Event-Related Gamma Activity in Schizophrenia Patients During a Visual Backward-Masking Task
Abstract
OBJECTIVE: Schizophrenia patients experience deficits in many aspects of cognition and perception. Abnormalities in gamma activity may underlie some of these deficits, including rapid processing of visual stimuli. This study examined event-related gamma range activity during a visual backward-masking task in schizophrenia patients and normal comparison subjects. METHOD: Event-related gamma activity was recorded in 15 normal comparison subjects and 32 schizophrenia patients. Participants had event-related gamma activity recorded while viewing 60 unmasked visual targets and 240 trials of visual backward masking. Effects of group, accuracy (correct versus incorrect), stimulus-onset asynchrony, and regional activity (left versus right hemisphere, anterior versus posterior regions) were assessed. RESULTS: Schizophrenia patients had significantly reduced gamma activity in relation to comparison subjects during the backward-masking task. Normal comparison subjects showed significantly greater gamma activity in the right hemisphere, whereas schizophrenia patients did not show this pattern of lateralization. For the unmasked target, there was no group effect and no significant interactions in gamma-band responses. CONCLUSIONS: These results extend previous findings of abnormal gamma range activity in schizophrenia patients. Patients showed overall less gamma activity and failed to show lateralization of activity to the right hemisphere during masking, but they showed comparable levels of gamma activity to unmasked stimuli. Schizophrenia patients’ poorer performance during a masking task may be partly influenced by this abnormal level and the distribution of gamma activity.
Schizophrenia patients exhibit a number of cognitive and basic perceptual abnormalities across all sensory modalities. It has been proposed that failure to maintain cortical-cortical oscillations in the gamma range (30–70 Hz, usually centered around 40 Hz) may subserve many of the perceptual and neurocognitive deficits seen in schizophrenia (1). This article examines the role of event-related gamma oscillations in the commonly reported visual backward-masking deficits in schizophrenia.
Backward masking occurs when a briefly presented visual target is followed shortly by a mask that interrupts processing of the target (2, 3). On the basis of an influential theoretical model, masking occurs when the transient channel activity elicited by the mask interrupts sustained channel processing elicited by the target (2). Schizophrenia patients consistently show deficits on visual backward-masking tasks in that they require a longer interval between the target and the mask to correctly identify the target (4–7).
Recent advances in the visual-masking literature have strongly suggested, on the basis of performance data, that sustained cells at the cortical level exhibit oscillations with activity in the gamma (40–70 Hz) range (8). Masking performance can fluctuate with these cortical oscillations because maximal masking occurs when the transient activity elicited by the mask coincides with the peak of the oscillating activity generated by the target (8–10).
Gamma abnormalities in schizophrenia have recently attracted interest. Two early studies examining gamma abnormalities in the auditory modality have demonstrated reduced gamma activity in schizophrenia patients (11, 12), indicative of a failure to maintain neural activity in the gamma range. More recent studies have shown event-related gamma deficits in schizophrenia in the visual modality as well (e.g., references 10, 13).
The purpose of the current study was to expand upon our preliminary findings of gamma abnormalities during masking in schizophrenia and examine in greater detail how gamma activity is influenced by the strength of the masking effect. The backward-masking task we employed in this study is an appropriate way to study gamma activity because participants must discriminate the form of a target from the mask and process contour information of that target. Perception of visual form presumably depends upon integrating basic visual elements (e.g., line or edge orientation) to construct higher-order conjunctions (e.g., angles or vertices), and finally, to form a coherent representation of an object. It is believed that a key role of gamma activity is to achieve this kind of binding together of visual features. In this study, we examined the effects of masking interval, unmasked versus masked trials, correct versus incorrect trials, and brain region on gamma activity in schizophrenia patients and healthy comparison subjects.
Method
Participants
Thirty-six patients with schizophrenia and 16 normal comparison subjects participated in the study. Data from three patients were excluded from the final analysis because of equipment malfunction, whereas data from one patient and one comparison subject were excluded because of invalid behavioral performance. Hence, the final group consisted of 32 patients (two women) and 15 normal comparison subjects (one woman).
All subjects were participating in a larger study of early visual processing (Early Visual Processing in Schizophrenia, M.F.G., principal investigator). The schizophrenia patients were recruited from outpatient treatment clinics at the Veterans Affairs (VA) Greater Los Angeles Healthcare System and through presentations in the community. Twenty-four patients were receiving atypical antipsychotic medication, four patients were receiving typical antipsychotic medication, and four were not taking antipsychotic medication at the time of testing. All patients were administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (14) and met diagnostic criteria for schizophrenia. The patients had to be between 18 and 60 years of age. They were excluded from participation if they had substance abuse or dependence in the last 6 months, mental retardation, a history of loss of consciousness for more than 1 hour, an identifiable neurological disorder, or were not sufficiently fluent in English.
The normal comparison participants were recruited through flyers posted in the local community and newspaper advertisements in local newspapers. They had to be between 25 and 55 years of age. An initial screening interview excluded potential normal comparison subjects who had any identifiable neurological disorder or head injury, had a first-degree relative with schizophrenia or another psychotic disorder, were not sufficiently fluent in English, had a history of schizophrenia or another psychotic disorder, bipolar disorder, recurrent depression, or a history of substance dependence or any substance abuse in the last 6 months. Potential normal comparison participants were also interviewed with the SCID and portions of the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) (15). Potential normal comparison subjects were excluded if they had any of the following axis II disorders: avoidant, borderline, paranoid, schizoid, or schizotypal personality disorders.
All SCID interviewers were trained by the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center to a minimum kappa of 0.75 for key psychotic and mood items. All participants had the capacity to give informed consent and provided written informed consent after all procedures had been fully explained in accordance with procedures approved by the institutional review boards at the University of California at Los Angeles and the VA Greater Los Angeles Healthcare System.
Table 1 lists the demographic characteristics of the patient and comparison groups, as well as symptom ratings on the Brief Psychiatric Rating Scale (BPRS) and the Scale for the Assessment of Negative Symptoms (SANS) (16) for the patients. The schizophrenia patients were significantly older than the normal comparison subjects, but the groups did not differ in the amount of education. Because most of our patient participants were recruited from VA clinics, the group was also predominantly men. The patients were clinically stable and exhibited mild clinical symptoms. Table 1 shows the mean scores of the BPRS clusters (17) and SANS global scores.
Procedures
Backward-masking procedure
The participants performed a computerized backward-masking task while simultaneously having their event-related gamma activity recorded. The participants identified targets in two separate blocks. The first block entailed identifying an unmasked target (60 trials). The second block included the masking procedure, consisting of identifying a masked target (240 trials). The target was a square with a gap in one of three locations (top, bottom, or left side) that could appear at one of four locations on the computer screen (for an example, see Figure 1). The mask was composed of squares that overlapped all four possible target locations. The subjects were required to name the direction of the gap.
Before testing, all participants were equated for unmasked performance with a psychophysical staircase method (18, 19) that adjusted the contrast of the target to obtain a critical stimulus intensity. This procedure entailed adjusting the grayscale of the target, presented for 13.3 msec (two screen sweeps at 150 Hz) until the participant was at 84% for accuracy of identification of the unmasked target. This procedure ensured that any masking differences between groups were not due to a basic visual deficit that prevented the subjects from seeing an unmasked target. The grayscale setting established during the critical stimulus intensity procedure was then used for the unmasked and masked trials.
On the masked trials, the mask was presented for 26.67 msec (four screen sweeps). The targets subtended 0.23° of the visual angle, and each of the four mask locations was 1.03° of the visual angle away from fixation. The background luminance of the computer screen measured 85.7 lux with a handheld light meter with a diffuser held against the screen. Sixty trials were presented in a quasi-random order for four stimulus-onset asynchronies of 0, 8, 16, or 24 screen sweeps, corresponding to stimulus-onset asynchronies of 0.0, 53.3, 106.7, and 160.0 msec. Mean percent correct values were computed separately for the unmasked and masked trials in the patient and comparison groups.
Event-related gamma recording
The participants had their EEG activity recorded during the unmasked and masked tasks. Stimulus presentation and data synchronization with the EEG were accomplished with E-Prime (Psychology Software Tools, Inc., Pittsburgh). EEG activity was collected continuously throughout the session and amplified with a Neuroscan NuAmps amplifier (Compumedics USA, El Paso, Tex.). Data were sampled at 1,000 Hz, with filter settings of 0 to 100 Hz. Thirty-two cap-mounted, sintered silver-silver chloride electrodes (Falk Minow Services, Herrsching-Breitbrunn, Germany) were positioned with a modified international 10–20 system-placement scheme. Additionally, four electrodes were used to measure a horizontal electrooculogram (EOG) (placed on the outer canthus of the left and right eyes) and a vertical EOG (placed above and below the left eye). All electrodes were referenced to the nose, and a forehead ground was employed.
All data were processed with Neuroscan Scan 4.3 software (Compumedics USA, El Paso, Tex.). Data were first high-pass filtered at 1 Hz and then epoched to 200 msec before and 823 msec after the stimulus. Artifact rejection was performed for any trial that exceeded ± 75 μV at electrode sites Fz, F3, F4, and Cz. For unmasked trials, an average of 14.3% of the trials were rejected for the schizophrenia patients and 7.5% for the normal comparison subjects. For the masked trials, an average of 16.7% of the trials were rejected for the schizophrenia patients and 7.2% for the normal comparison subjects.
Correct and incorrect responses to the unmasked and masked trials were examined separately at each stimulus-onset asynchrony. Electrode sites were averaged to examine regional effects: left anterior (F7, F3, FC5, FC1, C3, T7, and T1), right anterior (F8, F4, FC6, FC2, C4, T8, and T2), left posterior (CP5, CP1, P7, P3, TP9, PO9, and O1), and right posterior (CP6, CP2, P8, P4, TP10, PO10, and O2). Event-related band-power analysis was then performed to examine evoked gamma band activity in the 50–200 msec latency range. The 30–40 Hz gamma range was assessed (center frequency of 35 Hz, half-band width of 5 Hz, 24 dB, octave-squared Butterworth filter, warm-up from the right). The amplitude of the gamma response was converted to z scores for all analyses and graphing. The conversion to z scores was performed on a within-subject basis, separately for each electrode, and for each contrast of interest (e.g., correct versus incorrect, different stimulus-onset asynchronies, left versus right, anterior versus posterior). Conversion to z scores was used to standardize the range and to easily examine group differences and the topography of the responses.
For analyses, Greenhouse-Geisser epsilon corrections (e) were used for repeated-measures analyses with more than one degree of freedom. In these cases, we report the uncorrected degrees of freedom, the corrected p values, and e values. All mean values are presented as means and standard deviations. Because the groups differed in age, significant group differences were reanalyzed with age as a covariate (however, age did not significantly correlate with any of the gamma measures of interest for either group). For the gamma data, the results are presented in z score units. All statistical analyses used an a priori two-tailed significance level of 0.05 to determine significant results.
Results
Backward-Masking Performance
The patients were significantly older than the normal comparison participants (p<0.05) (Table 1). The patients tended to need a higher grayscale level (i.e., darker target) than the comparison subjects, although this difference did not reach statistical significance (t=1.86, df=45, p<0.07). The schizophrenia patients correctly identified an average of 80.2% (SD=9.3%) of the unmasked targets, whereas the normal comparison subjects identified an average of 85.3% (SD=10.3%)—a statistically insignificant difference (t=1.71, df=45).
The results of the performance data during the masked trials are shown in Figure 2. There were significant main effects of group (F=4.15, df=1, 45, p<0.05) and stimulus-onset asynchrony (F=125.77, df=3, 135, p<0.001, ε=0.644). The interaction between group and stimulus-onset asynchrony was not significant. The significant group effect held when age was entered as a covariate. These results show that the normal comparison subjects correctly identified a greater number of targets than the schizophrenia patients and that both groups showed the expected improvement in target detection as stimulus-onset asynchrony increased. These data are similar to what we have seen in previous behavioral studies (e.g., reference 10).
Event-Related Gamma Results for Unmasked Trials
The data for event-related gamma activity during the unmasked trials were subjected to a two-by-two-by-two-by-two (group [patients versus comparison subjects] by correct versus incorrect trials by anterior versus posterior by left versus right hemisphere) repeated-measures analysis of variance (ANOVA). There were no significant main effects or interactions, indicating that the patients and comparison subjects produced equal amounts of gamma activity to unmasked stimuli.
Event-Related Gamma Results for Masked Trials
The data for the event-related gamma activity during the masking task were subjected to a two-by-two-by-four-by-two-by-two (group [patients versus comparison subjects] by correct versus incorrect trials by stimulus-onset asynchrony by anterior versus posterior by left versus right hemisphere) repeated-measures ANOVA. The results showed a significant main effect of group (F=5.02, df=1, 45, p<0.03), a significant group-by-hemisphere interaction (F=4.29, df=1, 45, p<0.05), and a significant group-by-number correct-by-stimulus-onset asynchrony interaction (F=3.24, df=3, 135, p<0.03, ε=0.932).
The main effect of group revealed that, overall, the normal comparison subjects produced significantly greater gamma (mean=1.25, SD=0.27) than the schizophrenia patients (mean=1.05, SD=0.28). The significant group-by-hemisphere interaction revealed that the normal comparison subjects produced significantly greater gamma activity in the right hemisphere (mean=1.30, SD=0.31) than in the left (mean=1.20, SD=0.29), whereas the schizophrenia patients showed no significant differences between the right (mean=1.04, SD=0.31) and left (mean=1.06, SD=0.29) hemispheres (Figure 3). Both of these findings held when age was entered as a covariate.
The significant three-way interaction was explored with follow-up t tests. A difference score was computed between correct and incorrect gamma scores at each stimulus-onset asynchrony separately for the patients and the comparison subjects. The normal comparison subjects tended to produce greater gamma activity to incorrect versus correct trials at a stimulus-onset asynchrony of 53.3 msec (t=2.08, df=14, p<0.06), whereas the schizophrenia patients tended to produce greater activity to correct versus incorrect trials at a stimulus-onset asynchrony of 53.3 msec (t=1.69, df=31, p<0.11).
Discussion
The results of the present study provide further support for an event-related abnormality in gamma activity in schizophrenia patients. Group differences were seen both in the amount and distribution of gamma activity during the backward-masking procedure. These results, moreover, are specific to targets presented in the presence of a mask. The lack of a group difference in gamma activity to unmasked stimuli suggests comparable processing of unambiguous visual stimuli in patients and comparison subjects.
The finding of a group-by-hemisphere difference in gamma activity in masked trials is consistent with previous findings of a deficit in the right hemisphere in schizophrenia for processing visual information (e.g., references 20–22). For example, Nuechterlein and colleagues (23) scanned subjects with positron emission tomography (PET) during a perceptually demanding continuous performance test and found that the schizophrenia patients did not use the right hemisphere preferentially to process ambiguous visual stimuli as normal subjects did (24). With simple dot-pattern stimuli, Heckers et al. (22) found that normal comparison subjects showed significant decreases in PET activity in the right hemisphere from the first viewing to the last viewing of the dot pattern, suggesting that habituation of activity in the right hemisphere is indicative of processing simple visual stimuli. The schizophrenia patients showed a reversed asymmetry, suggesting that their left hemisphere was overactive in processing visual stimuli. On the basis of the gamma activity findings of the current study, it appears that schizophrenia patients lack the normal right hemisphere lateralization to process ambiguous visual information and that this deficit extends to the backward-masking task.
The finding of a significant interaction between group, stimulus-onset asynchrony, and accuracy (correct versus incorrect) was due to a tendency for the comparison subjects to produce greater gamma activity to incorrect trials at a stimulus-onset asynchrony of 53 msec, whereas the schizophrenia patients showed the reverse pattern. This pattern occurred at only one stimulus-onset asynchrony and was not predicted. We do not have an interpretation at this time but will see if the pattern replicates itself.
The findings in the present study add to a growing literature demonstrating that schizophrenia patients exhibit reduced gamma activity to simple stimuli. Previous studies with auditory stimulation have shown that schizophrenia patients are less able to engage or maintain gamma activity (11, 12). For example, Kwon et al. (11) found that schizophrenia patients failed to show EEG synchronization to 40-Hz steady-state auditory trains. In a separate study, Clementz et al. (12) found that gamma-band (e.g., 30–50 Hz) abnormalities in the auditory P50 suppression response were better than the broadband P50 response (e.g., 10–50 Hz) at detecting differences between patients and comparison subjects. Although these studies used a different modality than we did, they similarly examined time-locked, or evoked, gamma responses. The results across these studies suggest that schizophrenia patients may have abnormalities in establishing or maintaining gamma activity to simple auditory and visual stimuli. These abnormalities, in turn, may account for some of the perceptual deficits in schizophrenia.
Gamma oscillations at the neuronal level are thought to be modulated by γ-aminobutyric acid (GABA)-ergic interneuronal circuits (e.g., references 25, 26), as well as N-methyl-d-aspartic acid (NMDA)-modulated pyramidal cells (e.g., references 27, 28). Schizophrenia has been associated with abnormalities in the GABA and NMDA systems, which may explain the gamma abnormalities that have been observed. It is possible that such abnormalities in gamma synchronization could lead, through a cascade of processes, to mistakes in perceptual processing and misinterpretation of ambiguous stimuli.
Our study had a few limitations. The stimuli we used were very small (0.23° of the visual angle), which probably reduced the amount of gamma activity seen. Recent research has shown that the amount of gamma to visual stimuli increases with increasing size of the stimuli (29). For the current study, we intentionally selected stimuli that were identical to those used in our behavioral studies (30). These stimuli need to be relatively small and faint to establish each subject’s perceptual threshold.
Another limitation is that the study has a limited number of stimulus-onset asynchronies. Although this was intentional so as to make the length of the testing session tolerable to the participant, it had the disadvantage of making it more difficult to detect any stimulus-onset asynchrony-dependent, event-related gamma activity. Future studies that use a wider range of stimulus-onset asynchronies may be in a better position to detect stimulus-onset asynchrony-dependent gamma activity. On the basis of the results from the unmasked trials, we expect that gamma activity would be normal in schizophrenia patients at the longest stimulus-onset asynchronies when performance approaches unmasked levels.
The patients and comparison subjects differed in age. However, as we noted, covarying age did not change any of the significant results. Age also did not correlate with any gamma measures of interest (e.g., overall gamma activity, gamma to correct versus incorrect trials, etc.), further reducing the possibility that our results are due to age differences. Age effects on performance were minimized by matching all subjects for unmasked target discrimination with a psychophysical staircase method.
Although visual-masking procedures appear to be excellent probes for aberrant gamma activity in schizophrenia, this study does not clarify whether abnormal gamma activity is the main cause of masking deficits in schizophrenia. Evidence has accumulated showing that schizophrenia patients have abnormal transient activity (7, 31, 32), and this abnormality may partially account for visual-masking deficits. However, gamma-range activity in backward-masking tasks is considered to be a function of sustained channels (8, 9). Therefore, although the differences in gamma activity in the current study are provocative, the role that abnormal gamma activity plays in masking performance deficits in schizophrenia patients remains unclear.
Finally, our study examined only evoked—but not induced—gamma activity. Evoked gamma activity is phase-locked to the onset of a stimulus and can be detected by averaging EEG responses. This is indicative of early sensory registration or processing of stimuli (33). Induced gamma activity is a non-phase-locked activity, is not seen with traditional averaging techniques, and is indicative of perceptual organization or feature binding (33). Because backward masking interrupts the very early stages of target processing, we were primarily interested in the early evoked gamma response (50–200 msec). However, a close examination of induced gamma activity in future studies could provide further insight into the gamma deficits seen in schizophrenia patients.
In conclusion, the current study extends the findings of abnormal gamma activity in schizophrenia patients during a backward-masking task (10). The current study employed a masking paradigm to elicit gamma activity. Decreased gamma activity was seen in schizophrenia patients only during the masking task, consistent with theories that abnormalities of generating or maintaining gamma activity can contribute to perceptual aberrations in schizophrenia (34). We further found that these deficits are especially pronounced in the right hemisphere, an area that is preferentially responsible for processing ambiguous visuospatial information. Although the current study has several limitations, these results continue to demonstrate the usefulness of using backward-masking procedures to explore underlying neurophysiological abnormalities in schizophrenia.
 |
Presented in part at the 19th annual meeting of the Society for Research in Psychopathology, St. Louis, Oct. 7–10, 2004. Revised Nov. 24, 2004; revision received Feb. 9, 2005; accepted Feb. 22, 2005. From the Department of Psychology, UCLA, and the Department of Psychiatry and Biobehavioral Science, Geffen School of Medicine at UCLA, Los Angeles; the VA Greater Los Angeles Healthcare System; the Department of Psychiatry, University of California, San Diego; and the Department of Psychology, University of Houston, Houston. Address correspondence and reprint requests to Dr. Wynn, VA Greater Los Angeles Healthcare System, 11301 Wilshire Blvd. (MIRECC 210A), Bldg. 210, Los Angeles, CA 90073; [email protected] (e-mail). Supported by NIMH grants MH-43292 and MH-65707 (to Dr. Green) and by the Department of Veterans Affairs, Veterans Integrated Services Network 22 Mental Illness Research, Education, and Clinical Center. Jonathan K. Wynn was supported by a Ruth L. Kirschstein National Research Service Award (grant MH-14584) (Dr. Nuechterlein, principal investigator) during the preparation of this article. The authors thank Patrick Carlyle for technical assistance and Poorang Nori and Kelly Tillery for help in data collection.
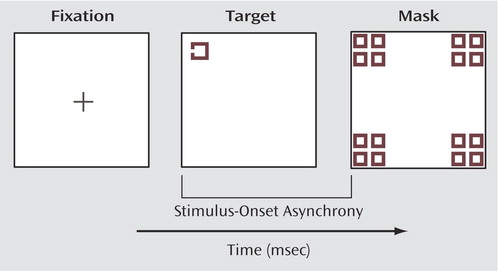
Figure 1. Examples of Computerized Visual Stimuli Used in a Visual Backward-Masking Task
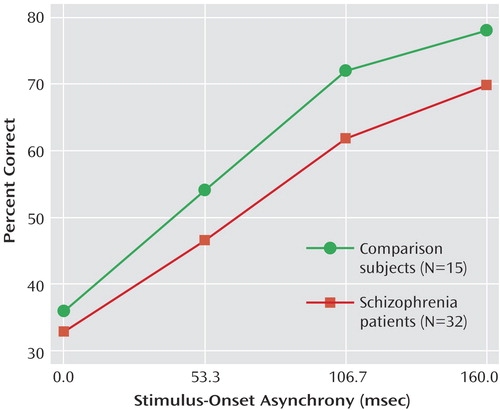
Figure 2. Performance Data on Visual Backward-Masking Tasks for Schizophrenia Patients and Normal Comparison Subjectsa
aSchizophrenia patients performed significantly worse (i.e., correctly identified fewer targets) than comparison subjects.
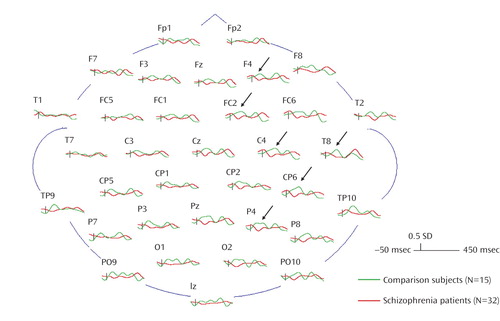
Figure 3. Grand Average z Score Gamma Waveforms for Schizophrenia Patients (red) and Normal Comparison Subjects (green)a
aSchizophrenia patients showed significantly less gamma activity in the 50–200 msec range, especially pronounced in the right hemisphere (indicated by the black arrows), than normal comparison subjects.
1. Green MF, Nuechterlein KH: Cortical oscillations and schizophrenia: timing is of the essence. Arch Gen Psychiatry 1999; 56:1007–1008Crossref, Medline, Google Scholar
2. Breitmeyer BG, Ganz L: Implications of sustained and transient channels for theories of visual pattern masking, saccadic suppression, and information processing. Psychol Rev 1976; 83:1–36Crossref, Medline, Google Scholar
3. Breitmeyer BG: Unmasking visual masking: a look at the “why” behind the veil of the “how.” Psychol Rev 1980; 87:52–69Crossref, Medline, Google Scholar
4. Green M, Walker E: Symptom correlates of vulnerability to backward masking in schizophrenia. Am J Psychiatry 1986; 143:181–186Link, Google Scholar
5. Saccuzzo DP, Schubert DL: Backward masking as a measure of slow processing in schizophrenia spectrum disorders. J Abnorm Psychol 1981; 90:305–312Crossref, Medline, Google Scholar
6. Braff DL, Saccuzzo DP, Geyer MA: Information processing dysfunctions in schizophrenia: studies of visual backward masking, sensorimotor gating, and habituation, in Neuropsychology, Psychophysiology, and Information Processing. Edited by Steinhauer JR, Gruzelier JH. New York, Elsevier, 1992, pp 305–334Google Scholar
7. Butler PD, DeSanti LA, Maddox J, Harkavy-Friedman JM, Amador XF, Goetz RR, Javitt DC, Gorman JM: Visual backward-masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res 2002; 59:199–209Crossref, Google Scholar
8. Purushothaman G, Ogmen H, Bedell HE: Gamma-range oscillations in backward-masking function and their putative neural correlates. Psychol Rev 2000; 107:556–577Crossref, Medline, Google Scholar
9. Breitmeyer BG, Ogmen H: Recent models and findings in visual backward masking: a comparison, review, and update. Percept Psychophys 2000; 62:1572–1595Crossref, Medline, Google Scholar
10. Green MF, Mintz J, Salveson D, Nuechterlein KH, Breitmeyer BG, Light GA, Braff DL: Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry 2003; 53:1113–1119Crossref, Medline, Google Scholar
11. Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW: Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry 1999; 56:1001–1005Crossref, Medline, Google Scholar
12. Clementz BA, Blumenfeld LD, Cobb S: The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport 1997; 8:3889–3893Crossref, Medline, Google Scholar
13. Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW: Abnormal neural synchrony in schizophrenia. J Neurosci 2003; 23:7407–7411Crossref, Medline, Google Scholar
14. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders Research Version (SCID-I). New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
15. First MB, Gibbon M, Spitzer RL, Williams JBW: Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II), version 2. New York, New York Psychiatric Institute, Biometrics Research, 1996Google Scholar
16. Andreasen NC: The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl 1989; 7:49–58Medline, Google Scholar
17. Overall JE, Hollister LE, Pichot P: Major psychiatric disorders: a four-dimensional model. Arch Gen Psychiatry 1967; 16:146–151Crossref, Medline, Google Scholar
18. Green MF, Nuechterlein KH, Breitmeyer BG: Development of a computerized assessment for visual masking. Int J Methods Psychiatr Res 2002; 11:83–89Crossref, Medline, Google Scholar
19. Wetherill GB, Levitt H: Sequential estimation of points on a psychometric function. Br J Math Stat Psychol 1965; 18:1–10Crossref, Medline, Google Scholar
20. O’Donnell BF, Potts GF, Nestor PG, Stylianopoulos KC, Shenton ME, McCarley RW: Spatial frequency discrimination in schizophrenia. J Abnorm Psychol 2002; 111:620–625Crossref, Medline, Google Scholar
21. Vanni S, Revonsuo A, Saarinen J, Hari R: Visual awareness of objects correlates with activity of right occipital cortex. Neuroreport 1996; 8:183–186Crossref, Medline, Google Scholar
22. Heckers S, Goff D, Weiss AP: Reversed hemispheric asymmetry during simple visual perception in schizophrenia. Psychiatry Res Neuroimaging 2002; 116:25–32Crossref, Medline, Google Scholar
23. Nuechterlein KH, Buchsbaum MS, Dawson ME: Neuropsychological vulnerability to schizophrenia, in The Neuropsychology of Schizophrenia. Edited by David AS, Cutting JC. Hove, UK, Lawrence Erlbaum Associates, 1994, pp 53–77Google Scholar
24. Hellige JB: Hemispheric asymmetry. Annu Rev Psychol 1990; 41:55–80Crossref, Medline, Google Scholar
25. Traub RD, Whittington MA, Stanford IM, Jeffreys JGR: A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 1996; 383:621–624Crossref, Medline, Google Scholar
26. Whittington MA, Traub RD, Jeffreys J: Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 1995; 373:612–615Crossref, Medline, Google Scholar
27. Whittington M, Faulkner HJ, Doheny HC, Traub RD: Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Ther 2000; 86:171–190Crossref, Medline, Google Scholar
28. Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW: NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci 1996; 16:2034–2043Crossref, Medline, Google Scholar
29. Busch NA, Debener S, Kranczioch C, Engel AK, Herrmann CS: Size matters: effects of stimulus size, duration and eccentricity on the visual gamma-band response. Clin Neurophysiol 2004; 115:1810–1820Crossref, Medline, Google Scholar
30. Green MF, Nuechterlein KH, Breitmeyer BG, Tsuang J, Mintz J: Forward and backward visual masking in schizophrenia: influence of age. Psychol Med 2003; 33:887–895Crossref, Medline, Google Scholar
31. Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC: Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr Res 2003; 64:91–101Crossref, Medline, Google Scholar
32. Green MF, Nuechterlein KH, Mintz J: Backward masking in schizophrenia and mania, II: specifying the visual channels. Arch Gen Psychiatry 1994; 51:939–944Crossref, Medline, Google Scholar
33. Tallon-Baudry C, Bertrand O: Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 1999; 3:151–162Crossref, Medline, Google Scholar
34. Green MF, Nuechterlein KH, Breitmeyer B, Mintz J: Backward masking in unmedicated schizophrenic patients in psychotic remission: possible reflection of aberrant cortical oscillation. Am J Psychiatry 1999; 156:1367–1373Abstract, Google Scholar



