Electrophysiological Correlates of Language Processing in Schizotypal Personality Disorder
Abstract
OBJECTIVE: This study examined whether the electrophysiological correlates of language processing found previously to be abnormal in schizophrenia are also abnormal in schizotypal individuals. The authors used the N400 component to evaluate language dysfunction in schizotypal individuals. METHOD: Event-related potentials were recorded in 16 comparison subjects and 17 schizotypal individuals (who met full DSM-III-R criteria) to sentences presented both visually and aurally; half of the sentences ended with an expected word completion (congruent condition), and the other half ended with an unexpected word completion (incongruent condition). RESULTS: In the congruent condition, the N400 amplitude was more negative in individuals with schizotypal personality disorder than in comparison subjects in both the visual and auditory modalities. In addition, in the visual modality, the N400 latency was prolonged in the individuals with schizotypal personality disorder. CONCLUSIONS: The N400 was found to be abnormal in the individuals with schizotypal personality disorder relative to comparison subjects. The abnormality was similar to the abnormality the authors’ laboratory reported earlier in schizophrenic subjects, in which the N400 amplitude was found to be more negative in both congruent and incongruent sentence completions. The N400 abnormality is consistent with the inefficient use of context.
Epidemiological studies suggest a common genetic factor in the clinical syndromes of schizophrenia and schizotypal personality disorder (1). For example, both schizophrenic patients and individuals diagnosed with schizotypal personality disorder have an equal probability of having a schizophrenic sibling. Schizotypal personality disorder is generally thought to be one of the schizophrenia spectrum disorders (1). Accordingly, similar, although less severe, impairments exist across other biological and cognitive domains (2, 3).
One of the most prominent clinical features of schizophrenia is impaired language. The speech of schizophrenic individuals is characterized by loose and bizarre associations, insensitivity to context, and inappropriate use of pronouns (4–9). These impairments have been attributed to a dysfunction in lexical networks and possibly in working memory (10, 11). Thus, if individuals with schizotypal personality disorder share a genetic vulnerability with schizophrenic patients, an impairment in the language system might also be expected in this group. Such impairments have been demonstrated both in verbal recall and in the ability to comprehend complex grammatical structures (3, 12–15).
In the current study we used the technique of event-related potentials to record electrical brain responses in subjects with schizotypal personality disorder and comparison subjects. Event-related potentials involve recording brain electrical activity averaged over many trials to provide an on-line measure of neurophysiological events associated with a particular stimulus in a cognitive task. The event-related potential is often analyzed in terms of the amplitude and latency of its major peaks, or components, associated with different aspects of information processing. Capitalizing on the existing reports, specific cognitive operations can now be associated with specific components, and thus it is possible to study these operations on the basis of their electrophysiological correlates rather than a behavioral response, which is often a more indirect index. The event-related potential technique is especially helpful in studying clinical populations in which the reaction times can be influenced by problems independent of the processes under investigation, e.g., slowed motor responses due to motor problems, rather than cognitive inability to make a judgment.
The event-related potential component that has been associated with aspects of language processing is the N400, a negative-going potential appearing about 400 msec after a target word. The N400 amplitude has been demonstrated to be more negative to words that do not fit well into the previous context or to words that are preceded by minimal context. Data suggest that the N400 reflects aspects of a brain search through the lexicon, as part of the process of recognizing the meaning of a word (16–18); the N400 may, indirectly, depend on working memory (19, 20). Recognition of a word in the sentence is quicker if the system has generated expectations of what this word might be; at a neural level, this process might be related to decreasing firing thresholds of neural assembles storing information about the word. In psychological terms, a quicker recognition time for a word brought about by a prior context is termed priming.
Since insensitivity to context, abnormal lexical search, and working memory deficits may contribute to the symptoms observed in schizophrenia, the N400 has been used to study brain electrical responses in language tasks comparing normal and schizophrenic individuals (21–26). Indeed, these studies report prolonged N400 latency and abnormal N400 amplitude (for discussion, see reference 23). In our laboratory’s recent study, relative to comparison subjects, schizophrenic individuals showed a prolonged latency and a more negative N400 amplitude to target words presented in sentences in the auditory and visual modalities.
In the current study we explored the possibility that a similar, albeit less severe, impairment might be found in schizotypal personality disorder. We hypothesized that if a failure to use context efficiently is one of the processes affected in schizophrenia spectrum disorders, then this deficit might reveal itself with less severity in schizotypal personality disorder, appearing in some but not all of the conditions. In our earlier study (23) we reported N400 abnormality in schizophrenic patients that suggested inefficient use of context in both the congruent and incongruent sentence conditions. If individuals with schizotypal personality disorder are similarly but less severely affected by a disease process, then less severe N400 abnormality might be expected. In accord with our initial hypothesis, we report the finding of a similar pattern of impairment in individuals with schizotypal personality disorder and with schizophrenia (i.e., progressing along the lines of severity, as indicated by the comparison of significant group results in the two studies), suggesting that the two groups suffer from a similar language dysfunction and thus strengthening the notion of a biological link between schizophrenia and schizotypal personality disorder.
METHOD
Subjects
Seventeen male, right-handed subjects were diagnosed with schizotypal personality disorder through use of the Structured Clinical Interview for DSM-III-R—Patient Version 1.0 (SCID-P) (27), the Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II) (28), and DSM-III-R criteria. Diagnostic reliability was evaluated by using the kappa statistics; the reliability between the rates was 0.87. Subjects with schizotypal personality disorder were selected for the study from individuals who responded to a newspaper advertisement soliciting male subjects who were shy, had few friends, and felt they had special powers. Overall, we screened 472 individuals who responded to the advertisement. The 17 men used in this study met full criteria for schizotypal personality disorder, i.e., they met the criteria for five or more of the nine DSM-III-R items. Sixteen male comparison subjects, matched for age and IQ, participated in the study and were selected from a pool of subjects recruited from a newspaper advertisement for male subjects interested in participating in an EEG study. They were also screened with the SCID-P (27) and SCID-II (28). English was the first language for all subjects.
The exclusion criteria for both groups of subjects were 1) history of ECT; 2) neurological illness; 3) history of traumatic brain injury with any known cognitive consequences or loss of consciousness greater than 5 minutes; 4) drugs or medications affecting cognitive function in the past year and any history of substance use or addiction, as defined by DSM-III-R; 5) use of neuroleptics at any time; and 6) hearing, vision, or upper body motor impairment. Additional exclusion criteria for the comparison group were histories of psychiatric disease in themselves or their first-degree relatives. The average age of subjects with schizotypal personality disorder was 40.7 years (SD=10.7, range=23–53), the average IQ was 110 (SD=14, range=80–131), and parental socioeconomic status was 3.4 (SD=1.3, range=1–5). The mean age of the comparison group was 35.8 (SD=8.8, range=22–51), IQ was 118 (SD=14, range=109–135), and parental socioeconomic status was 3.8 (SD=1.1, range=2–5). There were no statistically significant differences between the groups on any of these measures.
Stimuli and Experimental Task
Two hundred sentences were presented in the auditory and visual modalities. The sentences were 5–8 words long, followed the standard N400 paradigm (29), and were identical to the sentences used in the previous study (23). Half of the sentences ended with a word that made sense in terms of the previously presented context, and half of them ended with a word that did not make sense. The mean length of words was two syllables, 5.4 letters per word (SD=2.5). In the visual modality the words were presented in the middle of a computer screen for 300 msec, subtended 1.9 degrees, and had a 800-msec offset-to-onset interval. In the auditory modality the sentences were delivered by a male voice over Etymotic insert earphones. The average length of a word was 245 msec (SD=180), and the interword interval was the same as in the visual modality. The interval between the offset of one sentence and the onset of the next sentence was 2 seconds.
Two sets of sentences were generated to avoid repetition effects across modalities. Thus, if set A was presented in the visual modality, set B was presented in the auditory modality for each subject. Each set was prepared for presentation in the visual and auditory modality, so that across subjects, the same sets of sentences could be compared. In addition, to rule out effects of order of presentation, each of the sets was prepared in two randomized sequences. This design was used to ensure that the observed group differences might not be due to order of sentence presentation and that the differences that might be found between the two modalities were not due to different sentences used in the two modalities.
The subject’s task was to indicate whether or not the presented sentences made sense by pressing a “yes” or “no” response button in a design that counterbalanced right- and left-handed responses. The subjects were instructed to make a response after they “saw a star on the screen” 800 msec after the sentence ended. Thus, the recorded reaction time data did not reflect the speed of semantic decisions and were not used in the statistical analysis. The accuracy data, recorded along with the RT data, did not differ statistically in the two groups (t=0.54, df=25, p<0.59).
EEG Recording Procedures
The EEG was recorded through use of 28 tin-plate electrodes embedded in a Neuroscience Electrocap, with a left earlobe reference. The electrodes were positioned according to the International 10-20 system. The set of electrodes used for the study included all electrodes in the International 10-20 system and eight additional interpolated electrodes (23). Separate electrodes were placed at the left and right canthi and at supra- and infraorbital sites to record horizontal and vertical eye movements. The electrode impedance was maintained below 4 kΩ. The EEG was recorded by using a direct current to 40-Hz band pass (Neuroscan Inc., EEG amplifiers; 24 dB/octave low pass slope and 24 dB/octave high pass slope). The EEG was sampled over the epoch of 924 msec, starting at the onset of a target word, digitized at the rate of 256 samples per second. The data were stored on disk for further analysis.
Data Processing
All single-trial epochs were baseline corrected before further analyses. The contribution of vertical eye movements to scalp-recorded EEG was removed through use of subject-unique weighting coefficients (30). Single-trial epochs with voltage exceeding 75 or –75 V were rejected from further analysis. Single-subject averages were constructed from the EEG epochs recorded to the last words (congruent and incongruent relative to the context) in the visually and aurally presented sentences. All single-subject averages were band-pass filtered by using a digital filter of 0.01 to 8 Hz, the same as in our previous study of schizophrenic subjects. N400 peak latency was measured as the maximum negative voltage within 250–370 msec in the auditory modality. In the visual modality, the N400 peak latency was measured as the maximum negative voltage within 350–450 msec. These measurement windows were selected on the basis of the inspection of grand averages and individual average waveforms, in order to encompass the descending and ascending slope of a negative-going peak within the 300–500 msec poststimulus latency. The N400 amplitude was measured as the mean amplitude under the waveform curve within the same latency windows.
RESULTS
To test whether the more negative N400 amplitude in the grand average waveforms might be due to an earlier negativity, the N100 was measured as the most negative data point within 80–120 msec in the auditory and 120–180 msec in the visual modality at the sites where the peak was most prominent (Fz, Cz, F3/4, and C3/4 for the auditory and Pz, Oz, O1, and O2 for the visual modality) and, given the topographical and temporal differences between the two modalities, was subjected to separate repeated measures, mixed-model analyses of variance (ANOVAs), with group as a between-group factor (two) and condition (two) and electrode as within-group factors. In the auditory modality, no group differences were found; in the visual modality, a group-by-electrode interaction was found (F=3.54, df=3, 84, p<0.03). Group differences were found for Oz, O1, and O2.
The N400 peak latency, and the N400 mean area amplitude, measured at midline (Fz, Cz, Pz) were submitted to repeated measures, mixed-model ANOVAs in which group was the between-group factor and the within-group factors were modality (auditory, visual), condition (congruent, incongruent), and electrode (Fz, Cz, Pz). The Greenhouse-Geisser correction was used for significant interactions involving factors exceeding two levels.
The ANOVA on mean area amplitude revealed a main effect of group for the midline set of electrodes (F=9.3, df=1, 28, p<0.005) and an interaction between group and condition (F=6.1, df=1, 28, p<0.02). To follow up on the interaction and to test the prediction that the more negative N400 amplitude would be found in the congruent condition in individuals with schizotypal personality disorder relative to comparison subjects, the group differences were evaluated with repeated measures ANOVAs on the mean area amplitude in the congruent and incongruent conditions, with group as a between-group factor (schizotypal personality disorder and normal comparison) and electrode as a within-group factor.
As seen in Figure 1 and Figure 2 and Table 1 and Table 2, a more negative N400 amplitude was present in subjects with schizotypal personality disorder relative to normal comparison subjects in the congruent condition (F=13.8, df=1, 29, p<0.001) but not in the incongruent condition (F=1.6, df=1, 28, p<0.23). We also analyzed N400 amplitude difference waveforms for the two groups in the two modalities. As expected, less negative N400 difference amplitude was found in subjects with schizotypal personality disorder relative to comparison subjects (F=4.18, df=1, 28, p<0.05).
There was a main effect of group (F=18.9, df=1, 29, p<0.0001) and a significant interaction between group and modality (F=4.5, df=1, 29, p<0.04). Separate ANOVAs for auditory and visual modalities, with group as a between-group factor and condition and electrode as within-group factors, revealed that the latency difference was due primarily to a prolonged N400 latency in the visual modality in the schizotypal personality disorder group (F=17.6, df=1, 29, p=0.0001). In the auditory modality no statistically significant group differences were found (F=1.2, df=1, 30, p<0.31) (Figure 1 and Figure 2 and Table 3 and Table 4).
To test whether the more negative N400 might be due to a lower amplitude of the following positivity, the P600, an index of closure processing in language tasks, was measured as a mean area under the curve within 380–800 msec in normal subjects and 420–800 in subjects with schizotypal personality disorder and was analyzed for Fz, Cz, and Pz. The repeated measures, mixed-model ANOVAs revealed a group-by-electrode interaction at midline (F=3.82, df=2, 56, p=0.02). Tukey honestly significant difference post hoc t tests indicated significant group differences (p<0.05) at Pz in the auditory and visual modality. Smaller P600 amplitude was found in the schizotypal personality disorder group at this electrode.
DISCUSSION
Compared with normal subjects, subjects with schizotypal personality disorder showed a more negative N400 (larger) amplitude in the congruent condition in two modalities and a prolonged N400 amplitude in the visual modality. The group difference in the N100 in the incongruent condition at occipital sites suggests that subjects with schizotypal personality disorder may suffer from early visual system dysfunction. The absence of main group effects in the congruent condition in both the N100 and P600 suggests that the N400 greater negativity in the congruent condition cannot be attributed to a negative waveform spanning N100, N400, and P600 or to N400 effects deriving from N100 or P600. It is possible, however, that the diminished N400 amplitude and the smaller P600 amplitude may interact at some electrodes.
The pattern of abnormalities allows for a tentative hypothesis about language dysfunction in the schizotypal personality disorder group. A larger N400 amplitude is recorded to words that are less predicted by the context (less primed [16, 31]), so that a word that poorly fits the sentence context or that has little preceding context will elicit a larger N400 than a word that appears at the end of a highly constraining context such as, “She always had tea with sugar and cream.” The more negative N400 found to the congruent target words in the schizotypal personality disorder group might suggest a failure in priming. First, context may be poorly available because of an inability to keep it activated, or “on-line,” thus implicating a disturbance in a working memory system. Second, context, even if available, might fail to constrain the number of word candidates. The inefficient use of context to limit the number of activated words may be understood here as a faulty, context-dependent inhibition mechanism, a conclusion consistent with other reports (32, 33). Finally, even if the context-dependent inhibition mechanism functions correctly, the initial number of activated words may be too great to be efficiently limited to a few context-relevant choices. The last two types of impairment would make semantic network operations the locus of the language dysfunction.
The presence of N400 amplitude group differences in the congruent but not in the incongruent condition is more consistent with the failure of a context-dependent inhibitory mechanism (and with our previous finding [3] that subjects with schizotypal personality disorder failed to use semantic clustering to assist verbal learning, while they performed within normal range on other tests of language including reading) rather than a failure to maintain the sentence context in working memory. If working memory were involved directly, group differences would have been expected in both congruent and incongruent conditions, since across sentences, the memory load imposed by sentence stems preceding congruent and incongruent final words was similar. It is still possible that a working memory deficit (34) may have indirectly contributed to efficient context utilization.
Apparently, in the incongruent condition, the target word violated semantic expectations enough to be outside of the primed section of the network for both normal subjects and subjects with schizotypal personality disorder. In the congruent condition, the normal subjects were able to use context to constrain the network sufficiently and thus fit the final word more efficiently into the sentence so that the extensive search subroutines indexed by a large N400 did not need to be activated. In contrast, in individuals with schizotypal personality disorder, the words might have remained unconstrained by the context and thus be associated with a significantly more negative N400 amplitude in the congruent condition (Figure 1 and Figure 2).
Furthermore, the N400 latency was longer in the schizotypal personality disorder group in the visual modality for both congruent and incongruent words, implying that the processes indexed by the visual N400 took longer in subjects with schizotypal personality disorder than in comparison subjects regardless of sentence type, possibly because of either a slower search or a search through a larger section of the network. The statistical differences in the visual rather than the auditory modality may reflect a more complex processing indexed by visual N400 and hence more opportunity for delays in processing.
The present results do not allow us to address the issue of generators of N400 and their putative involvement in the abnormal N400 response seen in the schizotypal personality disorder group. However, some studies have reported N400-like waveform in depth-recording paradigms in medial temporal lobe structures (35–38). Current data suggest that associative networks are likely built through interaction of medial temporal lobe structures and neocortex (16, 38). These interactions may also play a role in guiding a process of context-generated expectancy. In a current model of schizophrenia pathology, a failure of recurrent inhibition is compatible with the observed dysfunction (39, 40). In addition, it has been demonstrated that schizophrenic individuals have structural abnormalities on magnetic resonance imaging in both the medial temporal lobe and superior temporal gyrus, especially in the language-dominant hemisphere (41).
This study, while demonstrating a less severe language dysfunction in schizotypal individuals, did not allow us to probe possible compensatory mechanisms. In addition, this study was not designed to, and did not, elucidate the relative contribution of working memory and semantic system disturbance in the population with schizotypal personality disorder. It did, however, demonstrate that the electrophysiological correlate of language processing, the N400, revealed a pattern of abnormality similar to that found in the schizophrenic population (23). These results appear to support the notion of a common biological factor contributing to the etiology of schizophrenia and schizotypal personality disorder.
Received July 14, 1998; revision received Jan. 22, 1999; accepted Feb. 5, 1999. From the Departments of Psychiatry, Harvard Medical School, Boston, Neuroscience Laboratory at Brockton/West Roxbury VA Medical Center, and Massachusetts Mental Health Center, Boston; and the Department of Radiology, Brigham and Women’s Hospital, Boston. Address reprint requests to Dr. McCarley, Department of Psychiatry 116A, VA Medical Center, 940 Belmont St., Brockton, MA 02401. Supported by the National Alliance for Research on Schizophrenia and Depression (Dr. Niznikiewicz); by grants MH-52807 and MH-40799 (Dr. McCarley), Research Scientist Development Award MH-00746, and First Award (Dr. Shenton) from NIMH; and by the Department of Veterans Affairs Center for Basic and Clinical Neuroscience Studies of Schizophrenia (Dr. McCarley).
 |
 |
 |
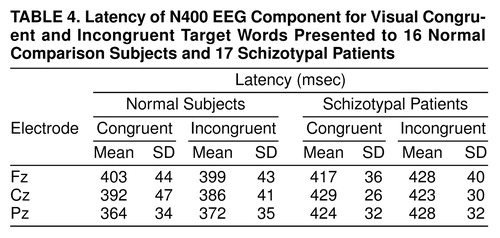 |
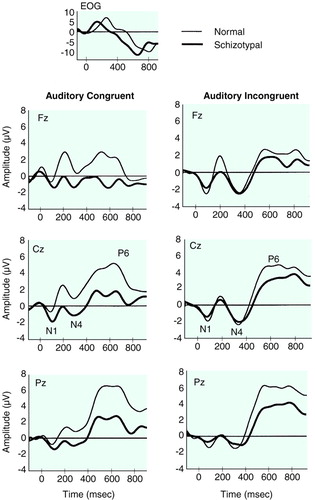
FIGURE 1. Grand Average Waveforms of 16 Normal Subjects and 17 Subjects With Schizotypal Personality Disorder in the Auditory Congruent and Incongruent Conditionsa
aWaveforms filtered with a low-pass-filter of 8 Hz to allow comparison with data reported in reference 23.
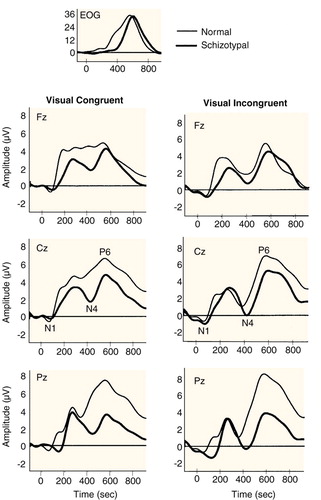
FIGURE 2. Grand Average Waveforms of 16 Normal Subjects and 17 Subjects With Schizotypal Personality Disorder in the Visual Congruent and Incongruent Conditionsa
aWaveforms filtered with a low-pass-filter of 8 Hz to allow comparison with data reported in reference 23.
1. Kendler KS, McGuire M, Gruenberg AM, Spellman M, O’Hare A, Walsh D: The Roscommon Family Study, II: the risk of nonschizophrenic nonaffective psychoses in relatives. Arch Gen Psychiatry 1993; 50:645–652Crossref, Medline, Google Scholar
2. Salisbury DF, Voglmaier M, Seidman LJ, McCarley RW: Topographic abnormalities of P3 in schizotypal personality disorder. Biol Psychiatry 1996; 40:165–172Crossref, Medline, Google Scholar
3. Voglmaier MM, Seidman LJ, Salisbury DF, McCarley RW: Neuropsychological dysfunction in schizotypal personality disorder: a profile analysis. Biol Psychiatry 1997; 41:530–540Crossref, Medline, Google Scholar
4. Chapman LJ, Chapman JP, Miller GA: A theory of verbal behavior in schizophrenia, in Progress in Experimental Personality Research, vol 1. Edited by Maher BA. New York, Academic Press, 1963, pp 49–77Google Scholar
5. Chapman LJ, Chapman JP, Daut R: Schizophrenic inability to disattend from strong aspects of meaning. J Abnorm Psychol 1976; 85:35–40Crossref, Medline, Google Scholar
6. Doherty N, Schnur M, Harvey P: Reference performance and positive and negative thought disorder: a follow-up study of manics and schizophrenics. J Abnorm Psychol 1988; 97:437–442Crossref, Medline, Google Scholar
7. Harvey PD: Speech competence in manic and schizophrenic psychoses: the association between clinically rated thought disorder and cohesion and reference performance. J Abnorm Psychol 1983; 92:368–377Crossref, Medline, Google Scholar
8. Kwapil T, Hegley DC, Chapman LJ, Chapman JP: Facilitation of word recognition by semantic priming in schizophrenia. J Abnorm Psychol 1990; 99:215–221Crossref, Medline, Google Scholar
9. Manschreck T, Maher B, Milavetz JJ, Ames D, Wesstein CC, Schneyer M: Semantic priming in thought disordered schizophrenic patients. Schizophr Res 1988; 1:61–66Crossref, Medline, Google Scholar
10. Maher BA: Language and schizophrenia, in Handbook of Schizophrenia, vol 5: Neuropsychology, Psychophysiology, and Information Processing. Edited by Steinhauer SR, Gruzelier JH, Zubin J. New York, Elsevier, 1991, pp 437–463Google Scholar
11. Spitzer M, Weisker I, Winter M, Maier S, Hermle L, Maher B: Semantic and phonological priming in schizophrenia. J Abnorm Psychol 1994; 3:485–495Crossref, Google Scholar
12. Lyons MJ, Merla ME, Young L, Kremen WS: Impaired neuropsychological functioning in symptomatic volunteers with schizotypy: preliminary findings. Biol Psychiatry 1991; 30:424–426Crossref, Medline, Google Scholar
13. Caplan R, Purdue S, Tanguay PE, Fish B: Formal thought disorder in childhood onset schizophrenia and schizotypal personality disorder. J Child Psychol Psychiatry 1990; 31:1103–1114Google Scholar
14. Condray R, Steinhauer SR: Schizotypal personality disorder in individuals with and without schizophrenic relatives: similarities and contrasts in neurocognitive and clinical functioning. Schizophr Res 1992; 7:33–41Crossref, Medline, Google Scholar
15. Siever LJ: Schizophrenia spectrum personality disorders, in American Psychiatric Press Review of Psychiatry, vol 11. Edited by Tasman A, Riba MB. Washington, DC, American Psychiatric Press, 1992, pp 25–42Google Scholar
16. Nobre AN, McCarthy G: Language-related potentials in the anterior-medial temporal lobe, II: effects of word type and semantic priming. J Neurosci 1995; 15:1090–1098Google Scholar
17. Holcomb PJ: Semantic priming and stimulus degradation: implications for the role of the N400 in language processing. Psychophysiology 1993; 30:47–60Crossref, Medline, Google Scholar
18. Van Petten C, Kutas M: Ambiguous words in context: an event-related potential analysis of the time course of meaning activation. J Mem Lang 1987; 26:188–208Crossref, Google Scholar
19. Kutas M, Hillyard SA: An electrophysiological probe of incidental semantic association. J Cognitive Sci 1989; 1:38–49Google Scholar
20. Kutas M, Van Petten C: Event-related potential studies of language, in Advances in Psychophysiology, vol 3. Edited by Ackles PK, Jennings JR, Coles MG. Greenwich, Conn, JI Press, 1988, pp 139–187Google Scholar
21. Adams J, Faux SF, Nestor PG, Shenton ME, Marcy B, Smith S, McCarley RW: ERP abnormalities during semantic processing in schizophrenia. Schizophr Res 1993; 10:247–257Crossref, Medline, Google Scholar
22. Nestor PG, Kimble MO, O’Donnell BF, Smith L, Niznikiewicz MA, Shenton ME, McCarley RW: Aberrant semantic activation in schizophrenia: a neurophysiological study. Am J Psychiatry 1997; 154:640–666Link, Google Scholar
23. Niznikiewicz MA, O’Donnell BF, Nestor PG, Smith L, Law S, Karapelou M, Shenton ME, McCarley RW: ERP assessment of visual and auditory language processing in schizophrenia. J Abnorm Psychol 1997; 1:85–94Crossref, Google Scholar
24. Grillon C, Ameli R, Glazer WM: N400 and semantic categorization in schizophrenia. Biol Psychiatry 1991; 29:467–480Crossref, Medline, Google Scholar
25. Mitchell PF, Andrews S, Fox A, Catts PB, McConghy N: Active and passive attention in schizophrenia: an ERP study of information processing in a linguistic task. J Abnorm Psychol 1991; 32:101–124Google Scholar
26. Koyama S, Nageishi Y, Shimokochi M, Hokama H, Myiazato Y, Miyatani M, Ogura C: The N400 component of event-related potentials in schizophrenic patients: a preliminary study. Electroencephalogr Clin Neurophysiol 1991; 78:124–132Crossref, Medline, Google Scholar
27. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version 1.0 (SCID-P). Washington, DC, American Psychiatric Press, 1990Google Scholar
28. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Washington, DC, American Psychiatric Press, 1990Google Scholar
29. Kutas M, Hillyard SA: Brain potentials during reading reflect word expectancy and semantic association. Nature 1984; 307:161–163Crossref, Medline, Google Scholar
30. Semlitsch HV, Anderer P, Schuster P, Presslich O: A solution for reliable and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology 1986; 23:695–703Crossref, Medline, Google Scholar
31. Van Petten C, Kutas M: Influences of semantic and syntactic context on open- and closed-class words. Mem Cognit 1991; 19:95–112Crossref, Medline, Google Scholar
32. McCarley RW, Hsiao J, Freedman R, Pfefferbaum A, Donchin E: Neuroimaging and the cognitive science of schizophrenia. Schizophr Bull 1996; 22:703–726Crossref, Medline, Google Scholar
33. Vinogradov S, Solomon S, Ober BA, Biggins CA, Shenaut GK, Fein G: Do semantic priming effects correlate with sensory gating in schizophrenia? Biol Psychiatry 1996; 39:821–824Google Scholar
34. Barch DM, Cohen JD, Servan-Schreiber D, Steingard S, Steinhauer SS, van Kammen DP: Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. J Abnorm Psychol 1996; 105:592–601Crossref, Medline, Google Scholar
35. Halgren E, Smith ME: Cognitive evoked potentials as modulatory processes in human memory formation and retrieval. Hum Neurobiol 1987; 6:129–139Medline, Google Scholar
36. Smith ME, Stapleton JM, Halgren E: Human medial temporal lobe potentials evoked in memory and language tasks. Electroencephalogr Clin Neurophysiol 1986; 63:145–159Crossref, Medline, Google Scholar
37. Halgren E, Baudena P, Heit G, Clarke M, Marinkovic K, Chauvel P: Spatio-temporal stages in face and word processing, 2: depth-recorded potentials in the human frontal and Rolandic cortices. J Physiol 1994; 88:51–80Google Scholar
38. McCarthy G, Nobre AN, Bentin S, Spencer D: Language-related field potentials in the anterior-medial temporal lobe, I: intracranial distribution and neural generators. J Neurosci 1995; 15:1080–1089Google Scholar
39. Grunze HC, Rainie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW: NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci 1996; 16:2034–2043Google Scholar
40. McCarley RW, Hsiao J, Freedman R, Pfefferbaum A, Donchin E: Neuroimaging and the cognitive neuroscience of schizophrenia. Schizophr Bull 1996; 22:703–726Crossref, Medline, Google Scholar
41. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Coleman M, Metcalf D, McCarley RW: Abnormality of the left temporal lobe and thought disorder in schizophrenia. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar



