An Extended Swedish Adoption Study of Anxiety Disorder and Its Cross-Generational Familial Relationship With Major Depression
Abstract
Objective:
To clarify, using an extended adoption design, the sources of parent-offspring transmission for anxiety disorder (AD) and its major subforms and their familial cross-generational relationship with major depression (MD).
Methods:
Offspring (born 1960–1992) and their parents, from six family types (intact, not-lived-with biological father or mother, lived-with step-father or step-mother, and adoptive), were ascertained from Swedish national samples. Diagnoses were obtained from national medical registers. We assessed three sources of parent-child resemblance: genes plus rearing, genes only, and rearing only. To test comorbidity effects, single diagnoses were assigned in comorbid cases based on frequency and recency.
Results:
For AD to AD parent-child transmission, best-estimate tetrachoric correlations for the three types of parent-offspring relationships genes plus rearing, genes only, and rearing only—equaled +0.16 (95% CI=0.16, 0.16), +0.12 (95% CI=0.10, 0.13), and +0.06 (95% CI=0.04, 0.07), respectively, with broadly similar results for MD to MD transmission. Cross-disorder cross-generation correlations were modestly lower, with genetic and rearing correlations for AD and MD estimated at +0.83 (95% CI=0.76, 0.90) and +0.83 (95% CI=0.69, 0.96), respectively. Analyses for panic disorder and generalized anxiety disorder (GAD) produced comparable findings, with the genetic correlation with MD modestly higher for generalized anxiety disorder than panic disorder. Applying a diagnostic hierarchy to comorbid cases resulted in a decline in cross-disorder cross-generation transmission with the estimated genetic correlation equaling +0.46 (95% CI=0.30, 0.62).
Conclusions and Relevance:
For AD and its major subforms, cross-generational transmission includes both genetic and rearing effects. In traditional analyses, AD and MD demonstrate highly correlated genetic and rearing effects. The genetic correlation weakened when applying a diagnostic hierarchy.
A range of family and twin studies have found that anxiety disorder (AD), among the most common of psychiatric conditions (1), is familial and moderately heritable (2, 3). Most, but not all twin studies, suggest that familial resemblance for AD arises solely from genetic effects (4–8). Genetic influences on AD have also been detected using genome wide association designs (3, 9). Multivariate analyses have suggested a substantial sharing of genetic risk factors across various specific anxiety disorders (10, 11).
However, to date, no adoption study of AD has been reported. While twin studies examine genetic and environmental influences within a generation, adoption studies address a different and equally important question: the magnitude and causes of cross-generational transmission.
AD is often comorbid with major depression (MD) and twin studies consistently suggest that the two disorders share much of their genetic risk (12–14). Family studies have shown that the offspring of individuals with MD have increased rates of AD (15)—but such designs cannot determine the degree to which these findings arise from genetic versus rearing effects.
We therefore report an expanded National Swedish adoption study of clinically diagnosed AD examining six family types: 1) intact nuclear families, 2) families with not-lived-with (NLW) biological fathers, 3) families with NLW biological mothers, 4) families with stepfathers, 5) families with stepmothers, and 6) biological and adoptive parents from adoption families. We address four major questions: first, what is the magnitude of the transmission of AD from parents to their offspring and to what degree does it result from genetic versus rearing effects? Second, what are the sources of the familial cross-generational relationship between AD and MD? Third, to what degree are the cross-generational genetic and rearing effects which contribute to liability to AD and to MD correlated? Fourth, how do results differ when we examine AD-subtypes or impose a hierarchy on MD and AD diagnoses?
Methods
Information for this study was collected from nationwide Swedish registers (as outlined in appendix Table 1 in the online supplement). Each person’s unique identification number, having been replaced with serial numbers for confidentiality, were used for registry linkages. Ethical approval was secured from the regional ethical review board in Lund, Sweden. Using the Swedish Hospital Discharge Register, Outpatient Care Register and nationwide primary care data, we diagnosed AD and MD using ICD-8, 9, and 10 codes and phobia, panic disorder, generalized anxiety disorder, and AD-not otherwise specified (NOS), using only ICD-10 codes as seen in appendix Table 2 in the online supplement. Individuals registered with bipolar disorder or schizophrenia (see appendix Table 2 in the online supplement) were censored. This study population included all individuals born in Sweden between 1960 and 1992, who were alive and resided in Sweden at least until the age of 20. For these individuals, here called offspring, we included the number of years, during ages 0–15, that they resided in the same household and geographic area as their biological mother, biological father, and possible stepfather, stepmother, adoptive mother, and adoptive father. Study end was on December 31, 2018 due to availability of medical register data. From 1960 to 1985 (every fifth year), we used household identification numbers from the Population and Housing Census to define family types. The household identification includes all individuals registered at the same residence. From 1986 onward (every year), we defined family type using the family identification from the Total Population Register. The family identification is defined by individuals registered at the same property who are also related, adopted, married, or had common children. Therefore, from 1986 we could not detect all stepparents, e.g., stepparents not married to a biological parent, nor having a common child with a biological parent. For years with no information on whether offspring and parents resided together, we used information from the closest year. Geographical areas called Small Areas for Market Statistics (SAMS) are defined by Statistics Sweden, the Swedish government-owned statistics bureau. There are approximately 9,200 SAMS throughout Sweden, and are characterized by homogeneous building types and limited by natural boundaries.
We defined the following types of families: intact families that included offspring residing from ages 0 to 15 years in the same household with both their biological mother and father; families with NLW (not-lived-with) father/mother, that included offspring who never resided in the same household or SAMS area as the biological father/mother; two types of stepfamilies (stepfather/stepmother) that included offspring who did not reside the entire period between ages 0 and 15 with the biological father/mother and resided at least 10 of these years with a biologically unrelated man/woman 18–50 years older; adoptive families that included offspring adopted at younger than 5 years, with information available on both adoptive parents and at least one biological parent. The adoptive parents had to be biologically unrelated to the offspring and the offspring had to reside with each adoptive parent for at least 10 years between the ages 0 and 15. As domestic adoptions declined substantially in Sweden in recent decades, we expanded modestly our adoption cohort to maximize sample size, including offspring born from 1955 to 1992. The NLW and stepparents were defined so that their relationships with their offspring resembled those of an adoptee and his/her biological and adoptive parents.
For all family types, parents had to be alive throughout 1975 and had to reside in Sweden during some period of time from 1976 and onwards. There are overlaps in the parent-offspring relationships between the NLW families and the stepfamilies. When doing joint statistical analyses across family types, parent-offspring relationships included in, for example, a stepfather family was removed from the NLW father family group, so that each relationship was only calculated once.
In our parent-offspring pairs, we calculated tetrachoric correlations—which represent the correlation in relatives for a latent underlying normally distributed liability to illness (16)—for AD transmission, MD transmission, AD to MD cross-transmission and MD to AD cross-transmission. We used this measure because of its ease of interpretability (17) and its insensitivity to changes in base rates (18), given the differing rates of AD and MD across our family types. To calculate weighted tetrachoric correlations and for testing for heterogeneity across families, we use a meta-analysis fixed-effects model (19). The model is fixed as samples of data come from the same population. For the heterogeneity tests, a significance level of 0.05 was utilized. We also calculated the genetic correlation and the rearing correlation between AD and MD (as outlined in appendix Table 3 in the online supplement).
In cases with both lifetime MD and AD diagnoses, we assigned a single diagnosis based on the relative number of the two diagnoses, and if those are close, giving greater emphasis to recent diagnoses (see appendix Table 4 in the online supplement). Data analysis was conducted from August 23, 2021, to February 11, 2022. Statistical analyses were performed using R, version 4.0.3 (20) (see appendix Table 5 in the online supplement) and SAS, version 9.4 (21).
Results
Sample Descriptives
The sample sizes of parents and offspring (born 1960–1992, mean age 41.5 [SD=10.0]), along with age and gender of offspring, in six family types are outlined in Table 1. The largest sample size is for intact families and the smallest for stepmother and NLW mother families. The prevalences of AD in the parents and offspring are shown in Table 2. We see the expected higher prevalence for AD in mothers versus fathers and daughters versus sons. Among offspring and parents, rates of AD are generally highest in the disrupted NLW and stepparent families, intermediate in adoptive families, and lowest in the intact families. We also examined the registries through which our AD cases were ascertained. The largest proportion (from 64% to 68% across offspring and parents) came only from primary care data, with the next largest (9%–17%) from both primary care data and specialist registries. For our main analyses, diagnoses of MD and AD were assigned without hierarchy.
| Measure | Intact Families | NLW Father Families | NLW Mother Families | Stepfather Families | Stepmother Families | Adoptive Families |
|---|---|---|---|---|---|---|
| Sample size offspring | 1,968,352 | 104,243 | 5,144 | 87,473 | 14,028 | 13,027 |
| Sample size biological mother | 1,968,352 | 77,450 | 5,144 | 65,458 | NA | 12,489 |
| Sample size biological father | 1,968,352 | 104,243 | 1,026 | NA | 4,042 | 7,662 |
| Offspring | ||||||
| Year of birth, mean (SD) | 1975.6 (9.6) | 1974.0 (9.5) | 1969.0 (7.8) | 1973.6 (8.4) | 1972.2 (8.5) | 1963.7 (7.2) |
| Age, mean (SD) | 41.3 (10.0) | 42.7 (10.1) | 47.3 (9.5) | 43.2 (9.2) | 44.2 (9.5) | 52.5 (9.2) |
| Male, % | 52.5 | 51.1 | 54.4 | 50.2 | 55.1 | 53.2 |
TABLE 1. Sample size, birth year, age, and sex distributions across the six family types included in the studya
| Family Type and Disorder | Intact Families (%) | NLW Father Families (%) | NLW Mother Families (%) | Stepfather Families (%) | Stepmother Families (%) | Adoptive Families (%) |
|---|---|---|---|---|---|---|
| All offspring | ||||||
| AD | 13.5 | 21.0 | 19.6 | 19.5 | 21.2 | 17.6 |
| MD | 14.3 | 22.7 | 22.0 | 21.8 | 23.7 | 20.4 |
| Female offspring | ||||||
| AD | 17.5 | 26.2 | 25.5 | 24.5 | 27.6 | 21.4 |
| MD | 18.8 | 28.8 | 28.4 | 27.8 | 31.3 | 26.3 |
| Male offspring | ||||||
| AD | 9.8 | 16.0 | 14.7 | 14.6 | 16.0 | 14.2 |
| MD | 10.2 | 16.9 | 16.6 | 15.8 | 17.4 | 15.2 |
| Biological mothers | ||||||
| AD | 11.4 | 19.8 | 17.8 | 16.4 | NA | 14.7 |
| MD | 15.8 | 25.8 | 22.6 | 22.5 | 20.1 | |
| Biological fathers | ||||||
| AD | 5.8 | 9.4 | 7.2 | NA | 7.0 | 7.0 |
| MD | 9.2 | 13.8 | 11.7 | 11.0 | 11.6 | |
| Stepmother or adoptive mother | ||||||
| AD | NA | NA | NA | NA | 12.6 | 11.3 |
| MD | 18.3 | 17.7 | ||||
| Stepfather or adoptive father | ||||||
| AD | NA | NA | NA | 7.0 | NA | 4.2 |
| MD | 11.0 | 8.5 | ||||
TABLE 2. Prevalences of anxiety disorder and major depression among the relatives from the six family types included in the studya
Cross-Generational Transmission of Anxiety Disorders
The top section of Table 3 presents parent-offspring tetrachoric correlations for AD to AD transmission, separately for mothers and fathers, across our six family types, divided into those that reflect genes plus rearing, genes only, and rearing only. The next columns present the weighted estimate across these parent-offspring relationships, and the final column a test for heterogeneity, which evaluates whether the findings used to calculate the weighted estimate were statistically heterogeneous. Significant heterogeneity was seen only for mothers providing both genes plus rearing. In mothers and fathers, respectively, transmission was strongest for genes plus rearing relationships (+0.17 and +0.15), intermediate in genes only relationships (+0.13 and +0.11) and weakest in rearing only relationships (+0.04 and +0.06).
| Transmission and Mode | Intact | NLW Father | NLW Mother | Stepfather | Stepmother | Adoptive | Weighted Estimate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tetrachoric Correlations | 95% CI | Tetrachoric Correlations | 95% CI | Tetrachoric Correlations | 95% CI | Tetrachoric Correlations | 95% CI | Tetrachoric Correlations | 95% CI | Tetrachoric Correlations | 95% CI | Tetrachoric Correlations | 95% CI | HET Testb | |
| AD to AD | |||||||||||||||
| Mother-offspring | |||||||||||||||
| Genes plus rearing | 0.17 | 0.16, 0.17 | 0.21 | 0.20, 0.22 | NA | NA | 0.18 | 0.16, 0.19 | NA | NA | NA | NA | 0.17 | 0.16, 0.17 | <0.001* |
| Genes only | NA | NA | NA | NA | 0.16 | 0.10, 0.21 | NA | NA | NA | NA | 0.12 | 0.08, 0.16 | 0.13 | 0.10, 0.16 | 0.32 |
| Rearing only | NA | NA | NA | NA | NA | NA | NA | NA | 0.04 | −0.00, 0.07 | 0.04 | −0.00, 0.08 | 0.04 | 0.01, 0.06 | 0.99 |
| Father-offspring | |||||||||||||||
| Genes plus rearing | 0.15 | 0.14, 0.15 | NA | NA | 0.07 | −0.10, 0.24 | NA | NA | 0.20 | 0.12, 0.28 | NA | NA | 0.15 | 0.14, 0.15 | 0.38 |
| Genes only | NA | NA | 0.12 | 0.10, 0.13 | NA | NA | NA | NA | NA | NA | 0.08 | 0.02, 0.14 | 0.11 | 0.10, 0.13 | 0.28 |
| Rearing only | NA | NA | NA | NA | NA | NA | 0.06 | 0.05, 0.08 | NA | NA | 0.07 | 0.01, 0.12 | 0.06 | 0.05, 0.08 | 0.93 |
| AD to MD | |||||||||||||||
| Mother-offspring | |||||||||||||||
| Genes plus rearing | 0.13 | 0.13, 0.14 | 0.16 | 0.14, 0.17 | NA | NA | 0.14 | 0.12, 0.15 | NA | NA | NA | NA | 0.13 | 0.13, 0.14 | 0.01 |
| Genes only | NA | NA | NA | NA | 0.12 | 0.07, 0.17 | NA | NA | NA | NA | 0.07 | 0.03, 0.11 | 0.09 | 0.06, 0.12 | 0.14 |
| Rearing only | NA | NA | NA | NA | NA | NA | NA | NA | 0.02 | −0.01, 0.06 | 0.04 | 0.00, 0.08 | 0.03 | 0.01, 0.06 | 0.42 |
| Father-offspring | |||||||||||||||
| Genes plus rearing | 0.12 | 0.11,0.12 | NA | NA | 0.09 | −0.07, 0.26 | NA | NA | 0.13 | 0.05, 0.21 | NA | NA | 0.12 | 0.11, 0.12 | 0.88 |
| Genes only | NA | NA | 0.08 | 0.06, 0.09 | NA | NA | NA | NA | NA | NA | 0.08 | 0.02, 0.13 | 0.08 | 0.06, 0.09 | 0.92 |
| Rearing only | NA | NA | NA | NA | NA | NA | 0.05 | 0.03, 0.07 | NA | NA | 0.05 | −0.01, 0.10 | 0.05 | 0.03, 0.07 | 0.83 |
| MD to AD | |||||||||||||||
| Mother-offspring | |||||||||||||||
| Genes plus rearing | 0.15 | 0.15, 0.15 | 0.18 | 0.16, 0.19 | NA | NA | 0.17 | 0.15, 0.18 | NA | NA | NA | NA | 0.15 | 0.15, 0.15 | <0.01* |
| Genes only | NA | NA | NA | NA | 0.07 | 0.02, 0.13 | NA | NA | NA | NA | 0.06 | 0.03, 0.10 | 0.07 | 0.04, 0.10 | 0.76 |
| Rearing only | NA | NA | NA | NA | NA | NA | NA | NA | 0.04 | 0.01, 0.08 | 0.01 | −0.03, 0.05 | 0.03 | 0.00, 0.05 | 0.19 |
| Father-offspring | |||||||||||||||
| Genes plus rearing | 0.13 | 0.12, 0.13 | NA | NA | 0.16 | 0.01, 0.30 | NA | NA | 0.08 | 0.01, 0.16 | NA | NA | 0.13 | 0.12, 0.13 | 0.40 |
| Genes only | NA | NA | 0.09 | 0.07, 0.10 | NA | NA | NA | NA | NA | NA | 0.03 | −0.03, 0.08 | 0.08 | 0.07, 0.10 | 0.03 |
| Rearing only | NA | NA | NA | NA | NA | NA | 0.06 | 0.04, 0.07 | NA | NA | 0.04 | −0.00, 0.09 | 0.06 | 0.04, 0.07 | 0.44 |
| MD to MD | |||||||||||||||
| Mother-offspring | |||||||||||||||
| Genes plus rearing | 0.17 | 0.16, 0.17 | 0.18 | 0.17, 0.20 | NA | NA | 0.18 | 0.17, 0.20 | NA | NA | NA | NA | 0.17 | 0.17, 0.17 | 0.03 |
| Genes only | NA | NA | NA | NA | 0.14 | 0.09, 0.19 | NA | NA | NA | NA | 0.04 | 0.01, 0.08 | 0.07 | 0.04, 0.10 | <0.01 |
| Rearing only | NA | NA | NA | NA | NA | NA | NA | NA | 0.04 | 0.00, 0.07 | 0.04 | 0.00, 0.07 | 0.04 | 0.01, 0.06 | 0.97 |
| Father-offspring | |||||||||||||||
| Genes plus rearing | 0.14 | 0.14, 0.14 | NA | NA | 0.17 | 0.03, 0.31 | NA | NA | 0.20 | 0.13, 0.27 | NA | NA | 0.14 | 0.14, 0.14 | 0.15 |
| Genes only | NA | NA | 0.08 | 0.07, 0.10 | NA | NA | NA | NA | NA | NA | 0.06 | 0.01, 0.11 | 0.08 | 0.07, 0.09 | 0.34 |
| Rearing only | NA | NA | NA | NA | NA | NA | 0.07 | 0.06, 0.09 | NA | NA | 0.02 | −0.02, 0.07 | 0.07 | 0.05, 0.08 | 0.03 |
TABLE 3. Parent-offspring tetrachoric correlations, weighted estimates, and heterogeneity tests for four extended adoption designsa
The top section of Table 4 and Figure 1a present the weighted estimates (along with 95% CIs and heterogeneity tests) from mothers and fathers reflecting genes plus rearing, genes only, and rearing only which were respectively, +0.16, +0.12, and +0.06.
| Transmission and Mode | Mothers | Fathers | Weighted Estimate | pb | |||
|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||
| AD to AD | |||||||
| Genes plus rearing | 0.17 | 0.16, 0.17 | 0.15 | 0.14, 0.15 | 0.16 | 0.16, 0.16 | <0.001* |
| Genes only | 0.13 | 0.10, 0.16 | 0.11 | 0.10, 0.13 | 0.12 | 0.10, 0.13 | 0.28 |
| Rearing only | 0.04 | 0.01, 0.06 | 0.06 | 0.05, 0.08 | 0.06 | 0.04, 0.07 | 0.10 |
| AD to MD | |||||||
| Genes plus rearing | 0.13 | 0.13, 0.14 | 0.12 | 0.11, 0.12 | 0.13 | 0.13, 0.13 | <0.001* |
| Genes only | 0.09 | 0.06, 0.12 | 0.08 | 0.06, 0.09 | 0.08 | 0.07, 0.09 | 0.59 |
| Rearing only | 0.03 | 0.01, 0.06 | 0.05 | 0.03, 0.07 | 0.05 | 0.03, 0.06 | 0.27 |
| MD to AD | |||||||
| Genes plus rearing | 0.15 | 0.15, 0.15 | 0.13 | 0.12, 0.13 | 0.14 | 0.14, 0.14 | <0.001* |
| Genes only | 0.07 | 0.04, 0.10 | 0.08 | 0.07, 0.10 | 0.08 | 0.07, 0.09 | 0.39 |
| Rearing only | 0.03 | 0.00, 0.05 | 0.06 | 0.04, 0.07 | 0.05 | 0.04, 0.06 | 0.04 |
| MD to MD | |||||||
| Genes plus rearing | 0.17 | 0.17, 0.17 | 0.14 | 0.14, 0.14 | 0.16 | 0.15, 0.16 | <0.001* |
| Genes only | 0.07 | 0.04, 0.10 | 0.08 | 0.07, 0.09 | 0.08 | 0.07, 0.09 | 0.65 |
| Rearing only | 0.04 | 0.01, 0.06 | 0.07 | 0.05, 0.08 | 0.06 | 0.05, 0.07 | 0.04 |
TABLE 4. Tests of transmission from mothers and fathers using weighted estimates across all family typesa
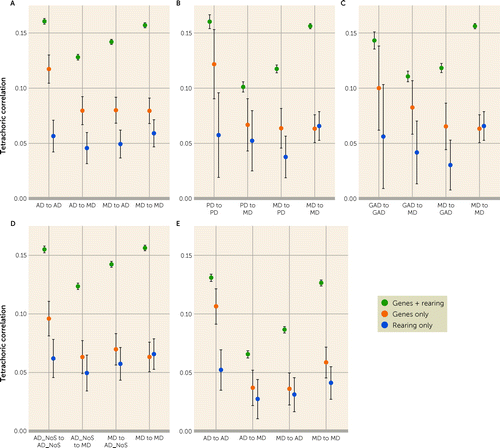
FIGURE 1. Summary results of the sources of the within and across disorder cross-generational transmissiona
a (A) Best weighted estimates (±95% CIs) of tetrachoric correlations on the Y-axis reflecting parent-offspring relationships due to 1) genes plus rearing, 2) genes only, and 3) rearing only for cross-generational transmission of anxiety disorders to anxiety disorders, anxiety disorders to major depression, major depression to anxiety disorders, and major depression to major depression. Diagnoses were assigned without a hierarchy. (B) Best weighted estimates (±95% CIs) of tetrachoric correlations on the Y-axis reflecting parent-offspring relationships due to 1) genes plus rearing, 2) genes only, and 3) rearing only for cross-generational transmission of panic disorder to panic disorder, panic disorder to major depression, major depression to panic disorder, and major depression to major depression. Diagnoses were assigned without a hierarchy. (C) Best weighted estimates (±95% CIs) of tetrachoric correlations on the Y-axis reflecting parent-offspring relationships due to 1) genes plus rearing, 2) genes only, and 3) rearing only for cross-generational transmission of generalized anxiety disorder to generalized anxiety disorder, generalized anxiety disorder to major depression, major depression to generalized anxiety disorder, and major depression to major depression. Diagnoses were assigned without a hierarchy. (D) Best weighted estimates (±95% CIs) of tetrachoric correlations on the Y-axis reflecting parent-offspring relationships due to 1) genes plus rearing, 2) genes only, and 3) rearing only for cross-generational transmission of anxiety disorders NoS to anxiety disorders NoS, anxiety disorders NoS to major depression, major depression to anxiety disorders NoS, and major depression to major depression. Diagnoses were assigned without a hierarchy. (E) Best weighted estimates (±95% CIs) of tetrachoric correlations on the Y-axis reflecting parent-offspring relationships due to 1) genes plus rearing, 2) genes only, and 3) rearing only for cross-generational transmission of anxiety disorders to anxiety disorders, anxiety disorders to major depression, major depression to anxiety disorders, and major depression to major depression. Diagnoses were assigned with a hierarchy depending on the total number of assigned diagnoses and if that number was close then the more recent diagnoses were given greater weight.
Cross-Transmission of Anxiety Disorders to Major Depression
The second section of Table 3 presents parent-offspring tetrachoric correlations for AD to MD transmission. No significant heterogeneity was seen across groups of families. In mothers and fathers, respectively, transmission was strongest for genes plus rearing relationships (+0.13 and +0.12), intermediate in genes only relationships (+0.09 and +0.08) and weakest in rearing only relationships (+0.03 and +0.05).
The second section of Table 4 and Figure 1a present the weighted estimates from mothers and fathers for AD to MD transmission reflecting genes plus rearing, genes only, and rearing only which were, respectively, +0.13, +0.08, and +0.05.
Cross-Transmission of Major Depression to Anxiety Disorders
The third section of Table 3 presents parent-offspring tetrachoric correlations for MD to AD transmission. Significant heterogeneity was seen again for mothers providing both genes plus rearing. In mothers and fathers, respectively, transmission was strongest for genes plus rearing relationships (+0.15 and +0.13), intermediate in genes only relationships (+0.07 and +0.08) and weakest in rearing only relationships (+0.03 and +0.06).
The third section of Table 4 and Figure 1a present the weighted estimates from mothers and fathers for AM to MD transmission reflecting genes plus rearing, genes only, and rearing only which were, respectively, +0.14, +0.08, and +0.05.
Cross-Generational Transmission of Major Depression
The final section of Table 3 presents parent-offspring tetrachoric correlations for MD to MD transmission. No significant heterogeneity was seen across groups of families. In mothers and fathers, respectively, transmission was strongest for genes plus rearing relationships (+0.17 and +0.14), intermediate in genes only relationships (+0.07 and +0.08) and weakest in rearing only relationships (+0.04 and +0.07).
The final section of Table 4 and Figure 1a present the weighted estimates from mothers and fathers for MD to MD transmission reflecting genes plus rearing, genes only and rearing only were, respectively, +0.16, +0.08, and +0.06.
Genetic and Rearing Correlation of MD and AD
From the genes only and rearing only results, we could calculate the genetic and rearing cross-generational correlations for MD and AD which equaled, respectively, +0.83 (95% CI=0.76, 0.90) and +0.83 (95% CI=0.69, 0.96).
Subtypes of Anxiety Disorder
As seen in appendix Table 6 in the online supplement, sufficient sample sizes were available to examine three AD subtypes: panic disorder, generalized anxiety disorder, and AD-NOS. Summary results for the analyses of these subtypes are presented, respectively, in Figures 1b–d and appendix Tables 7–12 in the online supplement. While confidence intervals are larger, the general patterns of results for the AD subtypes are similar to that seen for all the ADs. The estimated genetic and rearing cross-generational correlations for MD and panic disorder, generalized anxiety disorder, and AD-NOS were broadly similar to those seen for AD as a whole and estimated at respectively, +0.74 (95% CI=0.57, 0.90) and +0.69 (95% CI=0.39, 0.99), +0.91 (95% CI=0.69, 1.00) and +0.57 (95% CI=0.25, 0.90), and +0.86 (95% CI=0.75, 0.96) and +0.84 (95% CI=0.71, 0.98). While all these genetic correlations were substantial, they were highest for generalized anxiety disorder, intermediate for AD-NOS, and lowest for panic disorder.
Imposition of a Diagnostic Hierarchy
Offspring in our families had a total of 302,679 AD and 322,569 MD lifetime cases, of which 158,386 had both diagnoses (appendix Figure 1 in the online supplement and Table 13 in the online supplement). Applying our diagnostic hierarchy, 4.7% of comorbid cases could not be classified, as the timing of all MD and AD diagnoses were identical. Of the resulting 150,949 individuals, 49% and 51% were assigned, respectively, a primary AD and MD diagnosis. After applying the same diagnostic hierarchy to the parental groups, 2,139,378 offspring were retained in our analysis.
Summary results for the analysis of these cases—are seen in Figure 1e and appendix Tables 14–16 in the online supplement. For genetic effects, the MD to MD and AD to AD correlations are slightly attenuated with larger reductions for the MD to AD and AD to MD correlations. For rearing effects, the attenuations are modest and similar in the twin and cross-disorder correlations. The genetic correlation between MD and AD declined substantially to +0.46 (95% CI=0.30, 0.62), while the rearing correlation, although known imprecisely, declined modestly to +0.64 (95% CI=0.32, 0.96).
Discussion
We have conducted an extended adoption study of AD and MD using Swedish national samples. We review, in turn, our eight key findings.
First, assessed with no diagnostic hierarchy, AD is moderately transmitted from parents to their children with a correlation in intact families estimated at +0.16. From our extended adoption design, we are able, for the first time, to determine the sources of this transmission. Approximately 70% of the parent-offspring resemblance for AD results from genetic transmission—estimated at +0.12 and 30% from rearing effects—estimated at +0.06. Our findings are noteworthy as twin studies of anxiety disorders have shown, with near uniformity, that twin resemblance for AD results solely from genetic factors (4–8). These results are not necessarily contradictory, as twin studies are poorly powered to detect the modest sibling resemblance that arises from rearing effects (22).
We cannot, in our sample, easily clarify the mechanisms of the rearing effect of AD. Social learning may contribute. In humans and primates, the fear of objects or situations can be learned from observing fear responses in others (23). Parental anxiety disorder might be associated with suboptimal parenting (e.g., by causing overprotectiveness) and/or resulting in parental strife or separation which could predispose to AD in offspring. Indeed, high trait neuroticism—associated genetically with anxiety disorders (24, 25)—in parents is predictive of overprotective parenting (26) which predisposes offspring to anxiety disorder (22, 27–29).
Second, we observed parent-offspring cross-transmission from AD in parents to MD in children as well as from MD in parents to AD in children that were quantitatively only moderately lower than the parallel within-disorder effects. The magnitudes of these transmissions in intact families (+0.13 vs. +0.14) were very similar, as were the transmissions in genes-only (both +0.08) and rearing-only relationships (both +0.05). In Sweden, assuming no diagnostic hierarchy, MD and AD have a symmetrical cross-disorder cross-generation transmission of liability.
Third, we were able to calculate the correlation of the cross-generational genetic influences on MD and AD as equaling +0.83. This result is consistent with a number of twin studies, especially those that have examined generalized anxiety disorder, which report quite high genetic correlations, suggesting a substantial sharing of genetic risk factors between MD and AD (12–14). A prior twin study of lifetime MD and generalized anxiety disorder in Sweden, modelled separately by sex, reported a genetic correlation of the two disorders equal to 1.00 in females and +0.74 in males (13). The mean of these two figures would produce a figure close to that observed here. Of note, this twin study utilized interview-based DSM-IV diagnoses, while our study relied on registry diagnoses, yet produced very similar findings.
Fourth, we also calculated, for the first time to our knowledge, a rearing environmental correlation for MD and AD which was estimated at +0.83. Our findings suggest that the familial environmental factors that are both related to parental diagnoses of MD or AD and that influence risk for MD and AD in offspring are highly inter-correlated, albeit not identical. This high inter-correlation stands in contrast to the considerably lower resemblance of depressogenic and anxiogenic environmental exposures in adulthood as revealed both by twin modeling (12) and the assessment of specific classes of stressful life events (e.g., loss/humiliation versus danger) (30–32). The anxiogenic and/or depressogenic aspects of the home environment and enduring parent-child relationships that we capture in this study likely differ qualitatively from the acute environmental exposures in adulthood that predispose to MD or AD.
Fifth, our analyses permit us to compare the magnitude of the genetic and rearing influences for AD and MD. In most twin samples examining MD and one or more anxiety disorders, MD is more heritable (13, 33). That is not what we see here. Narrow-sense heritability in adoption designs equals twice the tetrachoric correlation in gene-only relationships which would be estimated as +0.24 for AD and +0.16 for MD, and this difference is statistically significant (p<0.001). These heritability estimates are lower than those seen in twin studies, but this occurs commonly (34–37), likely, at least in part, because adoption studies examine genetic effects across different generations, while relative pairs in twin analyses are always of the same age.
Sixth, our expanded adoption design assumes that 1) NLW fathers and mothers are good proxies for biological parents in an adoption design and 2) stepfathers and stepmothers are reasonable proxies for adoptive parents in an adoption design. We could test these assumptions. Of the 16 relevant comparisons of the weighted parent-offspring correlations provided in Table 3 (that is NLW with biological and step- with adoptive-parents), none were significant at chance-corrected levels, results which support the validity of our expanded adoption design.
Seventh, our sample contained three AD subtypes with sufficient sample size to examine individually with MD. Consistent with prior results showing high genetic correlations across different ADs (10, 11) and recent molecular genetic work which has focused on an aggregate AD diagnosis (9), the pattern of results was broadly similar across these three forms of AD, and the aggregate results for all AD. In accord with prior twin studies, generalized anxiety disorder and MD had a modestly stronger genetic relationship than did panic disorder and MD (33).
Finally, as seen previously (33, 38–40), we observed high levels of comorbidity between MD and AD. Previous twin studies of these syndromes have not typically taken this into account, in part because of uncertainty about an optimal approach. To attempt to address this knotty problem, we used a Procrustean approach by imposing a diagnostic hierarchy on comorbid cases based on number and recency of diagnoses so that all comorbid MD and AD cases were assigned a single diagnosis of either MD or AD. We then repeated our analyses. The major change in findings were reductions in the genetic cross-disorder parent-offspring correlations and, as a result thereof, a sizable drop in the MD-AD genetic correlation. Not surprisingly, these findings suggest that the substantial MD-AD comorbidity contributes to their high genetic correlation. Further conceptual and empirical work will be needed to determine an optimal approach to “correcting” for MD-AD comorbidity in genetically informed analyses.
Limitations
These results should be interpreted in the context of two potentially important methodological limitations. First, this study is restricted to subjects with physician-diagnosed MD and/or AD in Sweden. We could not evaluate affected individuals who never presented for treatment. Our treated cases were likely, on average, more severely affected than those who would be detected by personal interviews in community samples. It is difficult to determine whether such incomplete ascertainment is more likely to attenuate or upwardly bias estimates of parent-child resemblance. Obtaining our subjects as we did from hospital, specialist, and primary care registries, however, means that we did not need to rely on subject cooperation or accurate recall. Such data can produce false negative and false positive diagnoses, the nature of which is difficult to estimate. Sweden has no large-scale interview-based psychiatric epidemiological study with which to compare our treated prevalence rates. However, an epidemiologic survey in near-by Norway provided a lifetime estimate of MD of 17.8% and estimates for phobias, panic disorder, and generalized anxiety disorder ranging from 4.5% to 14.4% (41). 42,161 interviewed twins from the birth-certificate based Swedish twin registry produced an estimated lifetime prevalence of MD of 19.5% and generalized anxiety disorder of 6.8%. These compare to our estimates in our intact families for AD and MD of 13.5% and 14.3% respectively. The validity of both the AD and MD diagnosis from primary care data has been supported by its prevalence, sex ratio, sibling and twin correlations and associations with well-documented psychosocial risk factors (42, 43).
Second, to maximize power, we analyzed offspring born across a long 32-year period. To investigate cohort effects, we repeated our analyses for all AD cases in the older and younger half of our cohort. These results are summarized in Appendix Figures 2 and 3 in the online supplement and, respectively, Tables 17–20 and 21–24 in the online supplement. While the broad pattern of findings is quite similar across the two subcohorts, parent-offspring correlations from intact families (genes plus rearing) are significantly higher in our younger cohort. Of the eight correlations for rearing or genes only correlations across subcohorts, none were significantly different, correcting for multiple testing (see appendix Table 25 in the online supplement).
Conclusions
Using an expanded adoption design applied to a national Swedish sample, we showed that AD and its major subforms are moderately transmitted across generations largely as a result of genetic factors, but with rearing effects also playing a meaningful role. When examining diagnoses without hierarchy, the parent-offspring transmission from AD to MD and from MD to AD were nearly symmetrical and a result of a similar mixture of genetic and rearing effects, with relatively high genetic and rearing-environmental correlations. However, providing comorbid cases with only one diagnosis, based on frequency and recency, resulted in a moderate reduction in the AD-MD cross-generational genetic correlation.
1. : Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19Crossref, Medline, Google Scholar
2. : A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry 2001; 158:1568–1578Link, Google Scholar
3. : Genetic contributions to anxiety disorders: where we are and where we are heading. Psychol Med 2021; 51:2231–2246Crossref, Medline, Google Scholar
4. : Evidence for genetic influences common and specific to symptoms of generalized anxiety and panic. J Affect Disord 2000; 57:25–35Crossref, Medline, Google Scholar
5. : A population-based twin study of generalized anxiety disorder in men and women. J Nerv Ment Dis 2001; 189:413–420Crossref, Medline, Google Scholar
6. : Panic syndromes in a population-based sample of male and female twins. Psychol Med 2001; 31:989–1000Crossref, Medline, Google Scholar
7. : The structure of genetic and environmental risk factors for phobias in women. Psychol Med 2011; 41:1987–1995Crossref, Medline, Google Scholar
8. : Social phobia in a population-based female adolescent twin sample: co-morbidity and associated suicide-related symptoms. Psychol Med 2000; 30:797–804Crossref, Medline, Google Scholar
9. : Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry 2016; 21:1391–1399Crossref, Medline, Google Scholar
10. : The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry 2005; 62:182–189Crossref, Medline, Google Scholar
11. : Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. Br J Psychiatry 2009; 195:301–307Crossref, Medline, Google Scholar
12. : Major depression and generalized anxiety disorder. same genes, (partly) different environments? Arch Gen Psychiatry 1992; 49:716–722Crossref, Medline, Google Scholar
13. : The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol Med 2006; 37:453–462Crossref, Medline, Google Scholar
14. : The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. Am J Psychiatry 2011; 168:29–39Link, Google Scholar
15. : Offspring of depressed parents: 20 years later. Am J Psychiatry 2006; 163:1001–1008Link, Google Scholar
16. Ekström J: The phi-coefficient, the tetrachoric correlation coefficient, and the Pearson-Yule Debate. escholarshiporg, 2011Google Scholar
17. : Introduction to Quantitative Genetics, 3rd ed. New York, Wiley, 1989Google Scholar
18. : The influence of base rates on correlations: an evaluation of proposed alternative effect sizes with real-world data. Behav Res Methods 2016; 48:1021–1031Crossref, Medline, Google Scholar
19. : A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Method 2010; 1:97–111Crossref, Medline, Google Scholar
20. R Core Team: R: A language and environment for statistical computing (Version 4.0.3) [Computer software]. Vienna, Austria, R Foundation for Statistical Computing, 2019Google Scholar
21. SAS Institute: SAS/STAT Online Documentation, Version 9.4. Cary, NC, SAS Institute Inc., 2012Google Scholar
22. : Parenting and adult mood, anxiety and substance use disorders in female twins: an epidemiological, multi-informant, retrospective study. Psychol Med 2000; 30:281–294Crossref, Medline, Google Scholar
23. : Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ, Prentice-Hall, 1986Google Scholar
24. : Genetic and environmental sources of covariation between generalized anxiety disorder and neuroticism. Am J Psychiatry 2004; 161:1581–1587Link, Google Scholar
25. : A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry 2006; 163:857–864Link, Google Scholar
26. : The determinants of parenting: an epidemiological, multi-informant, retrospective study. Psychol Med 1997; 27:549–563Crossref, Medline, Google Scholar
27. : Parental representations of patients with anxiety neurosis. Acta Psychiatr Scand 1981; 63:33–36Crossref, Medline, Google Scholar
28. : Family factors in the development and management of anxiety disorders. Clin Child Fam Psychol Rev 2012; 15:69–80Crossref, Medline, Google Scholar
29. : Childhood parental loss and adult psychopathology in women. a twin study perspective. Arch Gen Psychiatry 1992; 49:109–116Crossref, Medline, Google Scholar
30. : Types of stressful life event and the onset of anxiety and depressive disorders. Psychol Med 1981; 11:803–815Crossref, Medline, Google Scholar
31. : Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J Nervous Ment Dis 1998; 186:661–669Crossref, Medline, Google Scholar
32. : Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry 2003; 60:789–796Crossref, Medline, Google Scholar
33. : The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Arch Gen Psychiatry 1995; 52:374–383Crossref, Medline, Google Scholar
34. : Genetic and environmental influences on antisocial behavior: a meta- analysis of twin and adoption studies. Psychol Bull 2002; 128:490–529Crossref, Medline, Google Scholar
35. : Sources of parent-offspring resemblance for major depression in a national Swedish extended adoption study. JAMA Psychiatry 2018; 75:194–200Crossref, Medline, Google Scholar
36. : An extended Swedish national adoption study of alcohol use disorder. JAMA Psychiatry 2015; 72:211–218Crossref, Medline, Google Scholar
37. : Heritability of personality: a meta-analysis of behavior genetic studies. Psychol Bull 2015; 141:769–785Crossref, Medline, Google Scholar
38. : Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry 2011; 72:341–348Crossref, Medline, Google Scholar
39. Maser JD, Cloninger CR, editors: Comorbidity of Mood and Anxiety Disorders. Washington, DC, American Psychiatric Press, 1990Google Scholar
40. : Comorbidity of mood and anxiety disorders. Depress Anxiety 2000; 12:69–76Crossref, Medline, Google Scholar
41. : A Norwegian psychiatric epidemiological study. Am J Psychiatry 2001; 158:1091–1098Link, Google Scholar
42. : The genetic epidemiology of treated major depression in Sweden. Am J Psychiatry 2018; 175:1137–1144Link, Google Scholar
43. : Common adult psychiatric disorders in Swedish primary care where most mental health patients are treated. BMC Psychiatry 2017; 17:235Crossref, Medline, Google Scholar



