in dopamine, it is intriguing to speculate that an abnormality in dopaminergic systems targeted by antipsychotic medication, or other factors related to increased striatal neural activity, might be a relevant pathophysiological process underlying these striatal changes (64, 65). Given that striatal volume has been shown to be low in first-episode patients (66) and that volume enlargement was not associated with age or illness duration effects in this region, it appears most likely that high neural and metabolic activity in the striatum after illness onset may increase striatal volumes early in the course of illness and then not progress appreciably after that time.
Several limitations in our study are important to recognize. First, the cross-sectional nature of our study limits any strong inference about age-related trajectories of brain changes. While we could not identify clinical or demographic factors related to illness duration that might artifactually induce differences from the comparison subjects in age-related effects, an unidentified factor related to patient age, rather than aging itself, might bias our findings. This limitation of our study design needs to be considered in balance with the impossibility of conducting prospective longitudinal studies of untreated patients. In this context, our study of a relatively large sample of never-treated patients with chronic schizophrenia who have a wide range of illness duration provides an important and novel approach for studying brain changes over the course of illness. The approach has two main advantages: it provides a window into later-course illness effects that will not be available from longitudinal studies for many years, and it avoids medication confounds that complicate interpretation of all ongoing longitudinal studies.
There are other limitations to be considered as well. First, while our sample of never-treated chronically ill patients was larger than those in previous studies, it was not large for precisely modeling age-related effects. In particular, the degree to which the age-related changes progress gradually or are prominent early or late is difficult to determine. Examination of the modeled age effects for different brain regions in Figure 2 provides somewhat conflicting clues in this regard. While the models of the regions of interest tend to cross around age 50 (with the patient data showing atrophic changes relative to the healthy subjects only after that point), the rate of change in the modeled age effects seems to increase not long after illness onset. Research with larger samples is needed to model age effects and the rate of change more precisely so this issue can be addressed. Second, our study design did not include a parallel matched group of chronically ill treated schizophrenia patients with comparable disease duration and demographic characteristics. Last, some age-related effects were not seen in regions where they might be expected, especially in the comparison subjects. This may be due to our sample size but perhaps more likely to the fact that the individuals in our sample were mostly under the age of 60 years, when atrophic age-related changes are notably less prominent than in elderly individuals.
In conclusion, by studying a rare sample of never-medicated chronically ill schizophrenia patients with a wide range of illness duration, we found a faster rate of prefrontal and temporal cortical thinning and striatal hypertrophy, which may represent core components of the pathophysiological process of schizophrenia over the course of illness, effects that could not be attributed to antipsychotic treatment. These findings may provide important insights into the course and regional specificity of progressive brain changes associated with schizophrenia in the decades after illness onset.
1 Kraepelin E: Dementia Praecox and Paraphrenia (1919). Translated by Barclay RM; edited by Robertson GM. New York, Robert E Krieger, 1971Google Scholar
2 : Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull 2013; 39:1129–1138Crossref, Medline, Google Scholar
3 : Are there progressive brain changes in schizophrenia? a meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry 2011; 70:88–96Crossref, Medline, Google Scholar
4 : Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 2011; 70:672–679Crossref, Medline, Google Scholar
5 : Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry 2011; 68:871–880Crossref, Medline, Google Scholar
6 : The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull 2008; 34:312–321Crossref, Medline, Google Scholar
7 : Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. Neuroimage 2012; 59:986–996Crossref, Medline, Google Scholar
8 : Three-year longitudinal population-based volumetric MRI study in first-episode schizophrenia spectrum patients. Psychol Med 2014; 44:1591–1604Crossref, Medline, Google Scholar
9 : Evidence for progressive brain abnormalities in early schizophrenia: a cross-sectional structural and functional connectivity study. Schizophr Res 2014; 159:31–35Crossref, Medline, Google Scholar
10 : Age-related brain trajectories in schizophrenia: a systematic review of structural MRI studies. Psychiatry Res 2013; 214:83–93Crossref, Medline, Google Scholar
11 : Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011; 68:128–137Crossref, Medline, Google Scholar
12 : Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry 2005; 62:361–370Crossref, Medline, Google Scholar
13 : A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med 2010; 40:1409–1422Crossref, Medline, Google Scholar
14 : The myth of schizophrenia as a progressive brain disease. Schizophr Bull 2013; 39:1363–1372Crossref, Medline, Google Scholar
15 : Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 2010; 67:728–734Crossref, Medline, Google Scholar
16 : A combined DTI and structural MRI study in medicated-naïve chronic schizophrenia. Magn Reson Imaging 2014; 32:1–8Crossref, Medline, Google Scholar
17 : Structural brain differences between never-treated patients with schizophrenia, with and without dyskinesia, and normal control subjects: a magnetic resonance imaging study. Arch Gen Psychiatry 2002; 59:332–336Crossref, Medline, Google Scholar
18 : Determining the chronology and components of psychosis onset: the Nottingham Onset Schedule (NOS). Schizophr Res 2005; 80:117–130Crossref, Medline, Google Scholar
19 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
20 : Positive and Negative Syndromes in Schizophrenia: Assessment and Research. New York, Psychology Press, 1991Google Scholar
21 : A classification of hand preference by association analysis. Br J Psychol 1970; 61:303–321Crossref, Medline, Google Scholar
22 : Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97:11050–11055Crossref, Medline, Google Scholar
23 : Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology 2002; 58:695–701Crossref, Medline, Google Scholar
24 : Thinning of the cerebral cortex in aging. Cereb Cortex 2004; 14:721–730Crossref, Medline, Google Scholar
25 : Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 2006; 32:180–194Crossref, Medline, Google Scholar
26 : Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage 1999; 9:179–194Crossref, Medline, Google Scholar
27 : Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355Crossref, Medline, Google Scholar
28 : Cortical surface-based analysis, II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999; 9:195–207Crossref, Medline, Google Scholar
29 : Automatically parcellating the human cerebral cortex. Cereb Cortex 2004; 14:11–22Crossref, Medline, Google Scholar
30 : A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004; 22:1060–1075Crossref, Medline, Google Scholar
31 : A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38:95–113Crossref, Medline, Google Scholar
32 : Association between psychotic symptoms and cortical thickness reduction across the schizophrenia spectrum. Cereb Cortex 2013; 23:61–70Crossref, Medline, Google Scholar
33 : Abnormal cortical growth in schizophrenia targets normative modules of synchronized development. Biol Psychiatry 2014; 76:438–446Crossref, Medline, Google Scholar
34 : Brain structural trajectories over the adult lifespan. Hum Brain Mapp 2012; 33:2377–2389Crossref, Medline, Google Scholar
35 : Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging 2005; 26:1245–1260Crossref, Medline, Google Scholar
36 : Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 2006; 30:730–748Crossref, Medline, Google Scholar
37 : Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007; 36:1065–1073Crossref, Medline, Google Scholar
38 : Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 1991; 48:996–1001Crossref, Medline, Google Scholar
39 : Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry 1998; 55:215–224Crossref, Medline, Google Scholar
40 : The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17–25Crossref, Medline, Google Scholar
41 : Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Res 2004; 130:57–70Crossref, Medline, Google Scholar
42 : Ten year progressive ventricular enlargement in schizophrenia: an MRI morphometrical study. Psychiatry Clin Neurosci 2001; 55:41–47Crossref, Medline, Google Scholar
43 : Global and regional cortical thinning in first-episode psychosis patients: relationships with clinical and cognitive features. Psychol Med 2011; 41:1449–1460Crossref, Medline, Google Scholar
44 : Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry 2013; 170:1308–1316Link, Google Scholar
45 : Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia. Schizophr Bull 2015; 41:201–210Crossref, Medline, Google Scholar
46 : Association between a longer duration of illness, age and lower frontal lobe grey matter volume in schizophrenia. Behav Brain Res 2008; 193:132–139Crossref, Medline, Google Scholar
47 : Smaller frontal gray matter volume in postmortem schizophrenic brains. Am J Psychiatry 2002; 159:1983–1991Link, Google Scholar
48 : Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry 2010; 15:823–830Crossref, Medline, Google Scholar
49 : Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol Psychiatry 2011; 70:1169–1178Crossref, Medline, Google Scholar
50 : Effects of social isolation rearing on the limbic brain: a combined behavioral and magnetic resonance imaging volumetry study in rats. Neuroscience 2009; 159:21–30Crossref, Medline, Google Scholar
51 : Localized gray matter volume reductions in the pars triangularis of the inferior frontal gyrus in individuals at clinical high-risk for psychosis and first episode for schizophrenia. Schizophr Res 2012; 137:124–131Crossref, Medline, Google Scholar
52 : Longitudinal progression of frontal and temporal lobe changes in schizophrenia. Schizophr Res 2012; 139:1–6Crossref, Medline, Google Scholar
53 : Extensive grey matter volume reduction in treatment-resistant schizophrenia. Int J Neuropsychopharmacol 2015; 18(7)Crossref, Google Scholar
54 : Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophr Bull 2011; 37:839–849Crossref, Medline, Google Scholar
55 : Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry 2009; 66:366–376Crossref, Medline, Google Scholar
56 : Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res 1998; 32:161–167Crossref, Medline, Google Scholar
57 : Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA 2001; 98:11650–11655Crossref, Medline, Google Scholar
58 : Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull 2008; 34:30–36Crossref, Medline, Google Scholar
59 : Cortical thickness in first-episode schizophrenia patients and individuals at high familial risk: a cross-sectional comparison. Schizophr Res 2013; 151:259–264Crossref, Medline, Google Scholar
60 : Parietal lobe volume deficits in schizophrenia spectrum disorders. Schizophr Res 2007; 89:35–48Crossref, Medline, Google Scholar
61 : Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex 2007; 17:415–424Crossref, Medline, Google Scholar
62 : Do antipsychotic drugs affect brain structure? a systematic and critical review of MRI findings. Psychol Med 2009; 39:1763–1777Crossref, Medline, Google Scholar
63 : Kraepelinian and non-Kraepelinian schizophrenia subgroup differences in cerebral metabolic rate. Schizophr Res 2002; 55:25–40Crossref, Medline, Google Scholar
64 : Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull 2010; 36:472–485Crossref, Medline, Google Scholar
65 : Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep 2007; 9:329–336Crossref, Medline, Google Scholar
66 : Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry 1998; 155:774–778Abstract, Google Scholar



