Anatomical and Functional Brain Abnormalities in Drug-Naive First-Episode Schizophrenia
Abstract
Objective
The authors sought to explore whether anatomical and functional brain deficits are present in similar or different brain regions early in the course of schizophrenia, before antipsychotic treatment, and whether these deficits are more severe or otherwise different in patients with prominent negative symptoms.
Method
A total of 100 drug-naive first-episode schizophrenia patients and 100 matched healthy comparison subjects underwent structural and resting-state functional MRI scanning. Gray matter volume and amplitude of low-frequency fluctuations during resting-state functional studies were measured.
Results
Group comparisons of gray matter volume showed significant differences mainly in thalamo-cortical networks, while alterations in the amplitude of low-frequency fluctuations were observed in fronto-parietal and default mode networks. Thus, different brain regions had alterations in gray matter volume and resting state physiology. These changes did not correlate with the duration of untreated illness, nor with acute clinical symptom severity. Patients with prominent negative symptoms had greater regional alterations in brain anatomy, particularly in the left dorsolateral prefrontal cortex, while the pattern of functional alterations was unrelated to severity of negative symptoms.
Conclusions
Anatomical and resting-state functional deficits were observed in different brain regions, indicating that anatomical and functional brain abnormalities are significantly dissociated in the early course of schizophrenia. The lack of association of these abnormalities with illness duration and episode severity suggests that these anatomical and functional changes may be early-evolving features of the illness that are relatively stable early in the course of illness. The different structural deficits of regional gray matter observed in patients with prominent negative symptoms may provide unique insight into the early regional neuropathology of this symptom dimension in schizophrenia.
Schizophrenia is a serious psychiatric illness with onset typically during adolescence and early adulthood and a lifetime incidence of about 1% in the general population (1). In recent decades, many studies have reported anatomical and functional brain abnormalities, most often in fronto-striato-thalamic networks, in schizophrenia patients relative to healthy comparison subjects (2).
One important question that remains largely unanswered is how anatomical and functional brain deficits are related early in the course of schizophrenia. Previous anatomical studies have revealed reductions of gray matter volume mainly in the thalamus and the temporal and frontal cortex (3–5), perhaps most notably in the temporal cortex in drug-naive first-episode schizophrenia (12–15). In contrast, the major functional deficits revealed by functional MRI (fMRI) in drug-naive first-episode schizophrenia patients have been localized more widely in prefrontal, temporal, and parietal regions (16–18), especially in the ventromedial prefrontal cortex (19) and in the default mode network, where deficits have been related to specific clinical features (20, 21). Thus, while there are overlaps and differences in brain regions where structural and functional abnormalities have been identified, few studies have examined these deficits together in the same sample. Another important question is whether anatomical and functional deficits are different in patients with prominent negative symptoms, which is important because these patients have different clinical manifestations and a diminished treatment response (22, 23).
Voxel-based morphometry (VBM) is a useful automatic technique for investigating the anatomy of the human brain (3, 15, 24, 25), and resting-state fMRI can be used to detect regional functional alterations. In resting-state fMRI, the amplitude of low-frequency (0.01–0.08 Hz) fluctuations of the blood-oxygen-level-dependent (BOLD) signal is considered to be physiologically meaningful and related to spontaneous neural activity (26). The two techniques have been widely used to explore morphometric and functional deficits in a range of psychiatric disorders, including schizophrenia.
Our aim in this study was to characterize alterations of gray matter volume and amplitude of low-frequency fluctuation in 100 drug-naive first-episode schizophrenia patients, the interrelationship of these abnormalities, and their relationship with illness duration and negative symptom severity.
Method
Participants
A total of 214 right-handed individuals were recruited for this study, including 107 treatment-naive first-episode schizophrenia patients and 107 healthy comparison subjects. The study was approved by the local research ethics committee, and written informed consent was obtained from all participants. Diagnoses of schizophrenia and duration of illness were determined by the consensus of two experienced clinical psychiatrists using the Structured Interview for the DSM-IV Axis I Disorder, Patient Edition (SCID). Psychopathology ratings were obtained using the Positive and Negative Syndrome Scale (PANSS) (27).
Healthy comparison subjects were recruited by poster advertisement. All comparison subjects were screened using the nonpatient edition of the SCID to confirm the lifetime absence of psychiatric illnesses. In addition, comparison subjects were interviewed to exclude individuals with a known history of psychiatric illness in first-degree relatives. The following exclusion criteria applied to all participants: history of drug or alcohol abuse, pregnancy, and any physical illness such as hepatitis, cardiovascular disease, or neurological disorders, as assessed by interview and review of medical records. Brain MR images (T1-weighted and T2-weighted images) were inspected by an experienced neuroradiologist, and no gross abnormalities were observed in any participant. Seven patients and seven comparison subjects with excessive head motion (translation of more than 1.5 mm or rotation of more than 1.5°) were excluded, resulting in samples of 100 patients and 100 comparison subjects for statistical analyses. There were no significant differences between groups in age, sex, height, weight, and years of education (see Table S1 in the data supplement that accompanies the online edition of this article) or in head motion during scanning. For analyses of associations of brain measures with negative symptom severity, patients were divided into two subgroups: 36 patients with prominent negative symptoms (a score ≥20 on the negative symptom subscale of the PANSS [27, 28]; 20 of them were female, and the mean age was 24.8 years) and 64 patients without prominent negative symptoms (a score < 20 on the negative symptom scale of the PANSS; 39 of them were female, and the mean age was 24.1 years). The mean time since onset of psychotic symptoms was 6.25 months (SD=11.0; range=0.1–46 months). For analyses of illness duration effects, patients were divided into two subgroups: 24 patients with untreated illness of a duration greater than 1 year (12–24 months, N=11; 24–36 months, N=11; 36–46 months, N=2) and 76 patients with a duration of untreated illness <12 months.
Data Acquisition
Scanning was conducted on a 3-T GE Signa EXCITE scanner (General Electric, Milwaukee). High-resolution T1-weighted volumetric three-dimensional images were obtained using a spoiled gradient recall sequence, and amplitudes of low-frequency (0.01–0.08 Hz) fluctuations of the BOLD signal were obtained using a gradient-echo echo-planar imaging sequence. The detailed scan parameters and methods for data analysis are presented in the online data supplement.
Data Analysis
VBM analyses of T1 MR images were performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm). The amplitude of low-frequency fluctuation was calculated using Data Processing Assistant for Resting-State fMRI (DPARSF), version 2.1, implemented with the MATLAB toolbox (http://www.restfmri.net). Voxel-wise group comparisons of gray matter volume and amplitude of low-frequency fluctuation were performed using two-sample t tests. For analyses comparing subgroups of patients in relation to negative symptoms and illness duration, voxel-based comparisons of gray matter volume and amplitude of low-frequency fluctuation were conducted using one-way analysis of variance comparing the two patient groups and the comparison group, followed by post hoc t tests. The statistical results of gray matter volume were corrected for multiple comparisons using the false discovery rate and those of amplitude of low-frequency fluctuation were corrected using the AlphaSim program (29). Our statistical threshold was a p value of 0.05, corrected for multiple comparisons. The average gray matter volume and amplitude of low-frequency fluctuation values of all voxels in brain areas in which abnormalities were detected were extracted, averaged, and correlated with PANSS scores, age, and untreated duration of illness. All analyses examining relationships of brain measures with clinical data were controlled for age and sex.
To test the influence of skull stripping on morphometric measures, the normalized images from 20 patients with the highest and 20 with the lowest VBM gray matter volume were selected, and the nonbrain tissue, including scalp, skull, and dura, were removed blindly and manually by an experienced neuroradiologist. The average gray matter volume of all voxels in abnormal areas revealed by VBM were extracted from these manually skull-stripped images and compared with the values of VBM findings from those without manual skull stripping using a paired t test. No significant difference was revealed between the findings with and without manual skull stripping.
Results
Relative to the comparison group, the patient group showed significantly decreased gray matter volume in the left paracentral and left inferior parietal lobules, and increased gray matter volume in the left and right thalamus, anterior cingulate cortex, insula, and orbital frontal gyrus (p<0.05 corrected with false discovery rate) (Table 1; Figure 1). For the amplitude of low-frequency fluctuation analysis, a decreased amplitude of low-frequency fluctuation was observed mainly in the right inferior and left superior frontal gyrus, the medial frontal gyrus bilaterally, the inferior parietal lobule bilaterally, and the precuneus, while an increased amplitude of low-frequency fluctuation was observed bilaterally in the putamen and occipital regions (p<0.05 corrected with AlphaSim) (Table 1; Figure 2).
| Coordinates | |||||
|---|---|---|---|---|---|
| Measure, Comparison, and Region | x | y | z | Cluster size | t |
| Gray matter volume | |||||
| Patients > healthy subjects | |||||
| Left thalamus | 9 | –31 | –5 | 99 | 4.85 |
| Left insula | –38 | –6 | 3 | 855 | 4.60 |
| Right thalamus | –8 | –31 | –6 | 12 | 5.18 |
| Right insula | 36 | –16 | –2 | 308 | 4.73 |
| Right anterior cingulate | 5 | 42 | 13 | 546 | 3.86 |
| Right orbital frontal gyrus | 6 | 53 | –20 | 763 | 3.96 |
| Healthy subjects > patients | |||||
| Left paracentral lobule | –14 | –25 | 67 | 76 | 4.51 |
| Left inferior parietal lobule | –56 | –36 | 39 | 385 | 4.32 |
| Amplitude of low-frequency fluctuation | |||||
| Patients > healthy subjects | |||||
| Left putamen | –24 | –3 | 12 | 165 | 5.62 |
| Left occipital gyrus | –36 | –72 | –6 | 356 | 4.47 |
| Right putamen | 27 | –6 | 9 | 102 | 4.18 |
| Right occipital gyrus | 39 | –81 | 9 | 56 | 4.00 |
| Right calcarine sulcus | 18 | –93 | 0 | 38 | 3.53 |
| Healthy subjects > patients | |||||
| Left superior frontal gyrus | –24 | 9 | 57 | 129 | 5.26 |
| Left inferior parietal lobule | –42 | –51 | 51 | 54 | 4.30 |
| Left rectus | 0 | 63 | –18 | 19 | 4.57 |
| Right inferior parietal lobule | 45 | –54 | 51 | 128 | 5.21 |
| Right inferior frontal gyrus | 48 | 12 | 21 | 127 | 4.52 |
| Right precuneus | 6 | –54 | 15 | 37 | 4.21 |
| Right insula | 42 | 9 | –12 | 31 | 4.16 |
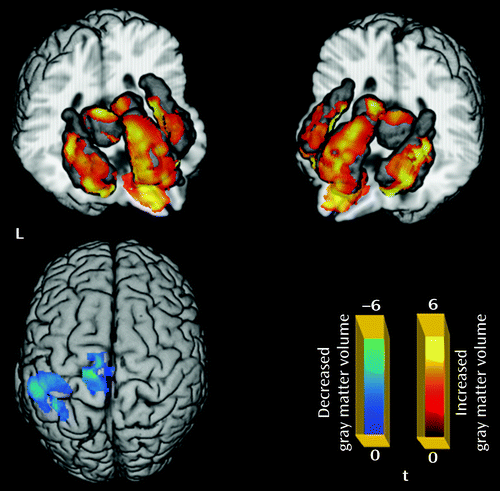
a Significant group differences were identified by t test (p<0.05 corrected for multiple comparisons with false discovery rate). The regions in blue indicate areas with decreased gray matter volume in first-episode schizophrenia, including the left postcentral gyrus extending to the paracentral lobule and the left inferior parietal gyrus; regions in red indicate areas with increased gray matter volume in first-episode schizophrenia, including the left and right thalamus, anterior cingulate cortex, insula, and orbital frontal gyrus.
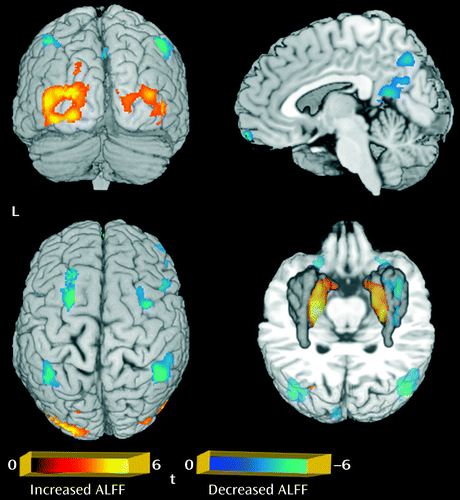
a Significant group differences were identified by t test (p<0.05 corrected for multiple comparisons with the AlphaSim program). The regions in blue showed a decreased amplitude of low-frequency fluctuation in first-episode schizophrenia, seen mainly in the right inferior and left superior frontal gyrus, the medial frontal gyrus, and the left and right inferior parietal lobule and precuneus; regions in red indicate areas with an increased amplitude in first-episode schizophrenia, including the left and right putamen and occipital regions. ALFF=amplitude of low-frequency fluctuation.
Subgroup analysis revealed that the patients with prominent negative symptoms showed significantly decreased gray matter volume in the right postcentral gyrus and the left inferior parietal lobule, and increased gray matter volume in the left and right thalamus, insula, and dorsolateral prefrontal cortex relative to comparison subjects (p<0.05 corrected with false discovery rate) (Figure 3; see also Table S2 in the online data supplement). Relative to comparison subjects, patients without prominent negative symptoms showed significantly decreased gray matter volume in regions including the right middle temporal gyrus, the left middle frontal gyrus, the left postcentral gyrus, and the right middle occipital gyrus, and increased gray matter volume in the left and right thalamus, insula, anterior cingulate cortex, and left inferior frontal gyrus (p<0.05 corrected with false discovery rate) (Figure 3; see also Table S2 in the data supplement). The direct comparison between patients without and with prominent negative symptoms revealed smaller gray matter volume in the middle frontal gyrus bilaterally and the left superior frontal gyrus in patients without prominent negative symptoms. For the amplitude of low-frequency fluctuation analysis, patients with and without prominent negative symptoms showed similar patterns of decreased amplitude of low-frequency fluctuation mainly in the left and right medial and middle prefrontal gyrus and the right inferior parietal gyrus, and increased amplitude of low-frequency fluctuation in the putamen and occipital regions bilaterally (p<0.05 corrected with AlphaSim) (Figure 4; see also Table S2 in the data supplement). Although the patients without prominent negative symptoms showed more regions with decreased function in the medial prefrontal cortex and the left parietal regions relative to comparison subjects, direct comparisons between the patients with and without prominent negative symptoms revealed no significant difference. Patients with longer and shorter duration of illness did not differ significantly in either the anatomical or functional brain data.
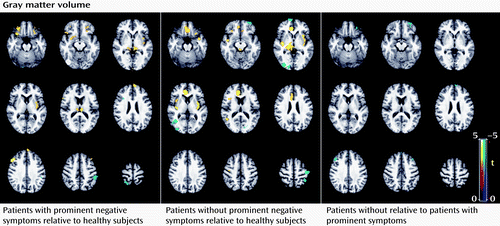
a The regions in blue denote areas with decreased gray matter volume and those in yellow indicate increased gray matter volume in the comparisons indicated (p<0.05 corrected with false discovery rate). Relative to healthy comparison subjects, patients with prominent negative symptoms showed significantly decreased gray matter volume in the right postcentral gyrus and the left inferior parietal lobule and increased gray matter volume in the left and right thalamus, insula, and dorsolateral prefrontal cortex. Relative to healthy comparison subjects, patients without prominent negative symptoms showed significantly decreased gray matter volume in regions including the right middle temporal gyrus, left middle frontal gyrus, left postcentral gyrus, and right middle occipital gyrus, and increased gray matter volume in the left and right thalamus, insula, anterior cingulate cortex, and left inferior frontal gyrus. The direct comparisons between patients without and with prominent negative symptoms revealed smaller gray matter volume in the left and right middle frontal gyrus and the left superior frontal gyrus in patients without prominent negative symptoms.
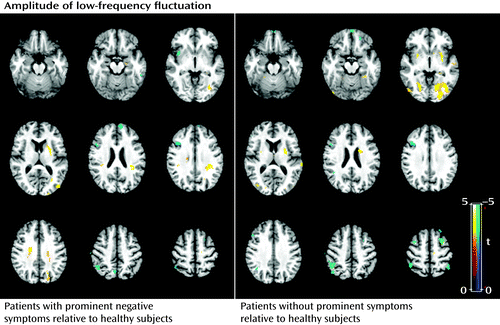
a The regions in blue denote decreased and those in yellow denote increased amplitude of low-frequency fluctuation in the comparisons indicated (p<0.05 corrected with false discovery rate). Patients with and without prominent negative symptoms showed similar patterns of decreased amplitude of low-frequency fluctuation, mainly in the left and right middle and inferior frontal gyrus and the right inferior parietal lobule, and increased amplitude in the left and right putamen and occipital regions.
To examine the overlap between gray matter volume and amplitude of low-frequency fluctuation findings, the regions with significant abnormalities in gray matter volume and amplitude of low-frequency fluctuation were overlaid on the same template. There was no overlap of areas with alteration in analyses of gray matter volume and amplitude of low-frequency fluctuation (see Figure S4 in the data supplement). Furthermore, the average gray matter volume values within the regions with abnormal functional measures were not significantly correlated with the amplitude of low-frequency fluctuation values in either the whole patient group or in subgroups defined by negative symptom severity or illness duration.
Significant positive correlations were observed between untreated illness duration and severity of psychotic symptoms, including PANSS positive symptom scores (r=0.59, p<0.05) and ratings of thought disturbance (r=0.48, p<0.05), activation (r=0.45, p<0.05), paranoia (r=0.50, p<0.05), and anergia (r=0.44, p<0.05). However, no significant correlations were observed between illness duration or positive symptom severity and either changes of gray matter volume or amplitude of low-frequency fluctuation in the patient group.
Discussion
Using the largest sample of drug-naive first-episode schizophrenia patients studied to date with MRI, this study revealed both cerebral anatomical and functional deficits early in the course of illness. However, there was no overlap in the regions with anatomical and functional abnormalities. Gray matter volume changes mainly involved thalamo-cortical networks, while functional changes reflected in the amplitude of low-frequency fluctuation were observed within a fronto-parietal network and the default mode network. Not only did different brain regions show abnormalities in anatomy and physiology measures, but average gray matter volume values within regions having anatomical abnormalities were not significantly associated with the amplitude of low-frequency fluctuation values in those regions. Brain changes in anatomy and function did not correlate with the duration of untreated illness or with positive symptom severity, but some anatomical differences, notably including the increased gray matter volume of the left dorsolateral prefrontal cortex, were identified that varied in relation to negative symptom severity.
It is noteworthy that the patient group showed widespread increases in gray matter volume in multiple brain areas, including the left and right thalamus, insula, anterior cingulate cortex, and orbital frontal gyrus. These findings differ from those of some previous studies that reported decreased gray matter volume within thalamo-cortical networks in chronic as well as first-episode schizophrenia patients (3–5, 8, 12–15). In fact, our previous study in a smaller sample (N=68) of drug-naive first-episode schizophrenia patients also revealed decreased gray matter volume in the right temporal and anterior cingulate cortex (12). Several factors merit consideration in explaining the regionally greater gray matter volume seen in the present study relative to several previous studies. Differences in patient characteristics across studies may be important, as some other studies have also reported increased gray matter volume in schizophrenia, including a report of such effects in the orbital frontal gyrus and anterior cingulate cortex in a study of 169 patients (30). One patient characteristic that the present data suggest may be important is severity of negative symptoms. This sample had a higher proportion of patients with prominent negative symptoms (36%) than the sample in our previous study (12%) (12). The results of the present study indicate that patients without prominent negative symptoms have more regions with decreased volume, especially in right temporal regions, than patients with prominent negative symptoms close to the time of illness onset, which may partly explain the discrepancy with our previous findings (12). Second, as the patients in the present study were early in the course of illness (average illness duration=6.25 months), possible early-course neuronal pathology, such as preapoptotic osmotic changes or hypertrophy, could increase regional volumes (31). Progressive gray matter volume loss might be expected after antipsychotic treatment (6–10) or in relation to secondary factors or course of illness effects (6, 32), which could contribute to the absence of reported hypertrophy in previous studies of patients who had a longer duration of illness before MRI scans were obtained (11). Of note, anatomical and functional changes did not correlate with untreated illness duration or with positive symptoms, and the direct comparison between patients with long and short untreated illness durations revealed no significant differences. These findings suggest a relatively static or slowly evolving process of anatomical changes, which is consistent with a recent study of treated schizophrenia patients (33) in suggesting the absence of progressive loss of gray matter volume during the early illness course before treatment has begun. Thus, the structural and functional brain changes observed in first-episode patients may be associated with neurodevelopmental problems such as neuronal overgrowth or a deficit in normal pruning during neurogenesis, as suggested by previous studies (34, 35) or, alternatively, with early illness pathophysiology effects, possibilities that need to be examined in future studies following at-risk individuals. Third, this study had a large sample and may have had the statistical power to detect hypertrophic effects not reported frequently in previous studies. Finally, this study used VBM8, which provides a more optimized method of segmentation and normalization than the VBM2 method in the analysis of gray matter volume (12). Furthermore, with concern for potential influences of skull-stripping artifacts, especially in the orbital frontal gyrus where deskulling is difficult, we contrasted computerized and manual skull stripping in a subsample of 40 participants and found no differences, indicating that artifacts induced by skull stripping procedures are not likely a cause of the group differences between patients and comparison subjects observed in this study. Although the exact mechanism for increased gray matter volume in first-episode schizophrenia before drug treatment is unclear, our findings indicate that regions within thalamo-cortical networks show early anatomical changes in the course of schizophrenia. Further research is needed on potential neuroinflammatory and other mechanisms that might contribute to these changes in brain anatomy and on how these anatomical changes influence clinical presentation and course of illness.
Deficits in the thalamus and frontal cortex have been widely reported in previous neuroimaging studies of schizophrenia (4, 9, 36–39). Alterations in the thalamus have been proposed to be associated with heterogeneous symptoms in schizophrenia, such as hallucinations, delusions, disorganized behaviors and speech, and thought disorder (5, 36). In contrast, prefrontal abnormalities have been related to negative symptoms and some deficits in higher cognitive function (40). Several other regions with altered gray matter volume were also identified in the present study, including the insula, inferior parietal lobule, and anterior cingulate cortex (41). All these regions are located in thalamo-cortical networks, where abnormalities are believed to be central to the pathogenesis of schizophrenia (10, 42, 43).
In contrast, in the analysis of functional data, abnormalities were located within fronto-parietal and default mode networks, which are thought to be important for decision making, working memory, and general monitoring of internal and external environments (20, 44, 45). In particular, the decreased amplitude of low-frequency fluctuation in medial prefrontal areas and the inferior parietal lobules bilaterally were consistent with previous studies in drug-naive schizophrenia patients (19). More interestingly, a small cohort study of antipsychotic-naive patients with first-episode schizophrenia showed significantly increased synchronous regional brain function after 6 weeks of antipsychotic treatment, especially in the left and right prefrontal, medial frontal, and parietal cortex, the left superior temporal cortex, and the right caudate nucleus (46). These regions include areas where pretreatment alterations were observed in the present study, consistent with the possibility that some of these changes may be shorter-term alterations associated with acute psychosis. Functional deficits in the medial frontal gyrus are thought to be part of the core pathogenesis of schizophrenia and may underlie a number of symptoms, including apathy, lack of emotion, and blunted affect (47). Besides medial prefrontal regions, we also observed an increased amplitude of low-frequency fluctuation in inferior parietal regions bilaterally. Both inferior parietal regions and prefrontal areas bilaterally play a key role in high-level cognitive and executive processing (48, 49), and functional activation studies have shown that deficits in these regions are greater during than after episodes of psychosis (50).
In summary, this study provides evidence that anatomical and functional deficits are seen in different brain regions in a large sample of antipsychotic-naive first-episode schizophrenia patients. An important caveat is that resting-state studies do not evaluate the functional integrity of all brain regions equally; default mode regions, for example, normally show more robust effects associated with the resting state, and thus sensitivity to illness effects may be greater in these regions. Activation studies in different regions of interest can provide complementary information with resting-state studies of structure-function relationships. Findings from the present study, along with future work clarifying the causes of the functional and structural changes reported and their dissociation, may provide new insight into the underlying neuropathology of the early course of schizophrenia.
1 : Catching up on schizophrenia: natural history and neurobiology. Neuron 2000; 28:325–334Crossref, Medline, Google Scholar
2 : Update on the use of MR for assessment and diagnosis of psychiatric diseases. Radiology 2010; 255:23–41Crossref, Medline, Google Scholar
3 : Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005; 162:2233–2245Link, Google Scholar
4 : A selective review of volumetric and morphometric imaging in schizophrenia. Curr Top Behav Neurosci 2010; 4:243–281Crossref, Medline, Google Scholar
5 : Thalamic pathology in schizophrenia. Curr Top Behav Neurosci 2010; 4:509–528Crossref, Medline, Google Scholar
6 : Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 2011; 70:672–679Crossref, Medline, Google Scholar
7 : Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. Neuroimage 2012; 59:986–996Crossref, Medline, Google Scholar
8 : Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull 2011; 37:177–188Crossref, Medline, Google Scholar
9 : The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry 2008; 165:1015–1023Link, Google Scholar
10 : The lifetime trajectory of schizophrenia and the concept of neurodevelopment. Dialogues Clin Neurosci 2010; 12:409–415Medline, Google Scholar
11 : The myth of schizophrenia as a progressive brain disease. Schizophr Bull (Epub ahead of print, Dec 7, 2012)Google Scholar
12 : Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry 2009; 166:196–205Link, Google Scholar
13 : Features of structural brain abnormality detected in first-episode psychosis. Am J Psychiatry 2000; 157:1829–1834Link, Google Scholar
14 : Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res 2000; 41:303–312Crossref, Medline, Google Scholar
15 : Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophr Res 2008; 104:44–60Crossref, Medline, Google Scholar
16 : Functional magnetic resonance imaging in schizophrenia. Dialogues Clin Neurosci 2010; 12:333–343Medline, Google Scholar
17 : Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 2010; 20:519–534Crossref, Medline, Google Scholar
18 : Functional brain imaging in schizophrenia: selected results and methods. Curr Top Behav Neurosci 2010; 4:181–214Crossref, Medline, Google Scholar
19 : Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage 2010; 49:2901–2906Crossref, Medline, Google Scholar
20 : Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res 2010; 117:21–30Crossref, Medline, Google Scholar
21 : Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev 2009; 33:279–296Crossref, Medline, Google Scholar
22 : Positive and negative schizophrenic symptoms and the role of dopamine. Br J Psychiatry 1980; 137:383–386Crossref, Medline, Google Scholar
23 ;
24 : The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol Psychiatry 2005; 58:457–467Crossref, Medline, Google Scholar
25 : Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 2002; 17:1711–1719Crossref, Medline, Google Scholar
26 : Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 2001; 22:1326–1333Medline, Google Scholar
27 : Reliability and validity of the Positive and Negative Syndrome Scale for schizophrenics. Psychiatry Res 1988; 23:99–110Crossref, Medline, Google Scholar
28 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
29 : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
30 : Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry 2008; 63:465–474Crossref, Medline, Google Scholar
31 : Changes in gray matter volume in patients with bipolar disorder. Biol Psychiatry 2005; 58:151–157Crossref, Medline, Google Scholar
32 : What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull 2008; 34:354–366Crossref, Medline, Google Scholar
33 : Duration of untreated illness in schizophrenia is not associated with 5-year brain volume change. Schizophr Res 2011; 132:84–90Crossref, Medline, Google Scholar
34 : Frontal white matter biochemical abnormalities in late-life major depression detected with proton magnetic resonance spectroscopy. Am J Psychiatry 2002; 159:630–636Link, Google Scholar
35 : Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol Psychiatry 2007; 61:776–781Crossref, Medline, Google Scholar
36 : Updated meta-analyses reveal thalamus volume reduction in patients with first-episode and chronic schizophrenia. Schizophr Res 2010; 123:1–14Crossref, Medline, Google Scholar
37 : A follow-up magnetic resonance imaging study of schizophrenia: relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry 1998; 55:145–152Crossref, Medline, Google Scholar
38 : Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 2003; 60:585–594Crossref, Medline, Google Scholar
39 : Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry 2008; 64:774–781Crossref, Medline, Google Scholar
40 : A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1–52Crossref, Medline, Google Scholar
41 : Structural neuroimaging in schizophrenia: from methods to insights to treatments. Dialogues Clin Neurosci 2010; 12:317–332Medline, Google Scholar
42 : Duration of illness, regional brain morphology, and neurocognitive correlates in schizophrenia. Ann Acad Med Singapore 2009; 38:388–395Medline, Google Scholar
43 : The role of the thalamus in schizophrenia. Can J Psychiatry 1997; 42:27–33Crossref, Medline, Google Scholar
44 : The social brain hypothesis of schizophrenia. World Psychiatry 2006; 5:77–81Medline, Google Scholar
45 : Parietal dysfunction is associated with increased outcome-related decision-making in schizophrenia patients. Biol Psychiatry 2002; 51:995–1004Crossref, Medline, Google Scholar
46 : Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry 2010; 67:783–792Crossref, Medline, Google Scholar
47 : Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain 2005; 128:2597–2611Crossref, Medline, Google Scholar
48 : Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry 2008; 165:1006–1014Link, Google Scholar
49 : Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br J Psychiatry 2003; 182:525–531Crossref, Medline, Google Scholar
50 : An fMRI study of visual attention and sensorimotor function before and after antipsychotic treatment in first-episode schizophrenia. Psychiatry Res 2009; 172:16–23Crossref, Medline, Google Scholar
51 : Emerging evidence of connectomic abnormalities in schizophrenia. J Neurosci 2011; 31:6263–6265Crossref, Medline, Google Scholar
52 : Does function follow form? Methods to fuse structural and functional brain images show decreased linkage in schizophrenia. Neuroimage 2010; 49:2626–2637Crossref, Medline, Google Scholar
53 : The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry 2004; 161:398–413Link, Google Scholar



