Smaller Left Heschl’s Gyrus Volume in Patients With Schizotypal Personality Disorder
Abstract
OBJECTIVE: Individuals with schizophrenia spectrum disorders evince similar genetic, neurotransmitter, neuropsychological, electrophysiological, and structural abnormalities. Magnetic resonance imaging (MRI) studies have shown smaller gray matter volume in patients with schizotypal personality disorder than in matched comparison subjects in the left superior temporal gyrus, an area important for language processing. In a further exploration, the authors studied two components of the superior temporal gyrus: Heschl’s gyrus and the planum temporale. METHOD: MRI scans were acquired from 21 male, neuroleptic-naive subjects recruited from the community who met DSM-IV criteria for schizotypal personality disorder and 22 male comparison subjects similar in age. Eighteen of the 21 subjects with schizotypal personality disorder had additional comorbid, nonpsychotic diagnoses. The superior temporal gyrus was manually delineated on coronal images with subsequent identification of Heschl’s gyrus and the planum temporale. Exploratory correlations between region of interest volumes and neuropsychological measures were also performed. RESULTS: Left Heschl’s gyrus gray matter volume was 21% smaller in the schizotypal personality disorder subjects than in the comparison subjects, a difference that was not associated with the presence of comorbid axis I disorders. There were no between-group volume differences in right Heschl’s gyrus or in the right or left planum temporale. Exploratory analyses also showed a correlation between poor logical memory and smaller left Heschl’s gyrus volume. CONCLUSIONS: Smaller left Heschl’s gyrus gray matter volume in subjects with schizotypal personality disorder may help to explain the previously reported abnormality in the left superior temporal gyrus and may be a vulnerability marker for schizophrenia spectrum disorders.
Schizotypal personality disorder is considered part of the schizophrenia spectrum (1) because it shares a common genetic diathesis with schizophrenia (2–4) and because individuals diagnosed with schizotypal personality disorder evince many of the biological features that are observed in schizophrenia, albeit in milder form. These include higher homovanillic acid levels, aberrant eye tracking, cognitive deficits, and electrophysiological abnormalities (5–12). Of note, however, persons with schizotypal personality disorder are not psychotic and generally have not been prescribed neuroleptic medications. Thus, schizotypal personality disorder affords researchers a unique opportunity to study the schizophrenia spectrum without the potential confounding factors of neuroleptic usage, repeated hospitalizations, and chronicity.
Interest in schizotypal personality disorder has increased in recent years concurrent with the ability to examine morphologic brain abnormalities with magnetic resonance imaging (MRI). Several laboratories have reported brain abnormalities in subjects with schizotypal personality disorder that were similar to findings reported in those with schizophrenia: corpus callosum shape differences (13), greater temporal horn size (14), enlarged CSF volume (15), thalamic abnormalities (16, 17), and greater incidence of large cavum septi pellucidi (18).
Our laboratory’s work has focused mainly on the temporal lobe, in particular the gray matter of the superior temporal gyrus. The superior plane of the superior temporal gyrus consists of three regions (moving posteriorly): the temporal pole (which has sensory-limbic integrative functions [19]), Heschl’s gyrus (responsible for processing of pure tones), and the planum temporale (which may be involved in phonemic processing). Of note, gray matter abnormalities in the superior temporal gyrus are the most robust finding in schizophrenia (all 12 of the studies examining gray matter have found abnormalities [20]). In our initial investigation of the temporal lobe in subjects with schizotypal personality disorder, we found the gray matter volume of the left superior temporal gyrus to be smaller and also found a higher rate of thought disorder in the subjects with schizotypal personality disorder (21). This finding was similar to what our laboratory (22) and other laboratories have shown in schizophrenia (23–25) (for review, see references 20, 26).
The goal of this study was to further delineate the superior temporal gyrus into Heschl’s gyrus and the planum temporale to determine whether individuals with schizotypal personality disorder exhibit abnormalities similar to those reported in patients with first-episode schizophrenia (27) and chronic schizophrenia (23, 28) as well as to explore whether there are correlations between region of interest volumes and clinical measures. By understanding the aberrant anatomy of the superior temporal gyrus in schizophrenia spectrum disorders, researchers may begin to understand further the associations between brain morphology, genetic phenotype, and symptoms/signs in schizotypal personality disorder.
Method
Subject Recruitment
A total of 21 male subjects with schizotypal personality disorder and 22 male comparison subjects recruited from the community were included in the current study. Methods for recruiting have been described in detail elsewhere (15, 18, 21). The diagnosis of schizotypal personality disorder was determined by using the nonpatient and axis II versions of the Structured Clinical Interview for DSM-IV (SCID). Fourteen of the schizotypal personality disorder subjects and 14 of the comparison subjects were common to those reported in previous studies (15, 18, 21), and five additional comparison subjects were common with another study (29). Comparison subjects were age matched (within 4 years) to the subjects with schizotypal personality disorder and group matched for years of education and parental socioeconomic status. After a complete description of the study, all subjects provided written informed consent.
All subjects were right-handed and met the following criteria, detailed elsewhere (15, 21): 1) age between 18 and 55 years old; 2) no history of neurological illness; 3) no history of alcohol or drug dependence in the past 5 years, abuse within the last year (as per DSM-IV criteria), or alcohol use in the 24 hours before testing; 4) no use of drugs/medication that would affect cognitive functioning or MRI results and no lifetime exposure to neuroleptics or ECT; 5) estimated IQ above 80, with English as primary language; and 6) no history of axis I psychotic disorder or bipolar disorder or current axis I mood disorder. Comparison subjects were additionally required to have no history of mental illness, learning disabilities, or an axis I disorder in a first-degree relative.
Clinical Measures
The Thought Disorder Index, a measure of the severity of thought disorder, was administered to 18 (85.7%) of the 21 schizotypal personality disorder subjects (three subjects were lost to follow-up) but not to comparison subjects because of “floor effects” (e.g., comparison subjects score <5 [30]). The California Verbal Learning Test (31) and, as an exploratory measure, the logical memory tests (immediate and delayed recall) from the Wechsler Memory Scale—Revised (on which schizotypal personality disorder subjects perform poorly [11, 12]), were used as verbal tests for correlations with the region of interest measures. These latter tests were selected because they are receptive language tasks that require subjects to listen to words and paragraphs, thereby recruiting the primary auditory cortex for initial processing. Clinical data were available for 20 (95.2%) of the 21 subjects with schizotypal personality disorder and 19 (86.4%) of the 22 comparison subjects.
MRI Procedures
Details regarding image acquisition and postprocessing are detailed elsewhere (15, 18, 21). Briefly, superior temporal gyrus gray matter was manually drawn (for boundaries, see references 21 and 22) on coronal 1.5-mm slices. Images were subsequently realigned and reformatted so that the voxel size became isotropic (0.9375 cm3) (27). Intracranial contents were determined from the double-echo spin-echo and original coronal spoiled gradient recall acquisition images (15, 21).
Boundary Identification
Landmarks used to identify Heschl’s gyrus and the planum temporale on reformatted images are described in detail elsewhere (27) and represent anterior and posterior extensions of the originally drawn superior temporal gyrus (see Figure 1 for delineation of the regions). Interrater reliability among three raters for the editing of the originally drawn superior temporal gyrus volumes into Heschl’s gyrus and planum temporale, with the extended boundaries, was high (intraclass correlation coefficient: r>0.99, number of cases=10).
Statistical Methods
To correct for differences in brain size, a linear regression procedure was used with absolute volume of each of the regions of interest as the dependent variable and intracranial contents as the independent variable, with residuals used in subsequent analyses. A test of normality, the Shapiro-Wilk test, demonstrated that left Heschl’s gyrus was not normally distributed; therefore, the nonparametric test, Mann-Whitney U, was used instead of an analysis of variance. To consider the possible confound of comorbidity, schizotypal personality disorder subjects without comorbid axis I disorders were examined separately with their corresponding comparison subjects. To measure region of interest asymmetry, we subtracted the right side from the left, since the left planum temporale is normally larger than the right (32). Two-tailed Student’s t tests were used to analyze between-group differences on demographic variables, since the data were normally distributed. Spearman correlations for the regions of interest were performed separately for the subjects with schizotypal personality disorder and the comparison subjects. To determine whether the magnitude of the correlation differed between the two groups, Fisher’s r-to-z transformation was employed. Similarly, exploratory Spearman correlations were performed on clinical/volumetric measures with a conservative significance level set at p<0.01.
Results
Subject Demographics
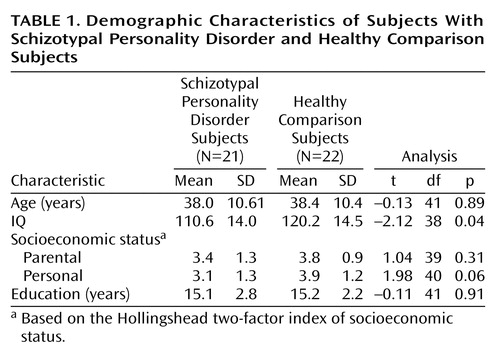
As seen in Table 1, there were no differences between the two groups in parental socioeconomic status. Lower personal socioeconomic status was seen in the subjects with schizotypal personality disorder, but this difference did not achieve statistical significance. The IQ of the comparison subjects was significantly higher than that of the schizotypal personality disorder subjects, but it is important to note that no difference was seen in level of education. Of note, only three schizotypal personality disorder subjects had neither an axis I disorder nor an additional axis II disorder. Consistent with other reports (33), most subjects had more than one comorbid diagnosis from axis I (major depression: N=5, dysthymia: N=4, panic disorder: N=2, past alcohol abuse or dependence: N=6, hallucinogen dependence or cocaine abuse: N=2) or axis II (paranoid [N=11], narcissistic [N=5], avoidant [N=5], and schizoid [N=4] personality disorders were most prevalent; all others combined: N=14). Interrater reliability for the diagnosis of schizotypal personality disorder diagnosis was high (kappa=0.89) (21).
Region of Interest Measures
No difference in whole brain volume was found between the two groups (t=–1.44, df=41, p<0.16). The Shapiro-Wilk test demonstrated that left Heschl’s gyrus gray matter volume was not normally distributed (Shapiro-Wilk statistic=0.92, df=43, p=0.01) and that this was due to the schizotypal personality disorder group (Shapiro-Wilk statistic=0.85, df=21, p=0.01) and not the comparison subjects (Shapiro-Wilk statistic=0.97, df=22, p<0.67). Left Heschl’s gyrus gray matter volume was 21% smaller in the schizotypal personality disorder subjects than in the comparison subjects (Figure 2), with an effect size (Cohen’s d) of 0.77. This finding held even when only those subjects without comorbid axis I disorders (N=11) and their age-matched comparison subjects (N=11) were included in the analysis (Mann-Whitney U=38, z=–1.48, p<0.02). (Only five schizotypal personality disorder subjects had no other axis II disorder; therefore, comparisons excluding those with comorbid axis II disorders were not performed.) There was no difference in right Heschl’s gyrus gray matter volume (Table 2). There was, however, a difference in Heschl’s gyrus asymmetry, with schizotypal personality disorder subjects exhibiting less asymmetry (i.e., less left> right asymmetry) than comparison subjects (Mann-Whitney U=148, z=–2.02, p<0.05).
Planum temporale gray matter volume, however, did not distinguish the two groups on either the right or left side (Table 2). Moreover, there was no difference in planum temporale asymmetry (Mann-Whitney U=212, z=–0.46, p=0.64) between the two groups; both groups showed left> right asymmetry.
Correlations Among Region of Interest Volumes
In the comparison subjects, a correlation between left Heschl’s gyrus and planum temporale gray matter volumes was found (rs=0.59, N=22, p=0.004) that was not present in the schizotypal personality disorder subjects (rs=–0.09, N=21, p=0.69). To verify that there was a significant difference in the correlation between these two groups, Fisher’s r-to-z transformation statistic was employed (z=–1.82, p<0.04). No other region-to-region correlation reached significance.
Correlations Between Clinical Measures and Region of Interest
Correlations were performed between the four regions of interest (right and left Heschl’s gyrus and planum temporale) and the three clinical measures (Thought Disorder Index [total score], California Verbal Learning Test [total words learned], and Wechsler Memory Scale—Revised [immediate and delayed recall performance]). The mean Thought Disorder Index score for the schizotypal personality disorder subjects (N=18) was 28.3 (SD=22.5, range=2.4–71.7). There were no correlations between Thought Disorder Index and any region of interest measure.
In the exploratory analyses, there were no statistically significant correlations between performance on the California Verbal Learning Test and region of interest volumes for either the subjects with schizotypal personality disorder or the comparison subjects. On the Wechsler Memory Scale—Revised, only one correlation (delayed recall performance and left Heschl’s gyrus volume) in subjects with schizotypal personality disorder surpassed our threshold of p<0.01 (rs=–0.57, N=20, p=0.008).
Discussion
We found smaller left Heschl’s gyrus gray matter volume in right-handed male subjects with schizotypal personality disorder relative to that of comparison subjects. To our knowledge, no other study has examined this structure in subjects with schizotypal personality disorder. This finding was a further refinement of our previous report of smaller gray matter volume in the left superior temporal gyrus (21).
In the comparison subjects there was a positive correlation between Heschl’s gyrus and planum temporale gray matter volume not found in the schizotypal personality disorder subjects. This lack of correlation was likely due to the 21% smaller left Heschl’s gray matter volume and the normal planum temporale volumes in the schizotypal personality disorder subjects rather than due to a difference in the variability of the data between the two groups. This reinforces the notion that the abnormality in subjects with schizotypal personality disorder is restricted to left Heschl’s gyrus.
This result is similar to our previous finding of smaller volumes of Heschl’s gyri (total volume) and left planum temporale in patients with first-episode schizophrenia (27). In chronic schizophrenia patients it was the left planum temporale, not left Heschl’s gyrus, that was smaller (28). The discrepancy between superior temporal gyrus findings is most likely due to subject or other methodological differences. For example, although schizotypal personality disorder and comparison subjects within this study had comparable education levels, the schizotypal personality disorder subjects had a lower IQ than did their counterparts. It may be that the disease or genetic liability of schizotypal personality disorder affects some measures of IQ. In a study of schizophrenic relatives, for example, Faraone et al. (34) noted deficits in the realms of abstraction, verbal memory, and auditory attention that were not attributable to educational level attained or parental socioeconomic status. It may be that subjects with schizotypal personality disorder have similar genetic loci affecting scores on neuropsychological measures as do the relatives (34). In addition, this report used fairly functional subjects recruited from the community, not patients, whereas a similar study of clinic-based schizotypal personality disorder subjects may demonstrate more extensive abnormalities. Therefore, how groups are matched may play a role in subtle differences in volume findings. The other factor that may account for the slightly diverging reports may be minimal differences in methodology. For example, in the present study, images were realigned, reformatted, and the anterior/posterior boundaries were extended compared with Kwon et al. (28). Also, despite good interrater reliability, different people were manually drawing the region of interest. Finally, in patients with chronic schizophrenia it is possible that with neuroleptic usage, the volume of the superior temporal gyrus may change over time (35) and that those effects would not be seen in these neuroleptic-naive schizotypal personality disorder subjects. Nonetheless, this report employed the same methodology as did Hirayasu et al. (27), thus strengthening the interpretation of finding a more constricted abnormality in schizotypal personality disorder than in schizophrenia (smaller left gray matter volume in left Heschl’s gyrus alone as contrasted with smaller volumes in both Heschl’s gyri and left planum).
The finding of smaller left Heschl’s gyrus gray matter volume in subjects with schizotypal personality disorder was a further specification of our previous finding of smaller left superior temporal gyrus volume and may have more precisely indicated a key area of abnormality in the schizophrenia spectrum. This may suggest that the less affected planum temporale may attenuate the language abnormalities seen in patients with schizophrenia. For example, schizotypal personality disorder subjects have thought disorder as measured by the Thought Disorder Index, but it was less severe than what has been shown in patients with schizophrenia (21).
The superior temporal gyrus consists of three components (anterior to posterior): the temporal pole, Heschl’s gyrus, and planum temporale. Receptive auditory processing is now considered to be hierarchical—not unlike the visual system—with highly specialized Heschl’s gyrus (primary auditory cortex) at the core and a surrounding belt region in the auditory cortex of the superior temporal plane with more integrative functions (36, 37). Heschl’s gyrus receives auditory sensory inputs via the thalamic medial geniculate nucleus and provides preattentive processing of pure tones (38, 39) in posterior and medial portions, while more extensive regions of this gyrus respond to band-passed noise (37).
In functional MRI (fMRI) studies, voice-selective regions have been located ventral to the belt region in the upper bank of the superior temporal sulcus, an area that receives direct inputs from the more dorsal surface of the superior temporal gyrus (36, 40) and is possibly analogous to the face recognition areas in the fusiform gyrus (41). The next stage of language processing, phonemic processing, which may be viewed as a fusing of multiple sound features, has been reported to occur in the auditory association cortex located in the superior temporal sulcus (40), although other reports place phonemic processing in the planum temporale and Wernicke’s area (32, 42), including the angular and supramarginal gyrus of the parietal lobe (43)—regions also found to be abnormal in schizophrenia (44–46).
The highest level of processing, that of semantic features, is the most highly left-lateralized and is thought to occur inferior to the superior temporal gyrus in the middle and inferior temporal gyri and in the temporal pole—areas less thoroughly explored in schizophrenia (40). Semantic processing also extends posteriorly to the angular gyrus (40).
Recent work has suggested a dual processing stream for auditory percepts (47), not unlike the dorsal and ventral pathways of the visual system. From the auditory core, located in A1 or Heschl’s gyrus, two parallel streams emerge: a ventral one dedicated to processing auditory patterns (“what”) located on the lateral belt of the superior temporal cortex, and a second dorsal pathway specific for processing spatial information of auditory inputs (“where”) that extends into the parietal region (39). According to this model, both streams converge with visual information in the prefrontal cortex in order to provide multimodal, integrative processing that can in turn be linked with the ability to abstract (39).
How these downstream language processes may be affected by an abnormality in the superior temporal gyrus or, more specifically, in Heschl’s gyrus is still uncertain. Moreover, the effect of pathology, such as that occurring in schizophrenia or schizotypal personality disorder, on the functional anatomy of these key language processing areas is just beginning to be explored. For example, using fMRI, Wible et al. (48) showed in schizophrenic subjects abnormal Heschl’s and posterior superior temporal gyrus activation to “mismatched” or unattended discordant tones, an example of an early perceptual processing abnormality in patients known to have abnormalities of language production. An increase in fMRI activation in Heschl’s gyrus during auditory hallucinations has also been reported (49) (although it has been suggested that the result might have been due to a shift of auditory attention as opposed to the hallucinations themselves [50]). Other data suggest that the superior temporal gyrus may be involved in the production of hallucinations (51, 52), and smaller superior temporal gyrus volume has been associated with both the severity of hallucinations (23, 53) and greater thought disorder (22, 25).
Our laboratory has previously shown that schizotypal personality disorder subjects, relative to comparison subjects, have language deficits (11, 12), thought disorder (21), verbal memory impairments (11), and, using event-related potentials, N400 abnormalities while processing normal sentences (54). In the present group of schizotypal personality disorder subjects, there was no correlation between any region of interest and thought disorder, as measured by total score on the Thought Disorder Index, or verbal learning, as measured by the California Verbal Learning Test. There was, however, a correlation between delayed recall, as measured by the Wechsler Memory Scale—Revised, and left Heschl’s gyrus volume that requires further study.
A potential limitation of this study was the inclusion of schizotypal personality disorder subjects with histories of nonpsychotic comorbid disorders. However, one would predict that inclusion would have diluted the results. For example, it may be that left planum temporale gray matter would have been similarly reduced, not unlike what was shown in patients with first-episode schizophrenia (27), had there not been comorbidity. Much remains to be learned about the seemingly high prevalence of comorbidity in this population (approximately one-half had axis I disorders and three-quarters had other personality disorders) and its impact on fundamental neuroanatomy.
The main finding of this study was the localization of deficits in the left superior temporal gyrus to Heschl’s gyrus, primary auditory cortex. This region operates early in the auditory processing stream and is important for processing of tones (37, 39, 40). Whether this volume deficit is directly associated with primary auditory processing problems and, downstream, contributes to language comprehension problems is not yet known. However, evidence of early processing problems in schizophrenia, such as mismatch abnormalities (structurally localized in or near Heschl’s gyrus), suggests that a focus on both structural and functional features associated with early auditory processing in schizotypal personality disorder may be a promising avenue of investigation.
 |
 |
Received June 6, 2001; revision received April 25, 2002; accepted May 1, 2002. From the Harvard Medical School (Clinical Neuroscience Division, Laboratory of Neuroscience, Department of Psychiatry and the VA Boston Healthcare System in collaboration with the Surgical Planning Laboratory, MRI Division, Department of Radiology, Brigham & Women’s Hospital). Address reprint requests to Dr. Shenton or Dr. McCarley, VA Boston Healthcare System, Psychiatry 116A, 940 Belmont St., Brockton, MA 02401; [email protected]; [email protected] (e-mail). Supported by grants from NIMH to Dr. McCarley (MH-52807, MH-40799) and Dr. Shenton (MH-01110, MH-50740) and from the Department of Veterans Affairs Center for Clinical and Basic Neuroscience Studies of Schizophrenia (Dr. McCarley); VA Merit Awards to Drs. McCarley and Shenton; a VA Psychiatry Research/Neuroscience Fellowship and Career Development Award (Dr. Dickey); and by a VA Psychiatry Research/Neuroscience Fellowship and a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression to Dr. Frumin. The authors thank Marie Fairbanks for administrative support and Iris Fischer, Anita Madan, and Sarah Toner for their support in the production of this project.
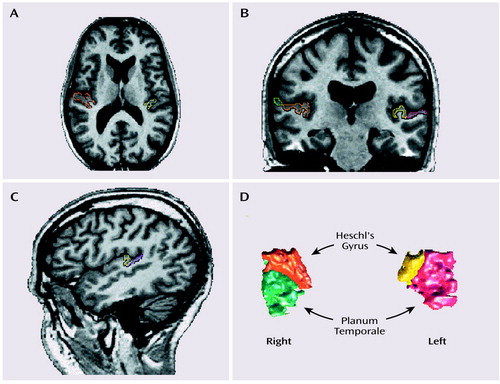
Figure 1. Delineation of Heschl’s Gyrus and the Planum Temporale in the Brain of a Subject With Schizotypal Personality Disordera
aThe axial image (part A) illustrates the most inferior slice boundary for Heschl’s gyrus, and the coronal image (part B) shows the separation of Heschl’s gyrus and the planum temporale. The sagittal view (part C) was used to confirm the delineation of the two regions of interest, which are shown in a three-dimensional rendering in part D. Following radiologic convention, the left side of the brain is on the right side of the image.
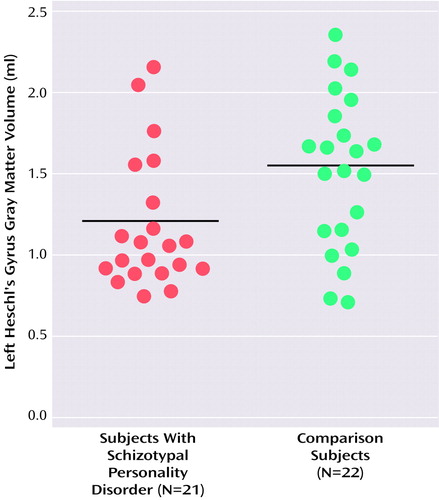
Figure 2. Absolute Gray Matter Volume of Left Heschl’s Gyrus in Subjects With Schizotypal Personality Disorder and Healthy Comparison Subjectsa
aHorizontal bars indicate mean volumes (schizotypal personality disorder subjects: mean=1.20 ml [SD=0.40]; comparison subjects: mean=1.51 ml [SD=0.48]).
1. Kety SS, Rosenthal D, Wender PH, Schulsinger F: The types and prevalence of mental illness in the biological and adoptive families of adopted schizophrenics, in Second Research Conference of the Foundation’s Fund for Research in Psychiatry. Edited by Rosenthal D, Kety SS. Elmsford, NY, Pergamon Press, 1967, pp 345-362Google Scholar
2. Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D: The Roscommon family study, I: methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch Gen Psychiatry 1993; 50:527-540Crossref, Medline, Google Scholar
3. Siever LJ, Silverman JM, Horvath TB, Klar H, Coccaro E, Keefe RS, Pinkham L, Rinaldi P, Mohs RC, Davis KL: Increased morbid risk for schizophrenia-related disorders in relatives of schizotypal personality disordered patients. Arch Gen Psychiatry 1990; 47:634-640Crossref, Medline, Google Scholar
4. Tsuang M, Stone W, Faraone S: Schizophrenia: a review of genetic studies. Harv Rev Psychiatry 1999; 7:185-207Crossref, Medline, Google Scholar
5. Clementz BA, Reid SA, McDowell JE, Cadenhead KS: Abnormality of smooth pursuit eye movement initiation: specificity to the schizophrenia spectrum? Psychophysiology 1995; 32:130-134Crossref, Medline, Google Scholar
6. Niznikiewicz MA, O’Donnell BF, Nestor PG, Smith L, Law S, Karapelou M, Shenton ME, McCarley RW: ERP assessment of visual and auditory language processing in schizophrenia. J Abnorm Psychol 1997; 106:85-94Crossref, Medline, Google Scholar
7. Salisbury D, Voglmaier M, Seidman L, McCarley R: Topographic abnormalities of P3 in schizotypal personality disorder. Biol Psychiatry 1996; 40:165-172Crossref, Medline, Google Scholar
8. Siever LJ, Amin F, Coccaro EF, Trestman R, Silverman J, Horvath TB, Mahon TR, Knott P, Altstiel L, Davidson M: CSF homovanillic acid in schizotypal personality disorder. Am J Psychiatry 1993; 150:149-151Link, Google Scholar
9. Siever LJ, Keefe R, Bernstein DP, Klar HM, Zemishlany Z, Peterson AE, Davidson M, Mahon T, Horvath T: Eye tracking impairment in clinically identified patients with schizotypal personality disorder. Am J Psychiatry 1990; 147:740-745Link, Google Scholar
10. Trestman RL, Keefe RS, Mitropoulou V, Harvey PD, deVegvar ML, Lees-Roitman S, Davidson M, Aronson A, Silverman J, Siever LJ: Cognitive function and biological correlates of cognitive performance in schizotypal personality disorder. Psychiatry Res 1995; 59:127-136Crossref, Medline, Google Scholar
11. Voglmaier MM, Seidman LJ, Niznikiewicz MA, Dickey CC, Shenton ME, McCarley RW: Verbal and nonverbal neuropsychological test performance in subjects with schizotypal personality disorder. Am J Psychiatry 2000; 157:787-793Link, Google Scholar
12. Voglmaier M, Seidman L, Salisbury D, McCarley R: Neuropsychological dysfunction in schizotypal personality disorder: a profile analysis. Biol Psychiatry 1997; 41:530-540Crossref, Medline, Google Scholar
13. Downhill J, Buchsbaum M, Wei T, Spiegel-Cohen J, Hazlett EA, Haznedar MM, Silverman J, Siever LJ: Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophr Res 2000; 42:193-208Crossref, Medline, Google Scholar
14. Buchsbaum MS, Yang S, Hazlett E, Siegel BV Jr, Germans M, Haznedar M, O’Flaithbheartaigh S, Wei T, Silverman J, Siever LJ: Ventricular volume and asymmetry in schizotypal personality disorder and schizophrenia assessed with magnetic resonance imaging. Schizophr Res 1997; 27:45-53Crossref, Medline, Google Scholar
15. Dickey CC, Shenton ME, Hirayasu Y, Fischer I, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Fraone S, McCarley RW: Large CSF volume not attributable to ventricular volume in schizotypal personality disorder. Am J Psychiatry 2000; 157:48-54Link, Google Scholar
16. Byne W, Buchsbaum M, Kemether E, Hazlett EA, Shinwari A, Mitropoulou V, Siever LJ: Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry 2001; 58:133-140Crossref, Medline, Google Scholar
17. Hazlett EA, Buchsbaum MS, Byne W, Wei T-C, Spiegel-Cohen J, Geneve C, Kinderlehrer R, Haznedar MM, Shihabuddin L, Siever LJ: Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry 1999; 156:1190-1199Abstract, Google Scholar
18. Kwon JS, Shenton ME, Hirayasu Y, Salisbury DF, Fischer IA, Dickey CC, Yurgelun-Todd D, Tohen M, Kikinis R, Jolesz FA, McCarley RW: MRI study of cavum septi pellucidi in schizophrenia, affective disorder, and schizotypal personality disorder. Am J Psychiatry 1998; 155:509-515Link, Google Scholar
19. Moran MA, Mufson EJ, Mesulam MM: Neural inputs into the temporopolar cortex of the rhesus monkey. J Comp Neurol 1987; 256:88-103Crossref, Medline, Google Scholar
20. Shenton M, Dickey C, Frumin M, McCarley R: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1-52Crossref, Medline, Google Scholar
21. Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Fischer I, Teh EK, Van Rhoads R, Jakab M, Kikinis R, Jolesz FA, Shenton ME: Schizotypal personality disorder and MRI abnormalities of temporal lobe gray matter. Biol Psychiatry 1999; 45:1393-1402Crossref, Medline, Google Scholar
22. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604-612Crossref, Medline, Google Scholar
23. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990; 147:1457-1462Link, Google Scholar
24. Marsh L, Suddath RL, Higgins N, Weinberger DR: Medial temporal lobe structures in schizophrenia: relationship of size to duration of illness. Schizophr Res 1994; 11:225-238Crossref, Medline, Google Scholar
25. Menon R, Barta P, Aylward E, Richards SS, Vaughn DD, Tien AY, Harris GJ, Pearlson GD: Posterior superior temporal gyrus in schizophrenia: grey matter changes and clinical correlates. Schizophr Res 1995; 16:127-135Crossref, Medline, Google Scholar
26. McCarley R, Wible C, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099-1119Crossref, Medline, Google Scholar
27. Hirayasu Y, McCarley R, Salisbury D, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME: Planum temporale and Heschl gyrus volume reduction in schizophrenia. Arch Gen Psychiatry 2000; 57:692-699Crossref, Medline, Google Scholar
28. Kwon JS, McCarley RW, Hirayasu Y, Anderson JE, Fischer IA, Kikinis R, Jolesz FA, Shenton ME: Left planum temporale volume reduction in schizophrenia. Arch Gen Psychiatry 1999; 56:142-148Crossref, Medline, Google Scholar
29. Anderson J, Wible C, McCarley R, Jakab M, Kasai K, Shenton M: Temporal lobe abnormalities and negative symptoms in chronic schizophrenia: a MRI study. Schizophr Res (in press)Google Scholar
30. Johnston MH, Holzman PS: Assessing Schizophrenic Thinking: A Clinical and Research Instrument for Measuring Thought Disorder. San Francisco, Jossey-Bass, 1979Google Scholar
31. Delis DC, Kramer JH, Kaplan E, Ober BA: The California Verbal Learning Test Manual. New York, Psychological Corp, 1987Google Scholar
32. Shapleske J, Rossell SL, Woodruff PW, David AS: The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev 1999; 29:26-49Crossref, Medline, Google Scholar
33. McGlashan TH: Schizotypal personality disorder: Chestnut Lodge follow-up study, VI: long-term follow-up perspectives. Arch Gen Psychiatry 1986; 43:329-334Crossref, Medline, Google Scholar
34. Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Lyons MJ, Tsuang MT: Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a diagnostic efficiency analysis. J Abnorm Psychol 1995; 104:286-304Crossref, Medline, Google Scholar
35. Keshavan MS, Haas GL, Kahn CE, Aguilar E, Dick EL, Schooler NR, Sweeney JA, Pettegrew JW: Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res 1998; 32:161-167Crossref, Medline, Google Scholar
36. Belin P, Zatorre R, Lafaille P, Ahad P, Pike B: Voice-selective areas in human auditory cortex. Nature 2000; 403:309-312Crossref, Medline, Google Scholar
37. Wessinger CM, VanMeter J, Tian B, Van Lare J, Pekar J, Rauschecker JP: Hierarchical organization of the human auditory cortex revealed by functional magnetic resonance imaging. J Cogn Neurosci 2001; 13:1-7Crossref, Medline, Google Scholar
38. Naatanen R: The role of attention in auditory information processing as revealed by event-related potentials and other measures of cognitive function. Behav Brain Sci 1990; 13:201-288Crossref, Google Scholar
39. Rauschecker J: Cortical processing of complex sounds. Curr Opin Neurobiol 1998; 8:516-521Crossref, Medline, Google Scholar
40. Binder J, Frost J, Hammeke T, Bellgowan PS, Springer JA, Kaufman JN, Possing ET: Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 2000; 10:512-528Crossref, Medline, Google Scholar
41. Kanwisher N, McDermott J, Chun M: The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 1997; 17:4302-4311Crossref, Medline, Google Scholar
42. Creutzfeldt O, Ojemann G, Lettich E: Neuronal activity in the human lateral temporal lobe. Exp Brain Res 1989; 77:451-475Crossref, Medline, Google Scholar
43. Peterson S, Fox P, Posner M, Mintun M, Raichle M: Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature 1988; 331:585-589Crossref, Medline, Google Scholar
44. Barta PE, Pearlson GD, Brill LB II, Royall R, McGilchrist IK, Pulver AE, Powers RE, Casanova MF, Tien AY, Frangou S, Petty RG: Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psychiatry 1997; 154:661-667Link, Google Scholar
45. Frederikse M, Lu A, Aylward E, Barta P, Sharma T, Pearlson G: Sex differences in inferior parietal lobule volume in schizophrenia. Am J Psychiatry 2000; 157:422-427Link, Google Scholar
46. Niznikiewicz M, Donnino R, McCarley RW, Nestor PG, Iosifescu DV, O’Donnell B, Levitt J, Shenton ME: Abnormal angular gyrus asymmetry in schizophrenia. Am J Psychiatry 2000; 157:428-437Link, Google Scholar
47. Wise R, Scott S, Blank S, Mummery C, Murphy K, Warburton E: Separate neural subsystems within “Wernicke’s area.” Brain 2001; 124:83-95Crossref, Medline, Google Scholar
48. Wible CG, Kubicki M, Yoo S-S, Kacher DF, Salisbury DF, Anderson MC, Shenton ME, Hirayasu Y, Kikinis R, Jolesz FA, McCarley RW: A functional magnetic resonance imaging study of auditory mismatch in schizophrenia. Am J Psychiatry 2001; 158:938-943Link, Google Scholar
49. Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W: Activation of Heschl’s gyrus during auditory hallucinations. Neuron 1999; 22:615-621Crossref, Medline, Google Scholar
50. Frith C: How hallucinations make themselves heard. Neuron 1999; 22:414-415Crossref, Medline, Google Scholar
51. David AS, Woodruff PW, Howard R, Mellers JD, Brammer M, Bullmore E, Wright I, Andrew C, Williams SC: Auditory hallucinations inhibit exogenous activation of auditory association cortex. Neuroreport 1996; 7:932-936Crossref, Medline, Google Scholar
52. Woodruff PWR, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SCR, Shapleske J, Rossell S, David AS, McGuire PK, Murray RM: Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry 1997; 154:1676-1682Link, Google Scholar
53. Levitan C, Ward P, Catts S: Superior temporal gyral volumes and laterality correlates of auditory hallucinations in schizophrenia. Biol Psychiatry 1999; 46:955-962Crossref, Medline, Google Scholar
54. Niznikiewicz MA, Voglmaier M, Shenton ME, Seidman LJ, Dickey CC, Rhoads R, Teh E, McCarley RW: Electrophysiological correlates of language processing in schizotypal personality disorder. Am J Psychiatry 1999; 156:1052-1058Abstract, Google Scholar



