Anterior Cingulate Cortex Activity and Impaired Self-Monitoring of Performance in Patients With Schizophrenia: An Event-Related fMRI Study
Abstract
OBJECTIVE: The authors examined brain activity associated with the internal monitoring of performance to test the hypothesis that error-related activity in the anterior cingulate cortex is impaired in patients with schizophrenia. METHOD: Seventeen patients with schizophrenia and 16 healthy comparison subjects underwent event-related functional magnetic resonance imaging during a continuous performance task; stimulus degradation was used to increase error rates. RESULTS: Comparison subjects, but not schizophrenic patients, showed error-related activity in the anterior cingulate cortex, and this difference in brain activity was significantly different across the two groups. Patients also showed less slowing of reaction time after error commission. CONCLUSIONS: Lower error-related activity in the anterior cingulate cortex and less performance adjustment after error commission are consistent with the hypothesis that disturbances in anterior cingulate cortex function are related to a specific alteration in an evaluative component of executive functioning—the internal monitoring of performance.
Executive functions are those cognitive processes necessary for controlled information processing and coordinated actions (1). Impaired executive functioning has served as the basis of a number of general theories of cognitive dysfunction in schizophrenia (2–6) and has been hypothesized to be related to the behavioral disorganization characteristic of this illness. With the growing awareness of the association between cognitive disability and poor outcome in schizophrenia (7), there has been a renewed interest in understanding the nature of impaired executive functioning in schizophrenia as well as its pathophysiological substrates.
Two broad sets of processes have been considered as contributors to executive functioning (1). Strategic processes are those involved in the top-down control of cognition. These functions include representing and maintaining goals and allocating limited attentional resources. These functions appear to rely on the integrity of regions of the dorsolateral prefrontal cortex (8, 9). A second set of processes, also essential for executive control, are those involved in the ongoing evaluation of performance. These evaluative functions are critical for the flexible adjustments of top-down control needed for a seamless adaptation to a constantly changing environment. A growing body of data implicate the anterior cingulate cortex on the medial surface of the frontal lobes in this function (1, 10).
Much of the theoretical and empirical work to date related to impaired executive functioning has focused on impaired strategic processes and their association with impairments in the dorsolateral prefrontal cortex and related circuitry (11–14). These studies have provided insights into the nature of impaired top-down cognitive control in patients with schizophrenia and focused attention on the potential role alterations in the local circuitry of this region and its development (15, 16) may have in cognitive disability. Relatively little attention has been paid to impaired performance monitoring functions in patients with schizophrenia. However, a number of studies that have used resting single photon emission computed tomography and positron emission tomography (PET) (14, 17–19) have suggested abnormalities of this region both at rest and during the performance of tasks engaging executive functions.
One way that the anterior cingulate cortex is thought to contribute to internal performance monitoring functions is through its sensitivity to errors. Event-related potential (10) and, more recently, event-related functional magnetic resonance imaging (fMRI) studies (20, 21) have shown activation in this region associated with error commission as well as evidence that this activity is coupled with performance adjustments following error commission (10). Preliminary electrophysiological evidence that error detection was impaired in patients with schizophrenia was reported by Kopp and Rist (22), who reported that these patients exhibited diminished error-related negativity, a transient response locked event-related potential signal that is observed concurrent with making an error during a speeded response task. In the present study, we used event-related fMRI and a speeded reaction time task to test the hypothesis that a reduction in brain performance monitoring activity in the anterior cingulate cortex contributes to impaired executive functioning in patients with schizophrenia.
Method
Subjects
Twenty-four schizophrenic patients and 23 healthy comparison subjects were studied. All subjects were right-handed, as determined by the first three questions of the Edinburgh Handedness Inventory (23). Subjects gave written informed consent for the study, which was approved by the Biomedical Institutional Review Board of the University of Pittsburgh. Three patients and three comparison subjects were excluded because of excessive movement, two patients and three comparison subjects were excluded because they did not perform the task correctly or made insufficient errors, and two patients and one comparison subject were excluded because they could not tolerate the scanner or data were lost for technical reasons. There were no significant differences between subjects who were excluded for the above reasons and those who remained in the analysis on gender, diagnosis, or total Positive and Negative Syndrome Scale (24) score (all p>0.30). Healthy comparison subjects who were excluded were nonsignificantly older than those who were included (mean=37.8 years [SD=3.6] versus 33.5 years [SD=6.0], respectively; t=1.8, df=22, p<0.08). Our final group consisted of 17 schizophrenic patients and 16 comparison subjects who were matched for age (mean=33.5 years [SD=5.8] and 34.1 years [SD=7.7], respectively; t=0.28, df=31, p<0.79), gender (patients: five women and 12 men, comparison subjects: five women and 11 men; χ2=0.01, df=1, p<0.91), and parental education (mean=13.5 years [SD=1.8] and 13.1 years [SD=3.6]; t=0.34, df=31, p<0.71). Patients were all clinically stable outpatients at the time of testing as per scores on the Positive and Negative Syndrome Scale (total score: mean=25.7 [SD=7.5]; positive symptom score: mean=7.1 [SD=3.2]; negative symptom score: mean=10.7 [SD=3.3]). All patients were receiving antipsychotic medications (haloperidol: N=4, fluphenazine: N=2, olanzapine: N=5, quetiapine: N=3, and clozapine: N=3) at stable doses for at least 30 days before scanning. All patients met DSM-IV criteria for schizophrenia as per the Structured Clinical Interview for DSM-IV (SCID) as well as review of their medical records and discussion with their treating clinician. Mean duration of illness for the patient group was 16.0 years (SD=9.1). Comparison subjects were evaluated with the nonpatient version of the SCID and were excluded for any lifetime history of axis I disorder as well as a family history of psychotic disorder. Exclusion criteria for both groups included a lifetime history of seizures or significant head trauma, a history of mental retardation, a history of substance abuse or dependence within the previous 6 months, and any contraindication for MRI scanning.
Event-Related fMRI Methods
Subjects performed 10 to 14 blocks of a 10-trial continuous performance task while in the scanner. The number of blocks varied from 10 to 14 depending upon the subjects’ ability to tolerate the procedure and their error rates. Subjects were presented with single letters at the center of a computer display and instructed to press a target button whenever an “A” (cue) was followed by an “X” (probe) and to press a nontarget button for all other stimuli. Subjects responded with their dominant (right) hand. In the version used, the majority of trials were valid “A followed by X” trials. Each stimulus appeared for 500 msec, followed by a 9500-msec interstimulus interval. Hence, the length of each trial (cue-probe pair) was 20 seconds. In alternating blocks, the stimuli were degraded by removing pixels from the display in order to increase error rates. We have previously shown that error-related brain activity, localized to the anterior cingulate cortex, is elicited when stimulus degradation is used to elicit errors. We have previously interpreted this result as reflecting response conflict, since transient, response-related activity has also been observed in the same region of the anterior cingulate cortex during correct trials in which response conflict is present (20). The degree of stimulus degradation was individually titrated during a practice session in the scanner to ensure that subjects were making errors and to equate the difficulty of the degraded condition for the two groups. There was no difference in the level of stimulus degradation used between the groups (patients: mean=85.9% [SD=6.4%], comparison subjects: mean=88.7% [SD=10.8%]). There was also no significant difference in the percentage of errors made (patients: mean=16.3% [SD=13.4%], comparison subjects: mean=13.1% [SD=9.4%]). Eight 2.5-second fMRI scans were acquired during each 20-second scan—four following the cue and four following the probe—and stimulus presentation was synchronized with scanning so that the data could be analyzed in an event-related manner.
Functional images were acquired by using a 1.5-T Signa whole body scanner (GE Medical Systems, Milwaukee) with a standard head coil. Functional scans were obtained by using a two-shot T2-weighted spiral-scan pulse sequence (TR=1250 msec, TE=35 msec, flip angle=60°, field of view=24 cm). Sixteen axial slices (3.75-mm3 voxels) were obtained parallel to the anterior commissure-posterior commissure line every 2.5 seconds. Scanning was event related, with acquisition of images synchronized to stimulus presentation. Structural images were obtained before functional scanning by using a standard T1-weighted pulse sequence. Images for all subjects were coregistered to a common reference structural MRI scan with a 12-parameter automated algorithm (25). They were then smoothed by using an 8-mm full width at half maximum three-dimensional Gaussian filter to accommodate individual differences in anatomy. Finally, data were pooled across subjects to increase the signal-to-noise ratio.
Data Analysis
After reconstruction, functional data were aligned to the first scan by using automated image registration (25). Data were then transformed to a common reference space (26), globally normalized and smoothed by using an 8-mm full width at half maximum Gaussian kernel. Group analyses were then performed by using a mixed-model analysis of variance (ANOVA) in which subject was a random factor and diagnosis, accuracy (correct versus incorrect response following probe presentation), and scan (the four sequential scans acquired beginning with the presentation of the probe) were fixed factors. F maps were thresholded for a significance value of p<0.005 with a contiguity threshold of eight contiguous voxels, corresponding to an image-wise false positive rate of p<0.05, corrected for multiple comparisons (27). Data were then transformed into Talairach space by using AFNI software (28) for display purposes and to localize the results in standard coordinates. For contrasts involving regions of interest identified in the above voxel-wise contrasts, data were averaged across all the voxels in the region of interest and contrasted by using mixed-model ANOVA.
Results
To ensure that signal-to-noise ratios were not impaired by nonspecific factors such as differences in head movement during scanning or other nonspecific factors, estimated movement parameters were extracted from the automated image registration log files, and signal-to-noise ratio maps were computed. Six subjects (three schizophrenic and three comparison) were excluded from the analysis because they had mean movement in one or more dimension of more than 4 mm or degrees (approximately one voxel). For the remaining groups, there were no significant differences on any dimension on the estimated movement parameters. When the groups were contrasted on signal-to-noise ratio maps across all regions of interest for which group contrasts were conducted, no significant differences were observed (patients: mean=736, SD=737, comparison subjects: mean=704, SD=720).
As in a previous study that used this paradigm (20), the healthy comparison subjects showed a robust, transient, response-related increase in the anterior cingulate cortex during error commission (75 voxels, centroid of activation [x, y, z]: 0, 27, 36). A small area of right anterior medial frontal cortex (Brodmann’s area 10) and left posterior parietal cortex (Brodmann’s area 40) also showed error-related activity in the healthy comparison subjects. Schizophrenic patients failed to show an increase in activity in the anterior cingulate cortex (or any other region of the brain) during commission of errors. In the direct contrast of the two groups, there was a large area of the anterior cingulate cortex, centered on the ventral bank of the cingulate sulcus (Brodmann’s area 24) and extending into adjacent Brodmann’s area 32, that showed a significant group-by-accuracy-by-scan interaction (54 voxels, centroid of activation: 2, 21, 36). The contrast between the error-related activity observed in the anterior cingulate cortex in comparison subjects and in the schizophrenic patients is shown in >Figure 1.
When normal subjects make an error, their reaction times are slower on the subsequent trial (29). This phenomenon, sometimes referred to as the “Rabbitt effect,” was considered to be evidence for central error monitoring processes long before cognitive neuroscience was able to show evidence of brain activity associated with such a process. It has previously been reported that the magnitude of the error-related negativity correlates with the degree of slowing following error commission (10). We contrasted reaction times before and after commission of errors across the two groups and found that the patients with schizophrenia exhibited significantly less slowing of reaction time after error commission. For comparison subjects, the mean reaction after an error was 708.13 msec (SD=216.6), whereas after a correct response it was 596.7 msec (SD=172.3). For the schizophrenic patients, mean reaction times after incorrect and correct responses were 801.7 (SD=167) and 774.9 (SD=157.3) msec, respectively. Hence, the mean Rabbitt effect for the comparison subjects was 111.4 msec, whereas for the schizophrenia patients it was only 26.8 msec (F=6.1, df=1, 31, p<0.02).
Discussion
This is the first study to our knowledge to use event-related fMRI to investigate brain activity associated with the internal monitoring of performance in patients with schizophrenia. We found that relative to matched comparison subjects, error-related activity in the anterior cingulate cortex was impaired in the patient group. Associated with this impairment, error-related performance adjustments also differed between patients and comparison subjects, with patients exhibiting less of the normal reaction time adjustment following errors or Rabbitt effect. These results are consistent with our hypothesis that a performance-monitoring deficit based in the anterior cingulate cortex contributes to impaired executive functioning in patients with schizophrenia.
A number of previous investigators have proposed models of impaired self-monitoring in patients with schizophrenia (30, 31). These models have proposed that the internal monitoring of actions is disrupted through an impairment of corollary discharge, the copy of a motor plan that is returned to a central monitor in order to evaluate a movement before or as it is executed. Some theories of error detection also assume an underlying mechanism involving a comparison of an efference copy of an action together with a representation of the correct action, referred to as the “comparator” hypothesis (32). From this perspective, our results are consistent with a disturbance of an efference copy mechanism. However, previous theories related to altered efference copy in schizophrenia have focused on this disturbance as a potential mechanism for positive symptoms in which putatively self-generated actions are attributed to an external agent, e.g., inner speech is mistaken for an external voice. Our hypothesis of impaired performance monitoring in patients with schizophrenia emphasizes the relevance that this deficit may have for impaired executive functioning in this illness. Furthermore, we have proposed that error detection in humans can be understood without the need for a comparator function involving efference copy mechanisms (33). On the basis of the observation that the same regions of the anterior cingulate cortex show activity during error commission and during tasks that elicit response conflict—as well as results from computational modeling studies that have shown that response conflict is particularly high during error commission—we have hypothesized that the anterior cingulate cortex contributes to evaluation of performance by detecting response conflict without the need for a comparator. In the present study, we could not examine activity related to response conflict because there were too few trials eliciting response conflict during the nondegraded blocks (generally fewer than five per subject). However, in a previous PET study that used the Stroop task (34), schizophrenic patients exhibited lower anterior cingulate cortex activity during the color-incongruent, response conflict-eliciting condition of this task. Further studies examining both error-related and response conflict-related activity in the anterior cingulate cortex are currently underway in our laboratory.
As previously mentioned, executive functioning appears to be implemented in the brain by a distributed network of regions, including the dorsolateral prefrontal cortex, and it is highly likely that disturbances are present in this region of the brain in patients with schizophrenia. Could the changes in error-related activity observed in the anterior cingulate cortex in the present study simply be secondary to changes in other regions, such as the dorsolateral prefrontal cortex? We cannot exclude this possibility, but we believe that it is unlikely. In a study of the error-related negativity in subjects with focal lesions of the dorsolateral prefrontal cortex but intact anterior cingulate cortices, the frontal patients showed an increase in the event-related potential signal associated with correct response and no change with respect to the amplitude of the error-related wave form. The pattern of reduction of the error-related negativity in the study by Kopp and Rist (22) and the pattern of MR signal change in the present study is quite different to that observed in the frontal patients. In these two studies there were no differences in activity associated with correct responses but markedly lower error-related effects. An examination of conflict-related activity in the anterior cingulate cortex may be even more informative with regard to this issue. An impairment in attentional control associated with dorsolateral prefrontal cortex dysfunction should elicit greater conflict. As such, impaired dorsolateral prefrontal cortex together with intact anterior cingulate cortex should produce greater cingulate response, while impaired conflict detection in this region would predict lower activity in this region. We have recently shown, using event-related methods, that top control functions of the dorsolateral prefrontal cortex and conflict-related activity in the anterior cingulate cortex can be dissociated in time (9). With this approach it should be possible to tease apart the relative contribution of these two regions to impaired executive functioning in schizophrenia, and these studies are also underway in our laboratory.
A caveat regarding the results of the present study, as well as the previous event-related potential study (22) suggesting diminished error-related brain activity in patients with schizophrenia, is that the patients were all treated with antipsychotic medications. Previous studies have shown that antipsychotic drugs decrease blood flow and metabolism in the anterior cingulate cortex. Reductions in anterior cingulate cortex resting metabolism and in the anterior cingulate cortex response during cognitive activation—which we would interpret as related to response conflict (19)—have previously been reported in unmedicated patients, but it is unknown how antipsychotic drugs might affect error-related activity in this region of the brain. Further studies examining error-related and response conflict-related activity in the anterior cingulate cortex in unmedicated patients before and after the initiation of treatment are needed in order to examine the possibility that antipsychotic medication may have a deleterious effect on the internal monitoring of performance in schizophrenia. An additional limitation is that data from several subjects had to be excluded from analysis because of excessive movement or because they were unable to perform the task. This raises an issue regarding the generalizability of results obtained from functional brain imaging studies to the broad population of schizophrenic patients. While, on the one hand, this limitation implies that results from cognitive fMRI studies of schizophrenia might not always be fully generalizable to the broad population of patients, on the other hand it is likely that such studies are biased against confirming hypotheses regarding the brain circuitry underlying impaired cognition in this illness, since the most severely affected patients cannot be studied. Hypotheses that are confirmed by these methods might then be considered to have survived a more stringent test by being confirmed in a subset of more mildly affected patients. The development of methods that permit more rapid data acquisition, as well as the use of simplified behavioral paradigms, may reduce the impact of this potential sampling bias in the future.
In conclusion, in the present study, patients with schizophrenia showed less error-related activity in the anterior cingulate cortex during event-related fMRI as well as associated impairments in performance adjustments following error commission. These data provide preliminary evidence for impaired anterior cingulate cortex-based evaluative function, and, hence, a specific contribution of disturbances in this region of the brain to impaired executive functioning in schizophrenia. Further studies examining response conflict-related activity during correct responses, medication effects, and the relative contributions of medial and lateral frontal cortical disturbances are likely to further increase our understanding of the functional neuroanatomy of cognitive disability in schizophrenia.
Received Nov. 8, 2000; revision received April 20, 2001; accepted April 25, 2001. From the Western Psychiatric Institute and Clinic, University of Pittsburgh. Address reprint requests to Dr. Carter, Western Psychiatric Institute and Clinic, Department of Psychiatry, University of Pittsburgh, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail). Supported by grants from the Stanley Foundation and NIMH (MH-01306) to Dr. Carter.The authors thank Deanna Barch, Ph.D., and Jonathan Cohen, M.D., Ph.D., for their assistance during the design and execution of this project.
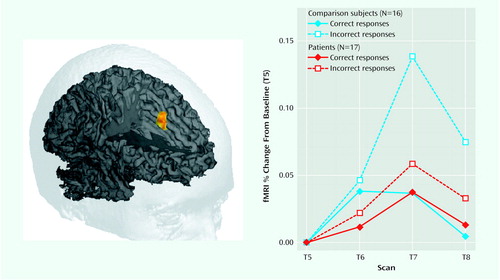
Figure 1. >Anterior Cingulate Cortex Activity in Patients With Schizophrenia and Healthy Comparison Subjects During a Continuous Performance Taska
aAfter incorrect responses, activity in the anterior cingulate cortex of the healthy subjects was significantly higher than activity seen after correct responses (F=11.3, df=1, 45, p<0.0001); this increase in anterior cingulate cortex activity after incorrect responses was not seen in the patients with schizophrenia (F=0.48, df=1, 48, p<0.70).
1. Carter CS, Botvinick MM, Cohen JD: The role of the anterior cingulate cortex in executive processes of cognition. Rev Neurosci 1999; 10:49-57Crossref, Medline, Google Scholar
2. Shakow D: Segmental set: a theory of the formal psychological deficit in schizophrenia. Arch Gen Psychiatry 1962; 6:1-17Crossref, Medline, Google Scholar
3. Calloway E, Naghdi S: An information processing model for schizophrenia. Arch Gen Psychiatry 1982; 39:339-347Crossref, Medline, Google Scholar
4. Braff D: Information processing and attention dysfunction in schizophrenia. Schizophr Bull 1993; 19:233-259Crossref, Medline, Google Scholar
5. Cohen JD, Barch DM, Carter CS, Servan-Schreiber D: Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol 1999; 108:120-133Crossref, Medline, Google Scholar
6. Liddle PF, Morris DL: Schizophrenic syndromes and frontal lobe performance. Br J Psychiatry 1991; 158:340-345Crossref, Medline, Google Scholar
7. Green MF: Schizophrenia From a Neurocognitive Perspective: Probing the Impenetrable Darkness. Boston, Allyn & Bacon, 1998Google Scholar
8. Smith EE, Jonides J: Storage and executive processes in the frontal lobes. Science 1999; 283:1657-1661Google Scholar
9. MacDonald AW, Cohen JD, Stenger VA, Carter CS: Dissociating the role of dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000; 288:1835-1838Google Scholar
10. Ghering WJ, Goss B, Coles MGH, Meyer DE, Donchin E: A neural system for error detection and compensation. Psychol Sci 1993; 4:385-390Crossref, Google Scholar
11. Weinberger DR, Berman K, Zec R: Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional cerebral bloodflow evidence. Arch Gen Psychiatry 1986; 43:114-124Crossref, Medline, Google Scholar
12. Weinberger DR, Berman K, Illowsky B: Physiological dysfunction of the dorsolateral prefrontal cortex, III: a new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry 1988; 45:609-615Crossref, Medline, Google Scholar
13. Weinberger DR, Gallhofer B: Cognitive dysfunction in schizophrenia. Int Clin Psychopharmacol 1997; 12(suppl 4):S29-S36Google Scholar
14. Andreasen NC, Rezai K, Alliger R, Swayze VW II, Flaum M, Kirchner P, Cohen G, O’Leary DS: Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with Xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943-958Crossref, Medline, Google Scholar
15. Lewis DA: Chandelier cells: shedding light on altered cortical circuitry in schizophrenia. Mol Psychiatry 1998; 3:468-471Crossref, Medline, Google Scholar
16. Akbarian S, Bunney WF, Ptokin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG: Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry 1993; 50:169-177Crossref, Medline, Google Scholar
17. Siegel BV Jr, Buchsbaum MS, Bunney WE Jr, Gottschalk LA, Haier RJ, Lohr JB, Lottenberg S, Najafi A, Nuechterlein KH, Potkin SG: Cortical-striatal-thalamic circuits and brain glucose metabolic activity in 70 unmedicated male schizophrenic patients. Am J Psychiatry 1993; 150:1325-1336Google Scholar
18. Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT: Limbic system abnormalities identified in schizophrenia with fluorodeoxyglucose and neocortical alterations with the deficit syndrome. Arch Gen Psychiatry 1992; 49:522-530Crossref, Medline, Google Scholar
19. Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RS, Grasby PM: Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature 1995; 378:180-182Crossref, Medline, Google Scholar
20. Carter CS, Braver TS, Barch DM, Botvinick M, Noll D, Cohen JD: Anterior cingulate cortex, error detection, and the on line monitoring of performance. Science 1998; 280:747-749Crossref, Medline, Google Scholar
21. Kiehl KA, Liddle PF, Hopfinger JB: Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology 2000; 37:216-223Crossref, Medline, Google Scholar
22. Kopp B, Rist F: An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. J Abnorm Psychol 1999; 108:337-346Crossref, Medline, Google Scholar
23. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97-113Crossref, Medline, Google Scholar
24. Kay S: Pyramidical model of schizophrenia, in Positive and Negative Symptoms in Schizophrenia: Assessment and Research. Edited by Kay S. New York, Brunner/Mazel, 1991, pp 181-193Google Scholar
25. Woods RP, Cherry SR, Mazziotta JC: Rapid automated algorithm for aligning and re-slicing PET images. J Comput Assist Tomogr 1992; 16:620-633Crossref, Medline, Google Scholar
26. Woods RP, Mazziota JC, Cherry SR: MRI-PET registration with automated algorithm. J Comput Assist Tomogr 1993; 17:536-546Crossref, Medline, Google Scholar
27. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33:636-647Crossref, Medline, Google Scholar
28. Cox R: AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162-173Crossref, Medline, Google Scholar
29. Rabbitt P: Errors and error correction in choice reaction time tasks. J Exp Psychol 1966; 71:264-272Crossref, Medline, Google Scholar
30. Feinberg I: Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull 1978; 4:636-640Crossref, Medline, Google Scholar
31. Frith CD, Done DJ: Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol Med 1989; 19:359-363Crossref, Medline, Google Scholar
32. Coles MGH, Sheffers MK, Fournier L: “Where did you go wrong?” errors, partial errors, and the nature of human information processing. Acta Psychol (Amst) 1995; 90:129-144Crossref, Medline, Google Scholar
33. Botvinick M, Braver TS, Barch DM, Carter CS, Cohen JD: Conflict monitoring and cognitive control. Psychol Rev (in press)Google Scholar
34. Carter CS, Mintun M, Nichols T, Cohen JD: Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: an [15O] H2O PET study during single trial Stroop task performance. Am J Psychiatry 1997; 154:1670-1675Google Scholar



