Altered White Matter/Gray Matter Proportions in the Striatum of Patients With Schizophrenia: A Volumetric MRI Study
Abstract
OBJECTIVE: Anatomical structures of the striatum were studied in 58 patients with schizophrenia and 56 healthy comparison subjects of both genders matched for age and handedness. METHOD: Magnetic resonance imaging scans were used to measure gray matter, white matter, and CSF volumes of the caudate, putamen, and nucleus accumbens in the left and the right hemispheres. RESULTS: White matter/gray matter ratios of the striatal structures were significantly lower in patients than in healthy subjects. In patients, relative white matter volumes in the caudate and nucleus accumbens were reduced, whereas gray matter in the putamen was increased. The total accumbens volume did not differ by diagnosis, but left side accumbens was larger than right in the healthy subjects. The proportion of white matter was greater in women in both the patient and healthy comparison groups. Total caudate and putamen volumes demonstrated no differences due to diagnosis or laterality, but a negative correlation was found in patients between white matter volumes and increasing age. There were no significant correlations among total striatal volumes, white matter/gray matter ratios, age at onset of illness, or illness duration. An estimate of lifetime neuroleptic consumption was positively correlated with right gray matter volume of the putamen in male schizophrenia patients who received typical neuroleptics. CONCLUSIONS: The proportion of white matter to gray matter tissue volumes of the caudate, putamen, and nucleus accumbens is altered in medicated chronic schizophrenia patients, but the total volumes are unchanged.
Since striatal structures play an important role in cognitive function and are highly related to neuroleptic treatment, attempts have been made to investigate cortical-striatal circuits in relation to cognitive deficits in schizophrenia (1). Quantitative magnetic resonance imaging (MRI) studies have focused on alterations of the striatal regions, i.e., the caudate, putamen, and nucleus accumbens. The possibility that striatal regions may be altered by typical neuroleptic medication has been suggested. However, volumetric MRI studies in the striatum of schizophrenia patients have yielded discrepant results. Many studies have reported enlargement of the caudate (2–6) and the putamen (6–8) in patients medicated with typical neuroleptics. Other investigators have found that the caudate volumes were not altered in longitudinal follow-up studies and suggested that the caudate was not changed by either neuroleptic medication or the disease process itself (9, 10). Furthermore, quantitative neuropathological studies comparing striatal structures in schizophrenia have yielded inconsistent results. In postmortem studies, Bogerts et al. (11, 12) and Falke et al. (13) reported that the volumes of the caudate and putamen did not differ between patients with schizophrenia and healthy subjects, whereas other studies have reported increased volume of striatal structures in schizophrenia (14–16). A rat study using dopamine D2 and dopamine D1 ligand binding showed that there were no significant differences in striatal volumes between the animals treated with haloperidol and untreated control rats (17). Thus, the question of whether striatal structures are changed or not in schizophrenia has not been fully answered.
Despite the important role of the nucleus accumbens in cognitive function (18, 19) as well as in reward function, and the specific dopamine D3 receptor distribution to this region, the nucleus accumbens has not been the focus of previous volumetric MRI studies.
In the present study, we measured for the first time gray matter, white matter, and CSF tissue class volumes from segmented magnetic resonance imaging (MRI) scans of the caudate, putamen, and nucleus accumbens in the right and left hemispheres of the same subjects. Gray and white matter proportions as well as the total volumes of each of the striatal structures were compared between patients with schizophrenia and healthy subjects of both genders.
Method
Fifty-eight patients (36 men and 22 women) diagnosed with schizophrenia according to DSM-IV and 56 healthy comparison subjects (34 men and 22 women), all Caucasians, were included in the study. All subjects lived within the area of northern Stockholm, Sweden. They were recruited at the Department of Clinical Neuroscience, Karolinska Hospital, Stockholm, Sweden. All subjects gave written informed consent for participation in this research, which was approved by the institutional review board (Research Ethics Committee) of the Karolinska Institutet. Exclusion criteria for the healthy subjects were current or past treatment for a psychiatric disorder or psychotic disorder in a first-degree relative. For all subjects, a history of alcoholism or drug addiction, head trauma with loss of consciousness for more than 5 minutes, or a history of somatic disorders affecting brain function were exclusion criteria.
Subject characteristics are presented in Table 1. The patients and the healthy subjects were matched by age and gender as closely as possible. The mean age at the time of scanning was 40.0 years (SD=7.3) for patients and 38.2 years (SD=8.0) for healthy subjects. There were no significant differences in gender and handedness between the groups. All participants were assessed with the Structured Clinical Interview for DSM-III-R (20). In addition, case notes were evaluated for diagnoses according to DSM-III-R and DSM-IV. The mean age at onset of illness was 25.4 years (SD=5.3) for the men and 24.8 years (SD=5.6) for the women. The mean duration of illness was 14.4 years (SD=6.8) and 14.1 years (SD=9.7) for men and women, respectively. At the time of investigation, 52 of the 58 patients were receiving neuroleptic medication. Twenty-five patients (10 women and 15 men) were being treated with typical neuroleptics (haloperidol, perphenazine, levomepromazine, thioridazine, or thiothixene). Twenty-seven patients (eight women and 19 men) were being treated with atypical neuroleptics (risperidone, olanzapine, or clozapine). An approximate estimate of total lifetime consumption of typical and atypical neuroleptics in haloperidol equivalent units (21) was calculated by multiplying duration of illness by the haloperidol equivalent units of current daily neuroleptic dose.
MR Acquisition
The subjects were examined with a 1.5-T General Electric Signa scanner (GE Medical Systems, Milwaukee) system at the MR Research Center, Karolinska Hospital, Stockholm. T1-weighted images that used a three-dimensional spoiled gradient recalled pulse sequence were acquired with the following parameters: 1.5-mm coronal slices, no gap, 35° flip angle, repetition time (TR)=24 msec, echo time (TE)=6.0 msec, number of excitations=2, field of view=24 cm, acquisition matrix=256×192. T2-weighted images were acquired with the following parameters: 2.0-mm coronal slices, no gap, TR=6000 msec, TE=84 msec, number of excitations=2, field of view=24 cm, acquisition matrix=256×192. From visual inspection, all scans were judged to be excellent without any obvious motion artifact. All the scans were found to lack gross clinical pathology as evaluated by a neuroradiologist. Quantitative analyses were performed blind with regard to subject diagnosis.
Tissue Segmentation
The segmentation was performed on Silicon Graphics Oxygen O2 computers, operative system: IRIX 6.5 (Silicon Graphics, Mountain Valley, Calif.) at the Psychiatry Section, Karolinska Hospital. The segmentation procedure classifies the imaged volume into gray matter, white matter, CSF, nonclass, and venous blood tissue class volumes using BRAINS (22, 23), an image analysis software program suite developed at the Iowa Mental Health Clinical Research Center. This is a semiautomatic system that utilizes MR image intensity. The intracranial volume is automatically obtained. Detailed information on the segmentation procedure and an evaluation of validity have been given by Harris et al. (24). Reproducibility and reliability of the segmentation procedure have also been ascertained previously by our research group (25, 26).
Tracing Methods
The caudate, putamen, and nucleus accumbens were delineated on coronal slices. Traces of the caudate and the putamen were automatically generated using an artificial neuronal network on continuous coronal slices (thickness: 1 mm) in the reconstructed image using the BRAINS software. A detailed description of the artificial neuronal network has been made by Magnotta et al. (27). In the current study, the traces of the artificial neuronal network of the caudate and the putamen were edited manually by following the trace guidelines, available from the University of Iowa Mental Health Clinical Research Center (http://iowa-mhcrc.psychiatry.uiowa.edu/mhcrc/IPLpages/manual_tracing.htm). Briefly, the caudate was delineated from the most anterior coronal section to the most posterior where the caudate structure was visible to the naked eye. The caudate tail was cut at the center of the mamillary body. The medial boundary was the lateral ventricles. The lateral boundary was the internal capsule. The nucleus accumbens was excluded from the caudate measurements.
The tracing of the putamen started from the anterior coronal slice on which the structure was first visible then continued posteriorly on coronal sections until it disappeared. The medial boundary was the internal capsule and the globus pallidus.
The delineation of the nucleus accumbens was done manually in coronal sections. The nucleus accumbens is a cell mass that is the most rostroventromedial part of the caudate-putamen complex (28). The posterior and anterior borders of the nucleus accumbens are hard to reliably delineate. In the anterior aspect, artifacts of the lateral ventricles are seen at the inferior part of the caudate and are hard to differentiate from the boundary of the nucleus accumbens. In the posterior aspect, it is difficult to discriminate the boundary between the nucleus accumbens and the substantia innominata (29) because of their contiguity. Therefore, we defined the posterior boundary of the nucleus accumbens as the slice anterior to the caudate separated from the nucleus accumbens (Figure 1). The delineation was continued anteriorly until the structure disappeared. Gray matter, white matter, and CSF tissue class volumes within the striatal volumes in both the right and the left hemispheres were measured in milliliters using the BRAINS software. The tissue class volumes of gray and white matter but not the CSF were added within each of the striatal structures to obtain the volume of each of the striatal structures. All measurements were completed by a single operator. The operator was blind to subject identity and diagnosis.
Reliability of Measurements
Ten scans were randomly selected for interoperator reliability. Two independent operators who were specialists in psychiatry with at least 1 year of postdoctoral training in working with the current image analysis program and who were blind to subject diagnosis and identity performed the tracing of the striatal structures. The volumes of left and right side for each structure were added to compute the intraclass coefficients (ICCs) (30) used to establish interoperator reliability for the entire striatum (ICC=0.93), caudate (ICC=0.96), putamen (ICC=0.85), and nucleus accumbens (ICC=0.79).
Statistical Analysis
Before conducting parametric comparisons, data were checked by the Kolmogorov-Smirnov test, which is an established method for checking normal distribution. Differences in age, height, weight, and body mass index among groups were analyzed by using analyses of variance (ANOVAs). Handedness was compared using chi-square tests with chi-square value for the basic analyses of group differences. Relative brain volume measures were used for all group comparisons. The absolute striatal gray matter, white matter, and absolute total striatal structure volumes were adjusted for interindividual differences in intracranial volume by dividing each striatal structure by intracranial volume multiplied by 1,000 to obtain the relative volumes. Two-way ANOVAs were performed on the relative striatal volumes, with gender and diagnostic groups (schizophrenia patients versus healthy subjects) as independent factors. Repeated ANOVAs with side as the repeated factor were performed to compare measurements of left and right side structures between the groups. We also investigated the ratios of white matter to gray matter of the striatal structures by two-way ANOVAs, with gender and diagnostic groups as independent factors. Since the left and right striatal structures were correlated in both patients and healthy subjects, left and right side measurements were summed up in the ratio comparison. Spearman’s rank correlation test was used for the statistical analyses between the striatal volumes and age at MRI, age at onset of schizophrenia, and the duration of illness. We examined the correlations between estimated lifetime neuroleptic consumption and the relative volumes of gray and white matter in each of the striatal structures by using linear regression analysis. Because of the number of tests performed, a conservative alpha level of 0.01 was used.
Results
There were no significant differences in gender, handedness, or distribution of age between the schizophrenia and comparison groups. The diagnostic groups did differ in body mass index, which was higher in the patients than the healthy subjects (Table 1). There were gender differences in height and weight, with men being taller and heavier than women. The intracranial volume did not significantly differ between the diagnostic groups, but was significantly different between genders (F=51.78, df=1, 112, p<0.001), with smaller volumes seen in the women. The schizophrenia men and women did not differ with regard to age at onset, illness duration, or estimated lifetime neuroleptic consumption.
Striatal Volumes and Volume Ratios
The absolute volumes are presented in Table 2. Absolute volumes were not compared across groups. The comparison of the relative total volumes of the caudate, putamen, and nucleus accumbens revealed no significant group differences across diagnostic or gender groups. As demonstrated in Table 3, relative white matter volumes of the caudate and nucleus accumbens were significantly smaller in the patients (p<0.001), whereas relative gray matter in the putamen was significantly larger in the patients (p<0.01). The results were equal across hemispheres. A significant gender effect was demonstrated for the relative white matter of the nucleus accumbens on the left and right side, with larger volumes in women than men (p=0.01). There were no significant diagnosis-by-gender interaction effects for any of the analyses.
As seen in Table 4, the white matter/gray matter ratios of the caudate, the putamen, and the nucleus accumbens were highly significantly lower in patients compared with healthy subjects (p<0.0001). There were no significant gender or diagnosis-by-gender interaction effects in the ratio comparisons.
Repeated ANOVA revealed a laterality difference in the nucleus accumbens of the healthy subjects only, in absolute as well as relative volumes, left being larger than right (absolute volume: F=11.55, df=1, 110, p=0.0009; relative volume: F=10.37, df=1, 110, p<0.002). There were no laterality differences in the caudate and the putamen in any of the subject groups.
There were negative correlations between age at MRI and white matter volume in the caudate (F=9.73, df=1, 56, p<0.003) and putamen (F=8.71, df=1, 56, p<0.005) in patients but not in healthy subjects. There were no other significant correlations between any of the other striatal tissue class volumes and age at MRI. Spearman rank correlation tests revealed that the striatal volumes were not correlated with age at onset of illness or illness duration. There was a significant correlation between the size of the relative right gray matter volume of the putamen and the estimated lifetime consumption of typical neuroleptics in male schizophrenia patients (F=10.18, df=1, 13, p<0.008). There were no other significant correlations between segmented striatal volumes or white matter/gray matter ratios and estimated lifetime neuroleptic consumption (atypical or typical, alone or combined).
Discussion
To our knowledge, this is the first study to investigate and report changes in gray matter and white matter tissue proportions in the striatum (i.e., the caudate, putamen, and nucleus accumbens) of the same subjects suffering from schizophrenia. We found the white matter/gray matter ratios of all three structures to be altered in the comparison between patients with chronic medicated schizophrenia and healthy subjects. The ratios were highly significantly lower in the patients for all three structures. When comparing the relative white and gray matter volumes across groups we could confirm that the deviant ratios in the patients were due to smaller white matter volumes in the caudate and nucleus accumbens, whereas in the putamen larger gray matter relative to white matter volumes were demonstrated. The changed proportions between the gray and the white matter volumes were symmetrical across hemispheres. White matter reduction within brain structures suggests a loss of myelin, which is formed and maintained by oligodendroglia (31), while the gray matter increase in the striatum may reflect neuronal changes. In schizophrenia, there is evidence for white matter abnormalities (32, 33). In gene expression studies of schizophrenia, genes associated with myelin abnormalities have been found to be down regulated (32). MR diffusion tensor imaging has indicated disruption of the white matter in a number of different brain regions (34, 35), although specific findings in the striatum have not been reported. An ultrastructural study by electron microscopy demonstrated cell death of oligodendroglia in the caudate of schizophrenia patients (36). The present white matter reduction in the caudate was in agreement with a study from Japan that used a similar method to investigate the caudate nucleus (37). Taken together, those and the present findings indicate that the white matter reductions seen in the caudate and nucleus accumbens underlie some of the pathophysiological changes observed in patients with schizophrenia. Abnormal myelination might affect connectivity in cortical-striatal circuits and thus contribute to the functional impairment in schizophrenia.
Despite the alterations of the proportion of the two main tissue types in the striatal structures, the total volumes were unchanged. The changes may represent neurodevelopmental abnormalities, pathophysiological effects of chronic disease, a differential effect of neuroleptic treatment in the striatum, or effects of some hitherto unknown factors, alone or combined. The gender effect for the white matter of the nucleus accumbens on both sides with larger volumes in women is interesting, since it cannot be ascribed to a neuroleptic effect or to the disease as such. We found a correlation between lifetime estimate of typical neuroleptic consumption—but not with atypicals nor typical/atypical combinations—and the gray matter of the right putamen in male schizophrenia patients. We used an approximate estimate of lifetime neuroleptic consumption, and the findings might be consistent with the hypothesis that the increase in gray matter seen in the putamen is associated with typical neuroleptic medication. One may assume that subjects with a long treatment history earlier in life would have received typical neuroleptics but later been switched to the atypical antipsychotics (i.e., the estimated lifetime consumption for atypical neuroleptics would mainly reflect treatment with typical neuroleptic medication). If so, a combined analysis of typical and atypical neuroleptics may be a more robust estimate.
Studies of neuroleptic-naive subjects could answer questions that relate to the effects of neuroleptic treatment on striatal volumes. On the other hand, such subjects are likely to have a much shorter duration of the disease, in which case it would be difficult to distinguish between effects of medication and putative chronic effects of disease itself. In the present study, however, we did not find a relationship between the segmented striatal structures and illness duration or age at onset. The recent Japanese study also found no correlation between duration of illness and segmented caudate volumes (37).
Contrary to the case of the caudate and the putamen, the nucleus accumbens has only been sparsely investigated in volumetric MRI studies. To our knowledge, there is only one previous volumetric MRI study of the nucleus accumbens, and in that study no significant differences were found in first-episode schizophrenia patients relative to healthy subjects (38). The result of our study confirms that the total volume of the nucleus accumbens is not altered in medicated schizophrenia patients. We found laterality effects in the healthy subjects (larger left side) and greater white matter proportions in women. The nucleus accumbens has been proposed as a critical station for cognitive deficits related to modulating gating of information flow and processing of information within the thalamocortical circuitry (19, 38, 39). Our findings of white matter reduction in the nucleus accumbens in schizophrenia may be related to such cognitive deficits.
The strengths of this study are that we have used a method that is well validated (24, 25). In several studies, it has been demonstrated to have a high interoperator as well as test-retest reliability. Semiautomatically detected striatal volumes have been combined with manual editing/delineation in each MR section. The novelty is that we have segmented MR images into different tissue types that were compared across groups and that we found highly significant differences in proportions between functionally different tissues among subject groups. This kind of investigation also has not been carried out in the nucleus accumbens or putamen before. The subject material is comparatively large and carefully clinically characterized. Both women and men were included. Our subjects are very moderate alcohol consumers, and we have ruled out significant effects of alcohol on the brain volumes in this subject material (unpublished 2005 study).
The limitations of this study are that we were not able to compare tissue class volumes of the striatal structures in initially drug-naive patients in a longitudinal prospective design. We also did not subgroup the patients according to validated information on lifetime neuroleptic medication. The measure of lifetime neuroleptic consumption that we used is an approximation, and we do not have a measure of its validity.
Conclusions
The proportion of white matter to gray matter tissue volumes of the caudate, putamen, and nucleus accumbens is altered in medicated chronic schizophrenia patients, but the total volumes are unchanged. Illness duration appears not to be related to this finding. Further studies are warranted to determine the underlying mechanisms of these changes in schizophrenia.
 |
 |
 |
 |
Received April 16, 2004; revision received Jan. 25, 2005; accepted Feb. 4, 2005. From Human Brain Informatics, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; the Department of Neuropsychiatry, Kansai Medical University, Moriguchi, Japan; and the Institute of Psychiatry, University of Oslo. Address correspondence and reprint requests to Dr. Agartz, Institute of Psychiatry, University of Oslo, PB 85 Vinderen, 0319 Oslo, Norway; [email protected] (e-mail). Supported by the Wallenberg Foundation and the Swedish Research Council (grant 2003-5845). The authors thank research nurse Monica Hellberg for her assistance.
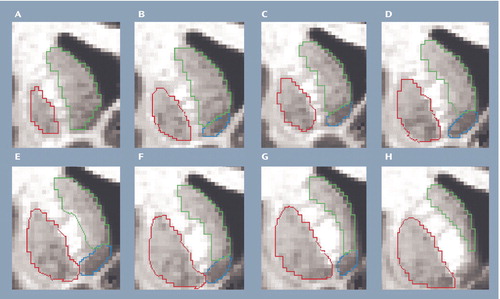
Figure 1. Delineation of the Striatuma
aCaudate=green; putamen=red; nucleus accumbens=blue.
1. Graybiel AM: The basal ganglia and cognitive pattern generators. Schizophr Bull 1997; 23:459–469Crossref, Medline, Google Scholar
2. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M: Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151:1430–1436Link, Google Scholar
3. Chakos MH, Lieberman JA, Alvir J, Bilder RM, Ashtari M: Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine (letter). Lancet 1995; 345:456–457Crossref, Medline, Google Scholar
4. Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW: Changes in caudate volume with neuroleptic treatment (letter). Lancet 1994; 344:1434Crossref, Medline, Google Scholar
5. Hokama H, Shenton ME, Nester PG, Kikinis R, Levitt JJ, Metcalf D, Wible CG, O’Donnell BF, Jolesz FA, McCarley RW: Caudate, putamen, and globus pallidus volume in schizophrenia: a quantitative MRI study. Psychiatry Res 1995; 61:209–229Crossref, Medline, Google Scholar
6. Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC: Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry 1999; 156:1200–1204Abstract, Google Scholar
7. Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC: Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155:1711–1717Link, Google Scholar
8. Noga JT, Vladar K, Torrey EF: A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Res 2001; 106:25–34Crossref, Medline, Google Scholar
9. DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R: Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res 1997; 74:129–140Crossref, Medline, Google Scholar
10. Tauscher-Wisniewski S, Tauscher J, Logan J, Christensen BK, Mikulis DJ, Zipursky RB: Caudate volume changes in first episode psychosis parallel the effects of normal aging: 5-year follow-up study. Schizophr Res 2002; 58:185–188Crossref, Medline, Google Scholar
11. Bogerts B, Meertz E, Schonfeldt-Baush R: Basal ganglia and limbic system pathology in schizophrenia: a morphometric study of brain volume and shrinkage. Arch Gen Psychiatry 1985; 42:784–791Crossref, Medline, Google Scholar
12. Bogerts B, Falkai P, Haupts M, Greve B, Ernst S, Tapernon-Franz U, Heinzman U: Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics: initial results from a new brain collection. Schizophr Res 1990; 3:295–301Crossref, Medline, Google Scholar
13. Falke E, Han LY, Arnold SE: Absence of neurodegeneration in the thalamus and caudate of elderly patients with schizophrenia. Psychiatry Res 2000; 93:103–110Crossref, Medline, Google Scholar
14. Heckers S, Heinsen H, Heinsen Y, Beckmann H: Cortex, white matter, and basal ganglia in schizophrenia: a volumetric postmortem study. Biol Psychiatry 1991; 29:556–566Crossref, Medline, Google Scholar
15. Heckers S: Neuropathology of schizophrenia: cortex, thalamus, basal ganglia, and neurotransmitter-specific projection systems. Schizophr Bull 1997; 23:403–421Crossref, Medline, Google Scholar
16. Lauer M, Beckmann H: The human striatum in schizophrenia: increase in overall relative striatal volume in schizophrenics. Psychiatry Res 1997; 68:87–98Crossref, Medline, Google Scholar
17. Lee H, Tarazi FI, Chakos M, Wu H, Redmond M, Alvir JM, Kinon BJ, Bilder R, Creese I, Lieberman JA: Effects of chronic treatment with typical and atypical antipsychotic drugs on the rat striatum. Life Sci 1999; 64:1595–1602Crossref, Medline, Google Scholar
18. Correa M, Carlson BB, Winsniecki A, Salamone JD: Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res 2002; 137:179–187Crossref, Medline, Google Scholar
19. Grace AA: Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Rev 200; 31:330–341Google Scholar
20. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Version (SCID-NP). New York, New York State Psychiatric Institute, Biometrics Research, 1986Google Scholar
21. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Schizophrenia. Am J Psychiatry 1997; 154(April suppl)Google Scholar
22. Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW II: Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci 1992; 4:125–133Crossref, Medline, Google Scholar
23. Andreasen NC, Cizadlo T, Harris G, Swayze VW II, O’Leary DS, Cohen G, Ehrhardt J, Yuh WT: Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci 1993; 5:121–130Crossref, Medline, Google Scholar
24. Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S: Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr 1999; 23:144–154Crossref, Medline, Google Scholar
25. Agartz I, Okugawa G, Greitz D, Nordström M, Magnotta V, Sedvall G: Reliability and reproducibility of brain tissue volumetry from segmented MR scans. Eur Arch Psychiatry Clin Neurosci 2001; 251:255–261Crossref, Medline, Google Scholar
26. Okugawa G, Takase K, Nobuhara K, Yoshida T, Minami T, Tamagaki C, Magnotta VA, Andreasen NC, Kinoshita T: Inter- and intraoperator reliability of brain tissue measures using magnetic resonance imaging. Eur Arch Psychiatry Clin Neurosci 2003; 253:301–306Crossref, Medline, Google Scholar
27. Magnotta VA, Heckel D, Andreasen NC, Cizadlo T, Corson PW, Ehrhardt JC, Yuh WT: Measurement of brain structures with artificial neural networks: two- and three-dimensional applications. Radiology 1999; 211:781–790Crossref, Medline, Google Scholar
28. Nieuwenhuys R, Voogd J, van Huijzen C: The so-called extrapyramidal motor system, in The Human Central Nervous System, 3rd revised ed. Berlin, Springer-Verlag, 1985, pp 258–260Google Scholar
29. Paxinos G: Current topics in basal ganglia neuroanatomy, in The Human Nervous System. New York, Academic Press, 1990, pp 485–496Google Scholar
30. Shrout PE, Fleiss JL: Intraclass correlations: using in assessing rater reliability. Psychol Bull 1997; 89:420–428Google Scholar
31. Adams JH, Graham DI: Oligodendroglia, in An Introduction to Neuropathology, 2nd ed. London, Churchill Livingstone, 1994, pp 32–33Google Scholar
32. Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V: White matter changes in schizophrenia: evidence for a myelin-related dysfunction. Arch Gen Psychiatry 2003; 60:443–456Crossref, Medline, Google Scholar
33. Okugawa G, Sedvall G, Agartz I: Reduced grey and white matter volumes in the temporal lobe of male patients with chronic schizophrenia. Eur Arch Psychiatry Clin Neurosci 2002; 252:120–123Crossref, Medline, Google Scholar
34. Lim KO, Helpern JA: Neuropsychiatric applications of DTI—a review. NMR Biomed 2002; 15:587–593Crossref, Medline, Google Scholar
35. Agartz I, Andersson JLR, Skare S: Abnormal brain white matter in schizophrenia: a diffusion tensor imaging study. Neuroreport 2001; 12:2251–2254Crossref, Medline, Google Scholar
36. Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V: Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull 2001; 55:597–610Crossref, Medline, Google Scholar
37. Takase K, Tamagaki C, Okugawa G, Nobuhara K, Minami T, Sugimoto T, Sawada S, Kinoshita T: Reduced white matter volume of the caudate nucleus in patients with schizophrenia. Neuropsychobiology 2004; 50:296–300Crossref, Medline, Google Scholar
38. Gunduz H, Wu H, Ashtari M, Bogerts B, Crandall D, Robinson DG, Alvir J, Lieberman J, Kane J, Bilder R: Basal ganglia volumes in first-episode schizophrenia and healthy comparison subjects. Biol Psychiatry 2002; 51:801–808Crossref, Medline, Google Scholar
39. Ballmaier M, Toga AW, Siddarth P, Rebecca EB, Levitt GJ, Lee M, Caplan R: Thought disorder and nucleus accumbens in childhood: a structural MRI study. Psychiatry Res Neuroimaging 2004; 130:43–55Crossref, Medline, Google Scholar



