The Anatomy of First-Episode and Chronic Schizophrenia: An Anatomical Likelihood Estimation Meta-Analysis
Abstract
Objective: The authors sought to map gray matter changes in first-episode schizophrenia and to compare these with the changes in chronic schizophrenia. They postulated that the data would show a progression of changes from hippocampal deficits in first-episode schizophrenia to include volume reductions in the amygdala and cortical gray matter in chronic schizophrenia. Method: A systematic search was conducted for voxel-based structural MRI studies of patients with first-episode schizophrenia and chronic schizophrenia in relation to comparison groups. Meta-analyses of the coordinates of gray matter differences were carried out using anatomical likelihood estimation. Maps of gray matter changes were constructed, and subtraction meta-analysis was used to compare them. Results: A total of 27 articles were identified for inclusion in the meta-analyses. A marked correspondence was observed in regions affected by both first-episode schizophrenia and chronic schizophrenia, including gray matter decreases in the thalamus, the left uncus/amygdala region, the insula bilaterally, and the anterior cingulate. In the comparison of first-episode schizophrenia and chronic schizophrenia, decreases in gray matter volume were detected in first-episode schizophrenia but not in chronic schizophrenia in the caudate head bilaterally; decreases were more widespread in cortical regions in chronic schizophrenia. Conclusions: Anatomical changes in first-episode schizophrenia broadly coincide with a basal ganglia-thalamocortical circuit. These changes include bilateral reductions in caudate head gray matter, which are absent in chronic schizophrenia. Comparing first-episode schizophrenia and chronic schizophrenia, the authors did not find evidence for the temporolimbic progression of pathology from hippocampus to amygdala, but there was evidence for progression of cortical changes.
The anatomical brain changes in first-episode schizophrenia are of interest for at least three reasons: 1) they may indicate a progression of brain changes after disease onset, 2) they may represent the core regions of pathological change in schizophrenia, and 3) they may provide a key to earlier diagnosis.
Changes in brain structure in first-episode schizophrenia have been identified by meta-analyses of MRI studies (1 – 3) . Compared with matched comparison groups, patients were found to have reduced whole brain volume (97%) and increased lateral ventricular volume (134% on the right and 125% on the left). In patients, hippocampal volumes were 92% on both sides (1) . However, amygdala volumes and temporal lobe volumes were not significantly different (3) .
These changes may be less widespread than those in chronic schizophrenia. Meta-analyses that included mainly patients with chronic schizophrenia (4 – 6) found that patients also had smaller mean cerebral volumes (98%) and greater total ventricular volumes (126%). Other volumes that were smaller were the hippocampus (93% on the left and 94% on the right), the parahippocampi (92% on the left and 89% on the right), the amygdala (91% on both sides), the frontal lobes (97% on both sides), and the temporal lobes (98% on the left and 97% on the right) (5) .
If there are more widespread brain changes in chronic schizophrenia than in first-episode schizophrenia, then this could imply a progression of brain anatomical changes after symptom onset. Since hippocampal deficits, but not amygdala deficits, appear to be present in first-episode schizophrenia, this has led to the hypothesis that there is a progression of temporolimbic involvement after disease onset (3) . Furthermore, if there are more localized changes in first-episode schizophrenia, then these may represent the core regions of pathology (or neurodevelopmental abnormality). They may represent the critical nodes in neurochemical circuits whose continuing dysfunction leads to further anatomical changes within the circuits over time.
Finally, the identification of specific anatomical brain changes in first-episode schizophrenia could provide a key to earlier diagnosis. Studies of brain MR images in individuals at high risk of developing a psychotic disorder have found gray matter changes (7 , 8) , and it is important to define which of these changes are indicative of first-episode schizophrenia. There is some evidence that earlier diagnosis and treatment of schizophrenia lead to improved outcomes (9 , 10) .
In this study, we conducted meta-analyses of voxel-based morphometry imaging studies by applying the technique of activation likelihood estimation (11) . Activation likelihood estimation was originally developed to identify the brain regions that were consistently activated by a cognitive task (12 , 13) , using coordinates reported by different functional imaging studies. It assumes that although each study reported the specific coordinates of activations, technical issues (such as interindividual variability in brain anatomy) and differences in investigators’ labels for anatomical regions lead to some uncertainty as to the actual locations of these peaks. Therefore, activation foci do not represent single points but rather “localization probability distributions” centered on the particular coordinates. In activation likelihood estimation, the foci reported by each study are modeled as a probability distribution. Then a map of the whole brain is constructed, assigning to each voxel a value equal to the probability that an activation lies within the voxel. This value is called the “activation likelihood estimation.” A statistical test of these values is then performed by comparing them with values in a null distribution obtained by permutation testing, correcting for multiple comparisons by controlling the false discovery rate. For example, a false discovery rate correction guarantees that in a set of voxels deemed significant for a test of α=0.05, the expected proportion of false positives is controlled (11) .
One of the difficulties when comparing imaging studies is that there is considerable variability when labeling neuroanatomical regions, and differences in nomenclature could obscure findings. An advantage of the activation likelihood estimation technique is that because it uses the coordinates of reported foci (rather than anatomical labels) for meta-analysis, it avoids the problem of any mislabeling of regions in the primary literature (14) .
In voxel-based morphometry, MR images are analyzed for structural change at the level of voxels—the individual elements within a three-dimensional digital image (15 , 16) . This automated technique analyzes the whole brain for changes rather than selecting a subsample of regions and so may be less likely to miss changes than the more traditional region-of-interest morphometry. We call this method anatomical likelihood estimation (ALE) when applied to voxel-based structural imaging studies, since the primary studies measure brain changes in gray matter structure rather than brain activations (as in functional imaging studies).
A previous meta-analysis of gray matter changes in schizophrenia (including mainly studies of chronic schizophrenia) found that deficits in patients were more frequently observed in various cortical and subcortical regions, including the superior temporal gyrus bilaterally, the medial frontal gyrus bilaterally, the anterior cingulate, the insular cortex bilaterally, the parahippocampal gyrus bilaterally, the thalamus, and the caudate bilaterally (17) .
The objectives of this meta-analysis were to use ALE to investigate gray matter structural brain changes in first-episode schizophrenia and to compare the distribution of these changes with those in chronic schizophrenia. We hypothesized that ALE analysis of first-episode schizophrenia studies would 1) identify the hippocampi but not the amygdala as regions affected by first-episode schizophrenia and 2) demonstrate more widespread cortical changes in chronic schizophrenia compared with first-episode schizophrenia.
Method
Study Ascertainment
Studies were considered for inclusion if they were published before June 2007 in article format (rather than as letters or abstracts), if they compared a group of subjects with schizophrenia (or schizophrenia and related diagnoses) and a comparison group (either related or unrelated to the subjects), if they utilized voxel-based morphometric analysis of MRI data sets to investigate differences in whole-brain structure (gray matter density), and if they reported the three-dimensional coordinates of brain changes in stereotactic space.
If measurements were reported for both an unrelated and a related comparison group, then the former measurements were used. If the comparison groups were unaffected dizygotic or monozygotic twins, then the former measurements were used (on the assumption that they showed genetic as well as environmental differences). Studies reporting results on a patient group in which patients had a diagnosis of schizophrenia but some patients had related diagnoses (e.g., schizoaffective disorder, first-episode psychosis) were also included. Studies that reported separate results for male and female subjects were entered as combined data. Studies in which the patients’ duration of illness was less than 1.5 years (e.g., with early-onset schizophrenia) were classified in the first-episode schizophrenia group.
Study data were excluded if insufficient data were reported to extract the number of subjects in each group, if there were fewer than six subjects in either the schizophrenia group or the comparison group, or if the data contributed to another publication, in which case the publication with the largest group size was selected.
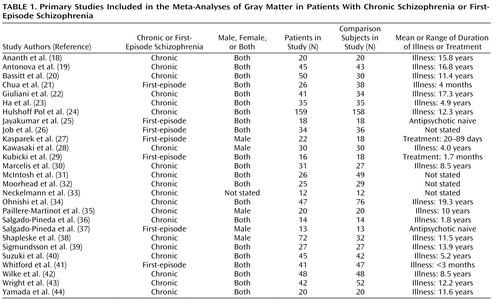
A systematic search strategy was used to identify relevant studies. First, we carried out a MEDLINE search using the following keywords: schizophrenia, psychosis, first episode, MRI, voxel, SPM (statistical parametric mapping), Talairach; the search was conducted in June 2007, and no time span was specified for date of publication. Second, a manual search was conducted of the titles of published papers in three psychiatric journals for the period January 2007 to May 2007: the American Journal of Psychiatry , Archives of General Psychiatry , and Biological Psychiatry . Finally, we searched the reference lists of the studies identified for inclusion. Studies were independently ascertained and checked by the authors. Table 1 lists the articles included in the meta-analysis (18 – 44) .
Coordinates that were reported in the stereotactic space of the Montreal Neurological Institute (MNI) were converted to Talairach coordinates using the Lancaster transform (icbm2tal) in GingerALE (11) . Talairach coordinates that had been generated by the Brett transform applied to statistical parametric mapping MNI coordinates were transformed back to MNI space in GingerALE and then to Talairach space using the Lancaster transform. Coordinates produced using small-volume correction were included.
Of the structural neuroimaging studies considered for the meta-analyses, two studies (45 , 46) were excluded because the subjects overlapped with other included studies.
Statistical Analysis
Meta-analyses were performed when at least two studies providing coordinates suitable for meta-analysis were available. Meta-analyses were carried out using the activation likelihood estimation technique (12) implemented in GingerALE ( 11 ; www.brainmap.org/ale/).
Meta-analyses were performed using the Talairach stereotactic coordinates derived from the studies listed in Table 1 . The likelihood of anatomical differences between groups was estimated on the basis of the equally weighted coordinates reported by the primary studies (12) . The reported loci of maximal anatomical difference were modeled as the peaks of three-dimensional Gaussian probability density functions with full-width half-maximum of 10 mm. The probabilities were combined to form a map of the ALE score at each voxel. Using a permutation test (5,000 permutations), nonparametric estimates for p values were derived for ALE scores. These probability maps were thresholded, controlling the false discovery rate at p<0.05 and a cluster extent threshold of 100 voxels.
For each cluster, the coordinate of the weighted center was generated and the maximum ALE value within the cluster was identified. The Talairach location of the cluster was assigned by identifying the location of the coordinate of the maximum ALE value in the automated Talairach atlas ( 47 ; http://www.talairach.org/), and this was manually checked in the Talairach atlas (48) .
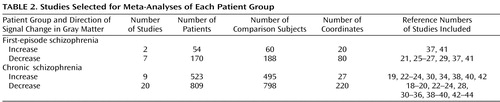
There were sufficient studies to perform meta-analyses (compared with comparison subjects) of decreases in gray matter in subjects with first-episode schizophrenia and in subjects with chronic schizophrenia, and increases in gray matter in subjects with first-episode schizophrenia and chronic schizophrenia.
In order to compare gray matter decreases in first-episode schizophrenia and chronic schizophrenia, a subtraction meta-analysis was performed (11) . Subtraction meta-analysis yields an ALE map that shows regions in which the two groups of foci are significantly different. Since there were more studies and considerably more coordinates for gray matter decreases in chronic schizophrenia (N=220) than in first-episode schizophrenia (N=80), this could have resulted in the appearance of more extensive changes in chronic schizophrenia. Therefore, a meta-analysis was performed using a random sample of 80 coordinates from the chronic schizophrenia gray matter decreases in comparison with the 80 coordinates from the first-episode schizophrenia gray matter decreases.
Results
A total of 27 articles were identified for inclusion in the meta-analyses ( Table 1 ); the articles selected for each meta-analysis are listed in Table 2 . The clusters identified in each meta-analysis were obtained after controlling the false discovery rate at p<0.05 and applying a cluster extent threshold of 100 voxels. The gray matter decreases in first-episode schizophrenia and chronic schizophrenia are displayed on brain templates ( Figures 1 and 2 ) using the MRIcron software program ( 49 ; www.sph.sc.edu/comd/rorden/mricron).
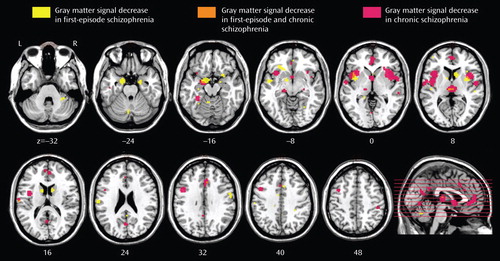
a Significant clusters thresholded with a false discovery rate at p<0.05 and a cluster extent threshold of 100 voxels displayed on a template brain. The right side of each section represents the right side of the brain. The z coordinate in Talairach space is indicated below each section; z=–16: left uncus/amygdala region; z=0 to +8: insula (left and right); z=+8: thalamus; z=+32: anterior cingulate.
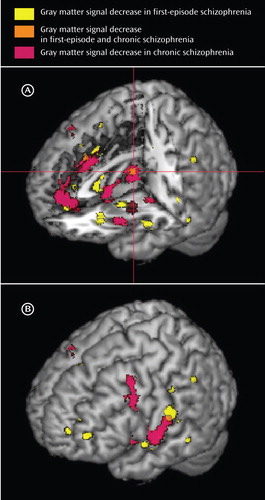
a Panel A shows regions of gray matter signal decrease displayed on a three-dimensional rendered brain with the left frontal lobe removed; the cross-hairs indicate gray matter decreases in the thalamus in both first-episode schizophrenia and chronic schizophrenia. Panel B shows the left hemisphere surface.
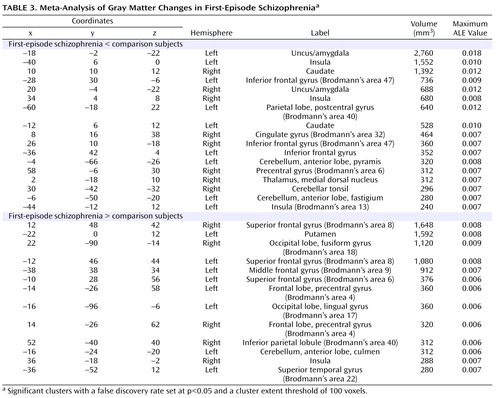
The coordinates for gray matter changes in first-episode schizophrenia are listed in Table 3 . (For chronic schizophrenia and the comparison between first-episode schizophrenia and chronic schizophrenia, gray matter changes are listed in full in the data supplement that accompanies the online edition of this article.) The p values cited in the text represent the uncorrected voxelwise probability estimates for the maximum ALE value within each cluster, derived by permutation testing.
First-Episode Schizophrenia
There were gray matter decreases in subcortical structures: the caudate head (left side, p=0.0002; right side, p<0.0002) and the thalamus (medial dorsal nucleus region) (p=0.001). There were cortical gray matter decreases including the insula (left side, p<0.0002; right side, p=0.0002), the anterior cingulate gyrus (p=0.0016), and the inferior frontal gyrus (left side, p=0.0004; right side, p=0.001) and limbic gray matter decreases in the uncus/amygdala (left side, p<0.0002; right side, p<0.0002). There were also cerebellar gray matter reductions including the pyramis (p=0.0008).
There were gray matter increases including the left putamen (p<0.0002).
Chronic Schizophrenia
There were gray matter decreases in the thalamus (medial dorsal nucleus region) (p<0.0002). There were cortical gray matter decreases including the insula (bilaterally) (p<0.0002), the anterior cingulate gyrus (p<0.0002), the left inferior frontal gyrus (p<0.0002), the left middle frontal gyrus (p<0.0002), the left temporal fusiform gyrus (p<0.0002), and the right superior/middle temporal gyrus (p=0.0018). There were limbic gray matter decreases including the left uncus/amygdala region (p<0.0002) and the right hippocampus region (p=0.0004).
There were gray matter increases including the right putamen (p<0.0002) and the left putamen (p=0.0002).
Comparison of Gray Matter Decreases Between First-Episode Schizophrenia and Chronic Schizophrenia
Gray matter decreases were greater in first-episode schizophrenia in the caudate head bilaterally (p<0.0002) and in the left uncus (p<0.0002).
Gray matter decreases were greater in chronic schizophrenia in the medial frontal gyrus (p=0.0004) and the left dorsolateral prefrontal cortex (p<0.0002). In cortical regions (frontal, parietal, temporal, occipital, and insular cortex) as opposed to limbic (uncus, amygdala), basal ganglia, and cerebellar regions, gray matter decreases were more extensive in chronic schizophrenia (4,400 voxels) than in first-episode schizophrenia (2,200 voxels).
Discussion
These meta-analyses have identified a network of gray matter changes in first-episode schizophrenia. The changes were more extensive than previously suggested by meta-analyses of region-of-interest studies that identified reduced whole brain volume and hippocampal volume and increased ventricular volume in first-episode schizophrenia (1 , 2) .
In addition, we observed considerable overlap in the regions affected in both first-episode schizophrenia and chronic schizophrenia. In both groups, we found gray matter decreases in the thalamus, the left uncus/amygdala region, the left and right insulae, the anterior cingulate, and the left inferior frontal gyrus.
There are some potential limitations of the ALE technique. The technique does not differentiate between the different voxel heights (z-values) and spatial extents of foci in primary studies or between different group sizes and probability threshold values of primary studies (50) . Many factors can influence the number of foci reported in primary studies. However, pooling multiple studies may be limited by such differences until rigorous standards of data reporting are developed for primary studies (11) . Another potential source of bias in primary studies is the analysis of data using small-volume correction in which a subvolume of the brain is tested for statistical changes. This may lead to findings of more positive results in selected brain regions. In our meta-analyses, although we included coordinates derived using small-volume correction, these only contributed to 6% of the total coordinates, and repeating our analyses with these coordinates excluded did not materially affect the results.
Thalamocorticostriatal Circuits and Schizophrenia
The network of changes identified by these meta-analyses included regions that have previously been strongly implicated in the pathology of schizophrenia. Meta-analyses of region-of-interest studies (of patients mainly with chronic schizophrenia) have identified volume reductions in the thalamus (5 , 51) , the anterior cingulate (52) , and the hippocampus (53) . Region-of-interest studies in first-episode psychosis have also found reduced thalamic volume in some (54 – 56) but not all (57) studies. A study of the anterior cingulate cortex in first-episode schizophrenia identified reduced cortical thickness (58) but not reduced gray matter volume.
Anatomically, the thalamus receives neural connections from the striatum (ventral striatum, caudate, putamen, and globus pallidus) and sends projections to (among others) the prefrontal and cingulate cortex, the hippocampus, and the limbic midbrain (59) . The striatum (especially the ventral striatum) receives topographical glutamatergic cortical (prefrontal, temporal cortex) and limbic input as well as midbrain dopaminergic input (which appears to modulate the responses of the striatum to cortical and thalamic afferent input). Therefore, there is an anatomical substrate for a neural circuit linking the thalamus, prefrontal cortex, limbic regions, and striatum. Functional neuroimaging studies suggest that executive functioning deficits in schizophrenia may be mediated by basal ganglia-thalamocortical circuitry disruptions (60) .
The “missing link” in this network is an anatomical change in the striatum. In first-episode schizophrenia, region-of-interest studies have found volume reductions in the caudate nucleus in some (61) but not all (54 , 62 , 63) studies. In this meta-analysis, we found decreases in gray matter in the caudate head (bilaterally) in first-episode schizophrenia, and these decreases were significantly greater than in chronic schizophrenia. Caudate volume deficits have been found in the offspring of patients with schizophrenia (64) , which may suggest a genetic contribution to this change.
Anatomical Differences Between the First-Episode and Chronic Patient Groups
We also investigated whether there were more extensive cortical changes in chronic schizophrenia compared with first-episode schizophrenia. We found that gray matter decreases were greater in chronic schizophrenia in the frontal cortex (medial frontal gyrus and left dorsolateral prefrontal cortex), the right insula cortex, and the left and right temporal cortex. These differences remained after correction for the number of available studies for chronic schizophrenia and first-episode schizophrenia by using a matched number of coordinates. Thus, although we did not find evidence for the temporolimbic progression of pathology from hippocampus to amygdala (3) , there was evidence for progression of cortical changes. Cortical gray matter deficits could arise from pathological disease progression, the effect of treatment (65) , or comorbidity (66) .
Dopaminergic Pathways and the Role of Antipsychotic Medication
In both first-episode schizophrenia and chronic schizophrenia, there were regions of gray matter increases. In voxel-based morphometry, an “increase” may reflect a relative preservation from deficit (which may be detected in the context of whole brain gray matter volume loss), or it may represent an absolute increase in gray matter.
In first-episode schizophrenia there were gray matter increases including the left putamen, and in chronic schizophrenia there were gray matter increases including the putamen bilaterally.
The regions in this study that showed gray matter changes in schizophrenia were in the distribution of dopaminergic effects (nigrostriatal, mesolimbic, and mesocortical pathways). The dopamine system is one target for antipsychotic drugs that may alter brain structure (67) . Antipsychotic treatment modulates putamen volume (63 , 68) and therefore may contribute to putamen volume increases in chronic schizophrenia. The brain changes in chronic schizophrenia may depend on whether patients receive treatment with conventional or atypical antipsychotic drugs, since conventional antipsychotics may be particularly associated with enlargement of the putamen (69) and whole brain gray matter reductions (65) , particularly in some cortical areas (69) .
A further implication of this study is that the caudate head gray matter deficit (identified in first-episode schizophrenia) “normalizes” as the course of schizophrenia becomes more chronic. If treatment with conventional antipsychotics results in gray matter volume increases in the basal ganglia (68) , then this may provide an iatrogenic mechanism for this effect.
The identification of these regions of gray matter changes in first-episode schizophrenia may assist in earlier diagnosis. Multivariate voxel-based morphometry has been used to differentiate patients with chronic schizophrenia from comparison subjects (28) . Voxel-based morphometry has been used to study progression of brain changes in subjects at high risk of developing schizophrenia (8) and has identified gray matter reductions over time that included the left uncus and the left inferior frontal gyrus (regions of change that this meta-analysis identified in first-episode schizophrenia). These regional changes had predictive value in identifying the subjects who went on to develop schizophrenia (70) . The anatomical changes identified in our meta-analysis warrant further study as potential disease markers. Improved diagnostic specificity may be achieved by multivariate techniques (71) or by shape analysis (72) , for example of the caudate head or uncus/amygdala.
In summary, these meta-analyses identified a distributed network of anatomical changes in first-episode schizophrenia. The pattern of results is consistent with theoretical models implicating thalamocorticostriatal circuits in the pathophysiology of schizophrenia. The network includes bilateral caudate head gray matter reductions, which are absent in chronic schizophrenia. There was also evidence for decreased frontal, insular, and temporal cortical gray matter in chronic schizophrenia, which is compatible with hypotheses of progressive anatomical abnormality over the course of the disorder.
1. Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA: Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry 2006; 188:510–518Google Scholar
2. Vita A, De Peri L, Silenzi C, Dieci M: Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res 2006; 82:75–88Google Scholar
3. Vita A, de Peri L: Hippocampal and amygdala volume reductions in first-episode schizophrenia. Br J Psychiatry 2007; 190:271Google Scholar
4. Lawrie SM, Abukmeil SS: Brain abnormality in schizophrenia: a systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry 1998; 172:110–120Google Scholar
5. Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET: Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 2000; 157:16–25Google Scholar
6. Shenton ME, Dickey CC, Frumin M, McCarley RW: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1–52Google Scholar
7. Borgwardt SJ, Riecher-Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M, Gschwandtner U, Haller S, Pfluger M, Rechsteiner E, D’Souza M, Stieglitz RD, Radu EW, McGuire PK: Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry 2007; 61:1148–1156Google Scholar
8. Job DE, Whalley HC, Johnstone EC, Lawrie SM: Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage 2005; 25:1023–1030Google Scholar
9. Killackey E, Yung AR: Effectiveness of early intervention in psychosis. Curr Opin Psychiatry 2007; 20:121–125Google Scholar
10. Buckley PE, Evans D: First-episode schizophrenia: a window of opportunity for optimizing care and outcomes. Postgrad Med 2006; spec no:5–19Google Scholar
11. Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT: ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 2005; 25:155–164Google Scholar
12. Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA: Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 2002; 16:765–780Google Scholar
13. Chein JM, Fissell K, Jacobs S, Fiez JA: Functional heterogeneity within Broca’s area during verbal working memory. Physiol Behav 2002; 77:635–639Google Scholar
14. Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT: A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp 2005; 25:6–21Google Scholar
15. Wright IC, McGuire PK, Poline JB, Travere JM, Murray RM, Frith CD, Frackowiak RS, Friston KJ: A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage 1995; 2:244–252Google Scholar
16. Ashburner J, Friston KJ: Voxel-based morphometry: the methods. Neuroimage 2000; 11:805–821Google Scholar
17. Honea R, Crow TJ, Passingham D, Mackay CE: Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005; 162:2233–2245Google Scholar
18. Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RSJ, Dolan RJ: Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel-based morphometry. Am J Psychiatry 2002; 159:1497–1505Google Scholar
19. Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R, Sharma T: The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol Psychiatry 2005; 58:457–467Google Scholar
20. Bassitt DP, Neto MR, de Castro CC, Busatto GF: Insight and regional brain volumes in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2007; 257:58–62Google Scholar
21. Chua SE, Cheung C, Cheung V, Tsang JT, Chen EY, Wong JC, Cheung JP, Yip L, Tai KS, Suckling J, McAlonan GM: Cerebral grey, white matter and CSF in never-medicated, first-episode schizophrenia. Schizophr Res 2007; 89:12–21Google Scholar
22. Giuliani NR, Calhoun VD, Pearlson GD, Francis A, Buchanan RW: Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr Res 2005; 74:135–147Google Scholar
23. Ha TH, Youn T, Ha KS, Rho KS, Lee JM, Kim IY, Kim SI, Kwon JS: Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res 2004; 132:251–260Google Scholar
24. Hulshoff Pol HE, Schnack HG, Mandl RC, van Haren NE, Koning H, Collins DL, Evans AC, Kahn RS: Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry 2001; 58:1118–1125Google Scholar
25. Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS: Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29:587–591Google Scholar
26. Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM: Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage 2002; 17:880–889Google Scholar
27. Kasparek T, Prikryl R, Mikl M, Schwarz D, Ceskova E, Krupa P: Prefrontal but not temporal grey matter changes in males with first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:151–157Google Scholar
28. Kawasaki Y, Suzuki M, Kherif F, Takahashi T, Zhou SY, Nakamura K, Matsui M, Sumiyoshi T, Seto H, Kurachi M: Multivariate voxel-based morphometry successfully differentiates schizophrenia patients from healthy controls. Neuroimage 2007; 34:235–242Google Scholar
29. Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW: Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 2002; 17:1711–1719Google Scholar
30. Marcelis M, Suckling J, Woodruff P, Hofman P, Bullmore E, van Os J: Searching for a structural endophenotype in psychosis using computational morphometry. Psychiatry Res 2003; 122:153–167Google Scholar
31. McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, Johnstone EC: Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry 2004; 56:544–552Google Scholar
32. Moorhead TW, Job DE, Whalley HC, Sanderson TL, Johnstone EC, Lawrie SM: Voxel-based morphometry of comorbid schizophrenia and learning disability: analyses in normalized and native spaces using parametric and nonparametric statistical methods. Neuroimage 2004; 22:188–202Google Scholar
33. Neckelmann G, Specht K, Lund A, Ersland L, Smievoll AI, Neckelmann D, Hugdahl K: MR morphometry analysis of grey matter volume reduction in schizophrenia: association with hallucinations. Int J Neurosci 2006; 116:9–23Google Scholar
34. Ohnishi T, Hashimoto R, Mori T, Nemoto K, Moriguchi Y, Iida H, Noguchi H, Nakabayashi T, Hori H, Ohmori M, Tsukue R, Anami K, Hirabayashi N, Harada S, Arima K, Saitoh O, Kunugi H: The association between the Val158Met polymorphism of the catechol- O -methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain 2006; 129:399–410 Google Scholar
35. Paillere-Martinot M, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, Recasens C, Attar-Levy D, Martinot JL: Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res 2001; 50:19–26Google Scholar
36. Salgado-Pineda P, Junque C, Vendrell P, Baeza I, Bargallo N, Falcon C, Bernardo M: Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. Neuroimage 2004; 21:840–847Google Scholar
37. Salgado-Pineda P, Baeza I, Perez-Gomez M, Vendrell P, Junque C, Bargallo N, Bernardo M: Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage 2003; 19:365–375Google Scholar
38. Shapleske J, Rossell SL, Chitnis XA, Suckling J, Simmons A, Bullmore ET, Woodruff PW, David AS: A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cereb Cortex 2002; 12:1331–1341Google Scholar
39. Sigmundsson T, Suckling J, Maier M, Williams SCR, Bullmore ET, Greenwood KE, Fukuda R, Ron MA, Toone BK: Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry 2001; 158:234–243Google Scholar
40. Suzuki M, Nohara S, Hagino H, Kurokawa K, Yotsutsuji T, Kawasaki Y, Takahashi T, Matsui M, Watanabe N, Seto H, Kurachi M: Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophr Res 2002; 55:41–54Google Scholar
41. Whitford TJ, Grieve SM, Farrow TF, Gomes L, Brennan J, Harris AW, Gordon E, Williams LM: Progressive grey matter atrophy over the first 2–3 years of illness in first-episode schizophrenia: a tensor-based morphometry study. Neuroimage 2006; 32:511–519Google Scholar
42. Wilke M, Kaufmann C, Grabner A, Putz B, Wetter TC, Auer DP: Gray matter changes and correlates of disease severity in schizophrenia: a statistical parametric mapping study. Neuroimage 2001; 13:814–824Google Scholar
43. Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK: Mapping of grey matter changes in schizophrenia. Schizophr Res 1999; 35:1–14Google Scholar
44. Yamada M, Hirao K, Namiki C, Hanakawa T, Fukuyama H, Hayashi T, Murai T: Social cognition and frontal lobe pathology in schizophrenia: a voxel-based morphometric study. Neuroimage 2007; 35:292–298Google Scholar
45. Whitford TJ, Farrow TF, Gomes L, Brennan J, Harris AW, Williams LM: Grey matter deficits and symptom profile in first episode schizophrenia. Psychiatry Res 2005; 139:229–238Google Scholar
46. Kawasaki Y, Suzuki M, Nohara S, Hagino H, Takahashi T, Matsui M, Yamashita I, Chitnis XA, McGuire PK, Seto H, Kurachi M: Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. Eur Arch Psychiatry Clin Neurosci 2004; 254:406–414Google Scholar
47. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT: Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000; 10:120–131Google Scholar
48. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
49. Rorden C, Karnath HO, Bonilha L: Improving lesion-symptom mapping. J Cogn Neurosci 2007; 19:1081–1088Google Scholar
50. Fox PT, Laird AR, Lancaster JL: Co-ordinate-based voxel-wise meta-analysis: dividends of spatial normalization: report of a virtual workshop. Hum Brain Mapp 2005; 25:1–5Google Scholar
51. Konick LC, Friedman L: Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry 2001; 49:28–38Google Scholar
52. Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P: Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophr Res 2007; 93:1–12Google Scholar
53. Nelson MD, Saykin AJ, Flashman LA, Riordan HJ: Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry 1998; 55:433–440Google Scholar
54. Crespo-Facorro B, Roiz-Santianez R, Pelayo-Teran JM, Gonzalez-Blanch C, Perez-Iglesias R, Gutierrez A, de Lucas EM, Tordesillas D, Vazquez-Barquero JL: Caudate nucleus volume and its clinical and cognitive correlations in first episode schizophrenia. Schizophr Res 2007; 91:87–96Google Scholar
55. Lang DJ, Khorram B, Goghari VM, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Honer WG: Reduced anterior internal capsule and thalamic volumes in first-episode psychosis. Schizophr Res 2006; 87:89–99Google Scholar
56. Ettinger U, Picchioni M, Landau S, Matsumoto K, van Haren NE, Marshall N, Hall MH, Schulze K, Toulopoulou T, Davies N, Ribchester T, McGuire PK, Murray RM: Magnetic resonance imaging of the thalamus and adhesio interthalamica in twins with schizophrenia. Arch Gen Psychiatry 2007; 64:401–409Google Scholar
57. Preuss UW, Zetzsche T, Jäger M, Groll C, Frodl T, Bottlender R, Leinsinger G, Hegerl U, Hahn K, Müller HJ, Meisenzahl EM: Thalamic volume in first-episode and chronic schizophrenic subjects: a volumetric MRI study. Schizophr Res 2005; 73:91–101Google Scholar
58. Fornito A, Yücel M, Wood SJ, Adamson C, Velakoulis D, Saling MM, McGorry PD, Pantelis C: Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Hum Brain Mapp (Epub May 24, 2007)Google Scholar
59. Gray’s Anatomy: The Anatomical Basis of Medicine and Surgery, 38th ed. Edited by Williams P. New York, Churchill Livingstone, 1995, pp 1186–1200Google Scholar
60. Camchong J, Dyckman KA, Chapman CE, Yanasak NE, McDowell JE: Basal ganglia-thalamocortical circuitry disruptions in schizophrenia during delayed response tasks. Biol Psychiatry 2006; 60:235–241Google Scholar
61. Keshavan MS, Rosenberg D, Sweeney JA, Pettegrew JW: Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry 1998; 155:774–778Google Scholar
62. Lang DJ, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Goghari VM, Honer WG: An MRI study of basal ganglia volumes in first-episode schizophrenia patients treated with risperidone. Am J Psychiatry 2001; 158:625–631Google Scholar
63. Glenthoj A, Glenthoj BY, Mackeprang T, Pagsberg AK, Hemmingsen RP, Jernigan TL, Baare WF: Basal ganglia volumes in drug-naive first-episode schizophrenia patients before and after short-term treatment with either a typical or an atypical antipsychotic drug. Psychiatry Res 2007; 154:199–208Google Scholar
64. Rajarethinam R, Upadhyaya A, Tsou P, Upadhyaya M, Keshavan MS: Caudate volume in offspring of patients with schizophrenia. Br J Psychiatry 2007; 191:258–259Google Scholar
65. Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M; HGDH Study Group: Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry 2005; 62:361–370Google Scholar
66. Deshmukh A, Rosenbloom MJ, De Rosa E, Sullivan EV, Pfefferbaum A: Regional striatal volume abnormalities in schizophrenia: effects of comorbidity for alcoholism, recency of alcoholic drinking, and antipsychotic medication type. Schizophr Res 2005; 79:189–200Google Scholar
67. Scherk H, Falkai P: Effects of antipsychotics on brain structure. Curr Opin Psychiatry 2006; 19:145–150Google Scholar
68. Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC: Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155:1711–1717Google Scholar
69. Dazzan P, Morgan KD, Orr K, Hutchinson G, Chitnis X, Suckling J, Fearon P, McGuire PK, Mallett RM, Jones PB, Leff J, Murray RM: Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology 2005; 30:765–774Google Scholar
70. Job DE, Whalley HC, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM: Grey matter changes can improve the prediction of schizophrenia in subjects at high risk. BMC Med 2006; 4:29Google Scholar
71. Davatzikos C, Shen D, Gur RC, Wu X, Liu D, Fan Y, Hughett P, Turetsky BI, Gur RE: Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry 2005; 62:1218–1227Google Scholar
72. Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG: Structural analysis of the basal ganglia in schizophrenia. Schizophr Res 2007; 89:59–71Google Scholar