Lateral and Medial Hypofrontality in First-Episode Schizophrenia: Functional Activity in a Medication-Naive State and Effects of Short-Term Atypical Antipsychotic Treatment
Abstract
OBJECTIVE: The dorsolateral prefrontal cortex and the anterior cingulate cortex are critical components of the brain circuitry underlying executive control. The objective of this study was to investigate control-related dorsolateral prefrontal cortex functioning and conflict-related anterior cingulate cortex functioning in a group of never medicated first-episode schizophrenia patients to determine whether both regions show dysfunction at illness onset. A second objective was to assess short-term effects of atypical antipsychotic medication on dorsolateral prefrontal cortex and anterior cingulate cortex functioning. METHOD: First-episode schizophrenia patients (N=23) and healthy comparison subjects (N=24) underwent event-related fMRI and performed a cognitive task designed to functionally dissociate the two regions. Four weeks after initiation of pharmacotherapy for patients, a subset of 11 patients and 16 comparison subjects underwent a repeat assessment. RESULTS: At baseline, patients exhibited hypoactivation in the dorsolateral prefrontal cortex and anterior cingulate cortex. After 4 weeks of antipsychotic treatment, the patients demonstrated improved functioning in the anterior cingulate cortex but not in the dorsolateral prefrontal cortex. CONCLUSIONS: These findings confirm the presence of dorsolateral prefrontal cortex dysfunction early in the course of schizophrenia and suggest that anterior cingulate cortex functioning may be altered at illness onset as well. Results also suggest that anterior cingulate cortex functioning may be especially sensitive to remedial antipsychotic treatment effects. These findings are consistent with an emerging literature documenting short-term benefits of atypical antipsychotic medication for the neural circuitry underlying cognitive deficits in schizophrenia.
Deficits in the executive control of cognition have long been a major research focus in schizophrenia. Functional neuroimaging studies have documented relationships between impairments in executive control and abnormal cortical activation in several regions of the prefrontal cortex. An extensive literature has linked impairments in strategic processes, such as representing and maintaining goals and allocating attentional resources, to dysfunction of the dorsolateral prefrontal cortex (1–9). A second frontal area reported to show altered activation in schizophrenia is the anterior cingulate cortex (10–13) on the medial surface of the frontal lobes. This region is posited to have a critical role in evaluative functions such as self-monitoring of performance (14). Although much has been learned about relationships between these components of executive control and their neural underpinnings, many questions remain concerning their respective roles in the etiology and course of schizophrenia. The present study examined dorsolateral prefrontal cortex and anterior cingulate cortex activity in never-medicated first-episode schizophrenia patients. We used a novel task designed to functionally dissociate the two regions and sought whether dysfunction of either or both of these regions would be observable at illness onset.
The failure of patients to activate the dorsolateral prefrontal cortex is among the most consistent findings in schizophrenia (1, 2, 5–7, 9). Functional magnetic resonance imaging (fMRI) studies of first-episode patients have shown dorsolateral prefrontal cortex hypoactivation (2–4), although hyperactivation has also been reported (8). The executive control function of interest in the present study was context processing (i.e., the ability to use context information to guide task-appropriate behavior), which robustly activates the dorsolateral prefrontal cortex in healthy subjects (2, 5, 15). Specific context-processing deficits have been reported in schizophrenia patients over a range of clinical states (16, 17), as well as in their healthy first-degree relatives (18). These deficits are associated with disorganization symptoms of the illness (4, 7, 17, 19) and are accompanied by reduced dorsolateral prefrontal cortex activity (2, 4, 5). We therefore predicted hypoactivation in the dorsolateral prefrontal cortex would be associated with context-processing deficits in this medication-naive, first-episode schizophrenia patient group.
With regard to functional alterations in the anterior cingulate cortex, neuroimaging studies in healthy participants have suggested that the anterior cingulate cortex is responsive to the occurrence of cognitive conflict and signals the need for recruitment of control processes to resolve conflict (14, 20). Schizophrenia patients show reduced error-related anterior cingulate cortex activity and less of a performance adjustment after error commission (11) or associated with response conflict (20), suggesting that an internal monitoring function of the anterior cingulate cortex is impaired in schizophrenia. To our knowledge, anterior cingulate cortex functioning in first-episode patients has not been reported, so it is unknown whether conflict-related anterior cingulate cortex dysfunction is present before possible effects of medication or illness chronicity arise.
Addressing the question of antipsychotic medication effects on dorsolateral prefrontal cortex and anterior cingulate cortex activity was another goal of the current study. Few neuroimaging studies have directly examined changes in functional activation due to treatment. In a recent PET study, Lahti et al. (21) found that clozapine, but not haloperidol, normalized the reduced anterior cingulate cortex activation pattern observed after medication withdrawal. Honey et al. (22) reported increased activation in the right dorsolateral prefrontal cortex and supplementary motor area/cingulate gyrus in chronic patients after substitution of risperidone for typical antipsychotics. Reviews of second-generation atypical antipsychotics agree that they may offer modest benefits for impaired cognition in schizophrenia (23, 24). If so, one would expect to observe corresponding changes between on- and off-medication states in functional brain activation. We tested this hypothesis in the current study, directly comparing dorsolateral prefrontal cortex and anterior cingulate cortex activation during the unmedicated state to that after 4 weeks of stabilization with second-generation antipsychotic medication.
Method
Participants
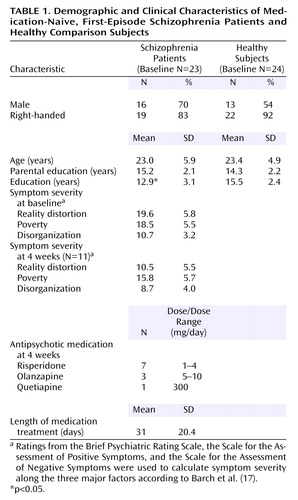
Participants at baseline were 23 medication-naive patients with first-episode schizophrenia and 24 healthy comparison subjects. Of these participants, a subset of 11 patients and 16 comparison subjects were scanned again after 4 weeks. All patients were part of a larger study of first-episode psychosis and were recruited if they were experiencing any type of psychotic symptom and it was their first psychiatric hospitalization or contact with outpatient psychiatric services. After baseline assessments, patients were treated naturalistically by their treating psychiatrists and followed longitudinally. Diagnostic confirmation occurred 6 months after index hospitalization through case conferences based on chart review and assessment with the Structured Clinical Interview for DSM-IV (SCID). Two of the patients had limited previous exposure to neuroleptics before baseline, but exclusion criteria were enforced by limiting total lifetime continuous treatment to 2 weeks or less with no more than three doses of oral neuroleptics during the month preceding study entry. Four patients had previous exposure to antidepressants, and two had been exposed to anxiolytics. All psychotropic medications were washed out at least 72 hours before the baseline scan. After baseline, five patients dropped out of the study, two were excluded from follow-up analysis because of poor medication compliance, and one was excluded because of poor comprehension of the task during the second scan.
Healthy comparison participants, recruited through community advertisements, were evaluated with the nonpatient version of the SCID and were excluded for any lifetime history of an axis I disorder or family history of psychotic disorders. Exclusion criteria for both patients and comparison subjects were the following: 1) age older than 50 or younger than 12, 2) DSM-IV mental retardation, 3) substance dependence within the past 6 months or substance abuse within the past month, or 4) lifetime history of significant neurologic disorder or head trauma or current medical condition that could influence CNS function or structure. See Table 1 for summary of demographic and clinical variables.
All participants provided written informed consent after the procedures had been fully explained. Additional safeguards to ensure that informed consent for patients was properly obtained included having them repeat back information about research participation to check comprehension and screening out patients who were floridly psychotic or exhibited obvious cognitive impairment.
Cognitive Task
Participants viewed a centrally presented red or green square at the beginning of each trial. An arrow probe appeared 7 seconds later in the same location pointing either to the left or to the right. The green square cue required subjects to respond to the probe with a button press using the ipsilateral hand (i.e., the hand on the same side toward which the arrow was pointing), whereas the red square cue required a response with the contralateral hand, a reversal of the usual stimulus-response mapping. This task, Preparing to Overcome Prepotency, exploits the Simon spatial incompatibility effect (25) in which an imperative stimulus appearing opposite to the response side results in slower response times than when stimulus and response appear on the same side. The Simon effect was augmented by an expectancy manipulation, such that same-sided responding was more frequent (green square cues were presented in 70% of the trials). During the period after a red square cue and before the arrow probe, subjects had to recall and maintain the instructions associated with the cue in order to successfully overcome the prepotent response tendency and respond with the contralateral hand. In this period we predicted a greater activation of the dorsolateral prefrontal cortex with red square cues relative to green square cues. At the time of response to the arrow probe, it was predicted that the conflict inherent in overriding the more automatic response would engage the anterior cingulate cortex.
The Preparing to Overcome Prepotency task was presented in five blocks of 24 trials. Stimulus durations were 500 msec, with a 7-second interval between cue and probe and an 11.5-second interval following the probe to allow for resolution of the hemodynamic response. Subjects practiced the task before being scanned and were instructed to respond as quickly and accurately as possible.
Neuroimaging Methods
Acquisition
Functional scans were acquired using a 3-T GE Signa whole body scanner with a standard head coil. Twenty-eight contiguous axial slices (thickness=3.2 mm) with 3.125 mm2 in-plane resolution were obtained beginning 12.8 mm below the anterior commissure-posterior commissure line. Scans used a single-shot T2*-weighted spiral scanning pulse sequence (TR=1500 msec, TE=18 msec, flip angle=70°, field of view=20 cm), allowing full image acquisition every 1.5 seconds. Thirteen scans were acquired during each 19.5-second trial. Structural images were obtained before and in the same plane as functional images by using a standard T1-weighted pulse sequence. High-resolution anatomical images were collected before functional images.
Preprocessing
Functional images were reconstructed and movement was corrected by using Automated Image Registration (26). A maximum movement criterion of 6 mm or degrees in any direction was applied, which resulted in two comparison subjects and two patients being excluded because of excessive movement. Following these exclusions there were no differences between groups in average movement in any direction (pitch, roll, yaw rotations, or x, y, z plane shift for absolute and incremental movement; baseline: Wilks’s lambda=0.66 [F=1.27, df=12, 30, p>0.28]; 4 weeks: Wilks’s lambda=0.53 [F=0.97, df=12, 14, p>0.50]). A six-parameter rigid-body algorithm (26) was used to register each participant’s structural T1-weighted image to their own high-resolution spoiled gradient-recall acquisition (SPGR) image. A 30-parameter nonlinear warping algorithm (27) was used to align each participant’s SPGR image to the Montreal Neurological Institute (MNI) single-subject high-resolution anatomical reference brain (28). Both sets of parameter estimates were applied to the functional T2*-weighted images to bring all participant data into alignment with the MNI reference brain. The data were then smoothed in three dimensions using an 8-mm full width at half maximum kernel to accommodate individual differences in brain morphology.
Statistical Analysis
Given the a priori hypotheses involving the dorsolateral prefrontal cortex and anterior cingulate cortex, we first conducted confirmatory analyses using previously identified functional regions of interest that showed control-related activation within the dorsolateral prefrontal cortex (Brodmann’s area 9, Talairach coordinates: x=–41, y=18, z=28; 37 voxels [15]) and conflict-related activation within the anterior cingulate cortex (Brodmann’s area 32, Talairach coordinates: x=0, y=15, z=41; 23 voxels [29]). These regions were brought into the common MNI reference space by using the same aforementioned transformation algorithms. Time series for each region of interest were then generated for each subject, and average change in activation from baseline for the cue period (scans 1–5) and the probe period (scans 6–13) were examined separately. Following the confirmatory analyses, exploratory analyses of the entire image were performed by using voxel-wise analyses of variance (ANOVAs) with subject as a random factor, group as a between-subject factor (comparison subjects versus schizophrenia patients), and cue (green versus red) and scan (1–5 [cue period] versus 6–13 [probe period]) as within-subject factors. fMRI data were analyzed for correct trials only, ensuring analysis of on-task performance. Voxel-wise statistical maps were thresholded for significance at p<0.005 (uncorrected) using an 8-voxel contiguity criterion (30). Correlations between individual differences in brain activity, symptoms, and task performance were also examined.
Results
Task Performance
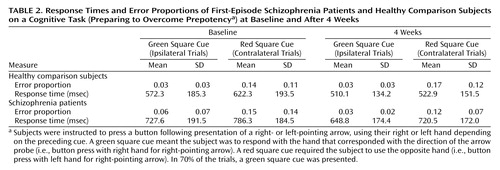
Table 2 shows basic performance data on the Preparing to Overcome Prepotency task. In a series of repeated-measures ANOVAs (two performance measures and two time points), main effects of condition were observed (all F>5.25, all p<0.03), indicating slower response times and higher error rates across groups on red cue (contralateral) trials compared with green cue (ipsilateral) trials. For response times at both time points, main effects of group were also observed (F>4.70, p<0.04), reflecting slower overall response times in patients. None of the ANOVAs revealed significant group-by-condition interactions (F<2.0, p>0.18).
Functional Neuroimaging at Baseline
Dorsolateral prefrontal cortex
Figure 1 illustrates the time series for dorsolateral prefrontal cortex activity following the cue presentation in the Preparing to Overcome Prepotency task. ANOVA revealed a significant main effect of scan (F=4.04, df=4, 180, p<0.005), a significant group-by-condition interaction (F=8.68, df=1, 45, p<0.01), and a significant group-by-condition-by-scan interaction (F=3.75, df=4, 180, p<0.01). These results reflect increasing activity within the dorsolateral prefrontal cortex in healthy subjects following the red square cue, which signaled the need to overcome the prepotent response to the upcoming probe. In schizophrenia patients, no increased dorsolateral prefrontal cortex activity was observed following either cue. Within-group analyses confirmed a significant cue-by-scan interaction (F=3.88, df=4, 92, p<0.01) for comparison subjects but not for patients (F=1.41, df=4, 88, p>0.23).
Anterior cingulate cortex
Figure 1 also shows the time series for anterior cingulate cortex activity following the arrow probe. ANOVA revealed main effects of condition (F=10.01, df=1, 45, p<0.005) and scan (F=37.91, df=7, 315, p<0.001), with a significant condition-by-scan interaction (F=2.71, df=7, 315, p<0.05) and a significant group-by-condition-by-scan interaction (F=2.10, df=7, 315, p<0.05), suggesting that conflict-related modulation of anterior cingulate cortex activity was reduced in patients relative to comparison subjects. Within-group ANOVAs indicated that the condition-by-scan interaction term was significant for healthy subjects (F=2.76, df=7, 161, p<0.01) but not for patients (F=1.95, df=7, 154, p<0.07).
Functional Neuroimaging at 4 Weeks
Dorsolateral prefrontal cortex
Figure 2 displays the time series for dorsolateral prefrontal cortex activity following the cue presentation for both groups after 4 weeks during which the patients had been receiving atypical antipsychotic medication. Between-group ANOVAs revealed a significant main effect of scan (F=6.89, df=4, 100, p<0.001) and a condition-by-scan interaction (F=3.37, df=1, 100, p<0.05). The group-by-condition-by-scan interaction observed at baseline was no longer significant at 4 weeks (F=0.67, df=4, 100, p>0.60), indicating no group difference in the extent to which dorsolateral prefrontal cortex activation was modulated by degree of cognitive control required.
Anterior cingulate cortex
Figure 2 also illustrates anterior cingulate cortex activity during the probe period. ANOVA resulted in main effects of condition (F=5.96, df=1, 25, p<0.05) and scan (F=25.72, df=7, 175, p<0.001), with a significant condition-by-scan interaction (F=4.19, df=7, 175, p<0.001) indicating that both groups showed similar conflict-related modulation of anterior cingulate cortex activity.
Effect of Medication
In order to more directly examine the effect of medication on dorsolateral prefrontal cortex and anterior cingulate cortex functioning, time-series data were analyzed only for the subset of patients who returned for follow-up testing (N=11). Baseline dorsolateral prefrontal cortex and anterior cingulate cortex activity for these patients is shown in the first panel in Figure 2. For dorsolateral prefrontal cortex activity, a two-by-two-by-five within-group ANOVA, with run (baseline versus 4-week), condition (red versus green square cue) and scan number (1 through 5) as within-subject factors resulted in no significant main effects or interactions (all p>0.25).
For anterior cingulate cortex activity, within-group ANOVAs revealed main effects of run (F=5.36, df=1, 10, p<0.05) and scan (F=13.26, df=7, 70, p<0.001), with a significant run-by-condition interaction (F=5.29, df=1, 10, p<0.05) and a significant run-by-condition-by-scan interaction (F=2.53, df=7, 70, p<0.05). The latter result reflects a greater anterior cingulate cortex response to trials with red square cues than green square cues at 4 weeks compared with baseline.
Exploratory Analysis
A within-group exploratory analysis across the entire image of the baseline data showed a network of control-related activation (red square cue > green square cue) in both groups during the cue period in lateral frontal regions (left Brodmann’s area 10), medial frontal regions (Brodmann’s area 8, rostral Brodmann’s area 32, and Brodmann’s area 24/23), and parietal regions (Brodmann’s area 40; right in healthy subjects, left in patients). During the arrow probe period, both groups activated medial frontal regions (right Brodmann’s area 9/10) and right primary motor and superior parietal regions (left Brodmann’s area 7/40). Healthy comparison subjects activated the caudal anterior cingulate cortex (Brodmann’s area 32). A between-group analysis was consistent with the confirmatory analysis for cue activity, with comparison subjects showing significantly greater left dorsolateral prefrontal cortex activity regions (Brodmann’s area 8 and Brodmann’s area 9) than patients. Bilateral superior parietal regions (Brodmann’s area 7 and Brodmann’s area 40/7) and right inferior parietal regions (Brodmann’s area 7) showed a similar pattern. Although patients failed to show significant anterior cingulate cortex activity during the probe, no significant between-group differences were observed in this region. Patients did show reduced activation in the left inferior frontal gyrus (Brodmann’s area 44), supplementary motor area (Brodmann’s area 6), right postcentral gyrus (Brodmann’s area 3), and left superior parietal cortex (Brodmann’s area 7).
Correlations Between Brain Activation, Symptoms, and Task Performance
Correlations between maximum dorsolateral prefrontal cortex and anterior cingulate cortex activation during red square trials and symptoms were examined in patients. At baseline, patients with greater disorganization symptoms had lower dorsolateral prefrontal cortex activation (r=–0.58, p<0.01), whereas the correlations with the other two symptom dimensions were not significant (reality distortion: r=0.16; poverty symptoms: r=–0.27). Conflict-related activation in the anterior cingulate cortex during the probe period was associated with both disorganization symptoms (r=–0.54, p<0.05) and poverty symptoms (r=–0.50, p<0.05) but not with reality distortion (r=0.20). At 4 weeks, no significant correlations were found between activation and symptoms. Furthermore, there were no significant correlations between change in symptom ratings and change in maximum dorsolateral prefrontal cortex or anterior cingulate cortex activation from baseline to 4 weeks.
Correlations in comparison subjects at baseline between response time interference and maximum fMRI signal for red square trials were consistent with those reported in MacDonald et al. (15), who used a similar task design (cue-period dorsolateral prefrontal cortex activity: r=–0.35, p<0.05, one-tailed; probe-period anterior cingulate cortex activity: r=0.33, p=0.06, one-tailed). In patients at baseline, neither of these correlations was significant nor were they significant at 4 weeks in either subject group.
Discussion
We used a novel task designed to probe independent functioning of the dorsolateral prefrontal cortex and anterior cingulate cortex in medication-naive, first-episode schizophrenia patients. Healthy subjects showed increased activity in the left dorsolateral prefrontal cortex following a cue that signaled the upcoming need to overcome a prepotent response, whereas patients failed to activate this region. These results indicate dorsolateral prefrontal cortex dysfunction early in the course of schizophrenia, before the initiation of medication, consistent with previous reports (1–4, 6). Moreover, patients rated as having severe disorganization symptoms tended to have lower dorsolateral prefrontal cortex activation, consistent with earlier work showing similar associations between disorganization symptoms and both hypofrontality (4, 7) and impaired context processing (17, 19).
We also observed a decrease in anterior cingulate cortex activation in patients relative to comparison subjects, an initial finding indicating, as with the dorsolateral prefrontal cortex, functional impairment at illness onset before long-term exposure to neuroleptic medication. This group difference was not detected, however, in the exploratory analysis for conflict-related activity in this region, suggesting the effect may not be as large as that observed in the dorsolateral prefrontal cortex (which did show parallel results in both the confirmatory and exploratory analyses). At baseline, decreased anterior cingulate cortex peak activation was associated with greater symptom expression, specifically with poverty and disorganization symptoms. The latter association is consistent with previous findings that anterior cingulate cortex functional abnormalities are associated with disorganization symptoms (31, 32). To what degree observed associations between disorganization symptoms and dorsolateral prefrontal cortex and anterior cingulate cortex dysfunction share common variance is unknown but may have implications for the role of impaired cognitive control at the systems level in behavioral and conceptual disorganization in schizophrenia.
Effects of Antipsychotic Medication
A subset of patients and healthy participants underwent repeat fMRI after the patients had received 4 weeks of atypical antipsychotic treatment. There was no significant improvement in dorsolateral prefrontal cortex function over this interval. In comparison, Honey et al. (22) indicated increased right prefrontal activation during a working memory task with substitution of risperidone for typical antipsychotic medications. However, the average time that patients were treated before the repeat scan (6 weeks) was longer than in the present study (31 days). We are currently conducting a further study with an 8-week stabilization period to ensure that medication effects on cognition and brain functioning, if present, have sufficient time to emerge.
We did observe a significant medication effect on anterior cingulate cortex functioning during the response period of the task. These results are consistent with those of Honey et al. (22) and Lahti et al. (21), who both reported greater supplementary motor area/cingulate (Brodmann’s area 6/32) activity with atypical relative to typical antipsychotics. Thus, the present findings contribute to an emerging literature whereby anterior cingulate cortex functioning appears to be among the most consistently sensitive to treatment effects. The mechanism by which short-term atypical antipsychotic regimens enhance task-related anterior cingulate cortex functioning is unknown, but a likely candidate is increased cortical dopamine availability (33), since there is evidence for modulation of impaired anterior cingulate cortex activation by experimental dopaminergic manipulation in schizophrenia (34).
Limitations of the present study include a small number of first-episode patients for the analysis of treatment effects, a relatively brief duration of treatment, and treatment with different atypical antipsychotics with different receptor binding profiles. Since the first two factors would result in lower power to detect treatment effects and the latter in increased variability, our reported negative finding with regard to a treatment effect on dorsolateral prefrontal cortex functioning should be interpreted with some reservation, whereas the treatment effect observed for anterior cingulate cortex functioning can be interpreted with more confidence. Another general limitation was the lack of clear behavioral effect in the scanner that would reflect a context-processing deficit (although some would consider this an advantage, since performance was not different across groups). In contrast, we found that data from the AX version of the Continuous Performance Test in these same participants at baseline indicated a specific context-processing deficit: patients had greater BX errors than healthy subjects (a high context-processing demand condition) but a similar number of AY errors (a task-difficulty control condition; see Barch et al. [17] for task details). We infer from these divergent behavioral results that the Preparing to Overcome Prepotency task is less sensitive to group differences in context processing than the AX version of the Continuous Performance Test possibly because of the long interval between cue and probe used in the scanner. This long delay might have enabled subjects to use a different strategy to maintain a level of performance comparable to that of healthy subjects, such as encoding and rehearsing the cue as an item independent of context, and then retrieving the appropriate stimulus-response mapping at the onset of the probe.
In conclusion, the current study found evidence for hypoactivation of both the dorsolateral prefrontal cortex and anterior cingulate cortex at illness onset. Impairments in frontal lobe functions were associated with core clinical features of schizophrenia before the initiation of antipsychotic medication. Short-term medication effects on conflict-related anterior cingulate cortex activation were observed, whereas medication effects on control-related dorsolateral prefrontal cortex activation were not significant. These findings contribute to an emerging picture in the literature of the demonstrated short-term benefit of atypical antipsychotics to the brain circuitry that underlies impaired cognition in schizophrenia.
 |
 |
Presented in part at the ninth annual meeting of the Organization for Human Brain Mapping, New York, June 18–22, 2003. Received Nov. 1, 2004; revision received Feb. 16, 2005; accepted March 7, 2005. From the Department of Psychiatry, University of Pittsburgh Medical School, Pittsburgh; the Department of Psychology, University of Minnesota, Minneapolis; the Department of Psychology, Princeton University, Princeton, N.J.; and the Department of Psychiatry, University of California, Davis. Address correspondence and reprint requests to Dr. Carter, UC Davis Imaging Research Center, 4701 X St., Sacramento, CA 95817; [email protected] (e-mail). Supported by funds received from NIMH (MH-59883 and MH-45156) and NIH/NCRR/GCRC grant M01 RR-00056. The authors thank Drs. Matcheri S. Keshavan, Gretchen Haas, and Debra Montrose and the clinical core staff of the Conte Center for the Neuroscience of Mental Disorders (MH-45156, D. Lewis, principal investigator) for their assistance in diagnostic and psychopathological assessments. The authors also thank Eric Yablonsky, Avram Holmes, and Jean Miewald for their assistance in data collection and management.
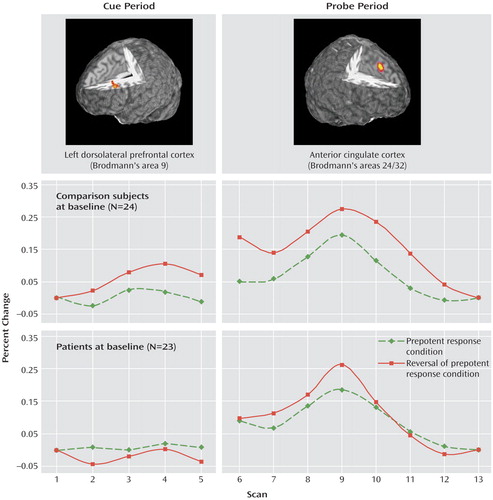
Figure 1. Dorsolateral Prefrontal Cortex and Anterior Cingulate Cortex Activity During Performance of a Cognitive Task in Medication-Naive, First-Episode Schizophrenia Patients and Healthy Comparison Subjectsa
aCue period time series shows control-modulated activation in the dorsolateral prefrontal cortex (in which greater activation would be expected during preparation to overcome a prepotent response). Probe period time series shows conflict-modulated activation in the anterior cingulate cortex (in which greater activation would be expected with the conflict inherent in overcoming the prepotent response).
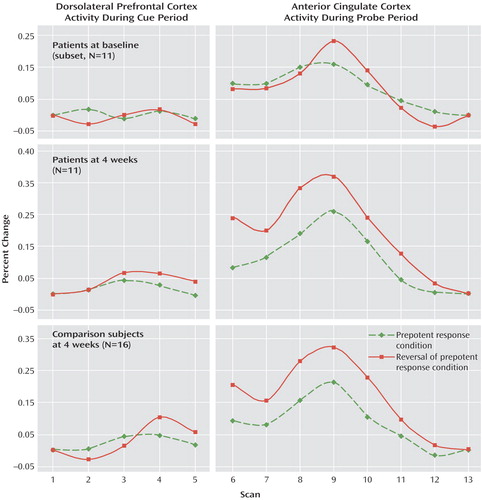
Figure 2. Performance on the Preparing to Overcome Prepotency Task at Four Weeks in Medication-Naive, First-Episode Schizophrenia Patients and Healthy Comparison Subjects, With Direct Change From Baseline to Four Weeks in Patientsa
aBaseline time series are presented only from the subset of N=11 patients with 4-week scans.
1. Andreasen NC, Rezai K, Alliger R, Swayze VW II, Flaum M, Kirchner P, Cohen G, O’Leary DS: Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with Xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943–958Crossref, Medline, Google Scholar
2. Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald AW, Noll DC, Cohen JD: Selective deficits in prefrontal cortex function in medication naive patients and schizophrenia. Arch Gen Psychiatry 2001; 58:280–288Crossref, Medline, Google Scholar
3. Goodman JM, Seidman LJ, Patti M, Strous RD, Strauss M, Caplan B, Patel JK, Zimmet S, Jenkin B, Green AI: A functional MRI study of working memory in first-episode schizophrenic patients. Schizophr Res 1999; 36:221–222Google Scholar
4. MacDonald AW III, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD: Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry 2005; 162:475–484Link, Google Scholar
5. MacDonald A, Carter C: Event-related fMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J Abnorm Psychol 2003; 112:689–697Crossref, Medline, Google Scholar
6. Parellada E, Catafau AM, Bernardo M, Lomena F, Catarineu S, Gonzalez-Monclus E: The resting and activation issue of hypofrontality: a single photon emission computed tomography study in neuroleptic-naive and neuroleptic-free schizophrenic female patients. Biol Psychiatry 1998; 44:787–790Crossref, Medline, Google Scholar
7. Perlstein WM, Carter CS, Noll DC, Cohen JD: Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry 2001; 158:1105–1113Link, Google Scholar
8. Ramsey NF, Koning HA, Welles P, Cahn W, van der Linden JA, Kahn RS: Excessive recruitment of neural systems subserving logical reasoning in schizophrenia. Brain 2002; 125:1793–1807Crossref, Medline, Google Scholar
9. Weinberger DR, Berman K, Illowsky B: Physiological dysfunction of the dorsolateral prefrontal cortex, III: a new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry 1988; 45:609–615Crossref, Medline, Google Scholar
10. Andreasen NC, Arndt S, Swayze VW II, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WTC: Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 1994; 266:294–298Crossref, Medline, Google Scholar
11. Carter CS, MacDonald AW III, Ross LL, Stenger VA: Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry 2001; 158:1423–1428Link, Google Scholar
12. Heckers S, Weiss AP, Deckersbach T, Goff DC, Morecraft RJ, Bush G: Anterior cingulate cortex activation during cognitive interference in schizophrenia. Am J Psychiatry 2004; 161:707–715Link, Google Scholar
13. Holcomb HH, Lahti AC, Medoff DR, Weiler M, Dannals RF, Tamminga CA: Brain activation patterns in schizophrenic and comparison volunteers during a matched-performance auditory recognition task. Am J Psychiatry 2000; 157:1634–1645Link, Google Scholar
14. Botvinick M, Braver TS, Barch DM, Carter CS, Cohen JD: Conflict monitoring and cognitive control. Psychol Rev 2001; 108:624–652Crossref, Medline, Google Scholar
15. MacDonald AW, Cohen JD, Stenger VA, Carter CS: Dissociating the role of dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000; 288:1835–1838Crossref, Medline, Google Scholar
16. Stratta P, Daneluzzo E, Bustini M, Casacchia M, Rossi A: Schizophrenia deficits in the processing of context. Arch Gen Psychiatry 1998; 55:186–187Crossref, Medline, Google Scholar
17. Barch D, Carter C, MacDonald A, Braver T, Cohen J: Context processing deficits in schizophrenia: diagnostic specificity, four-week course, and relationships to clinical symptoms. J Abnorm Psychol 2003; 112:132–143Crossref, Medline, Google Scholar
18. MacDonald A, Pogue-Geile MF, Johnson M, Carter C: A specific deficit in context processing in the unaffected siblings of patients with schizophrenia. Arch Gen Psychiatry 2003; 60:57–65Crossref, Medline, Google Scholar
19. Cohen JD, Barch DM, Carter CS, Servan-Schreiber D: Schizophrenic deficits in the processing of context: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol 1999; 108:120–133Crossref, Medline, Google Scholar
20. Kerns JG, Cohen JD, MacDonald AWI, Johnson MK, Stenger VA, Aizenstein H, Carter CS: Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry 2005; 162:1833–1839Link, Google Scholar
21. Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Frey KN, Hardin M, Tamminga CA: Clozapine but not haloperidol re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology 2004; 29:171–178Crossref, Medline, Google Scholar
22. Honey GD, Bullmore ET, Soni W, Varatheesan M, Williams SC, Sharma T: Differences in frontal cortical activation by a working memory task after substitution of risperidone for typical antipsychotic drugs in patients with schizophrenia. Proc Natl Acad Sci USA 1999; 96:13432–13437Crossref, Medline, Google Scholar
23. Green MF: Recent studies on the neurocognitive effects of second-generation antipsychotic medications. Curr Opin Psychiatry 2002; 15:25–29Crossref, Google Scholar
24. Harvey PD, Keefe RSE: Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry 2001; 158:176–184Link, Google Scholar
25. Simon JR: The effects of an irrelevant directional cue on human information processing, in Stimulus-Response Compatibility: An Integrated Perspective. Edited by Proctor RW, Reeve TG. Amsterdam, North-Holland, 1990, pp 31–86Google Scholar
26. Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC: Automated image registration, I: general methods and intrasubject, intramodality validation. J Comput Assist Tomogr 1998; 22:139–152Crossref, Medline, Google Scholar
27. Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC: Automated image registration, II: intersubject validation of linear and nonlinear models. J Comput Assist Tomogr 1998; 22:153–165Crossref, Medline, Google Scholar
28. Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM:3D statistical neuroanatomical models from 305 MRI volumes. Proceedings of the IEEE Nuclear Science Symposium and Medical Imaging Conference1993:1813–1817Google Scholar
29. Carter CS, MacDonald A III, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD: Parsing executive processes: strategic vs evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 2000; 97:1944–1948Crossref, Medline, Google Scholar
30. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33:636–647Crossref, Medline, Google Scholar
31. Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RSJ: Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992; 160:179–186Crossref, Medline, Google Scholar
32. Ebmeier KP, Blackwood D, Murray C, Souza V, Walker M, Dougall N, Moffoot APR, O’Carroll RE, Goodwin GM: Single-photon emission computed tomography with 99mTc-exametazime in unmedicated schizophrenic patients. Biol Psychiatry 1993; 33:487–495Crossref, Medline, Google Scholar
33. Ichikawa J, Dai J, Meltzer HY: Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5-HT1A receptor agonism. Brain Res 2002; 956:349–357Crossref, Medline, Google Scholar
34. Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RS, Grasby PM: Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature 1995; 378:180–183Crossref, Medline, Google Scholar



