Verbal and Nonverbal Neuropsychological Test Performance in Subjects With Schizotypal Personality Disorder
Abstract
OBJECTIVE: The authors contrasted verbal and nonverbal measures of attention and memory in patients with DSM-IV-defined schizotypal personality disorder in order to expand on their previous findings of verbal learning deficits in these patients and to understand better the neuropsychological profile of schizotypal personality disorder. METHOD: Cognitive test performance was examined in 16 right-handed men who met diagnostic criteria for schizotypal personality disorder and 16 matched male comparison subjects. Neuropsychological measures included verbal and nonverbal tests of persistence, supraspan learning, and short- and long-term memory retention. Neuropsychological profiles were constructed by standardizing test scores based on the means and standard deviations of the comparison subject group. RESULTS: Subjects with schizotypal personality disorder showed a mild to moderate general reduction in performance on all measures. Verbal measures of persistence, short-term retention, and learning were more severely impaired than their nonverbal analogs. Performance on measures of memory retention was independent of modality. CONCLUSIONS: The results are consistent with previous reports that have suggested a mild, general decrement in cognitive performance and proportionately greater involvement of the left hemisphere in patients with schizotypal personality disorder. The findings provide further support for a specific deficit in the early processing stages of verbal learning.
Schizotypal personality disorder is characterized by oddities in appearance, perception, and behavior that appear to represent a milder variant of schizophrenia (1). A biological relationship between chronic schizophrenia and schizotypal personality disorder has been supported by genetic and family studies (2), by similarities in performance on measures of attention and information processing (3), and by neurochemical similarities (4).
Similarities between schizophrenia and schizotypal personality disorder have also been demonstrated on clinical measures of neuropsychological function. Generally, the neuropsychological deficits evident in schizophrenia include impaired abstraction, attention, language, and verbal learning and memory, which are thought to reflect involvement of frontal and left temporal-limbic brain regions (5–9). Until recently, neuropsychological function in schizotypal personality disorder was relatively poorly understood, since few studies included subjects who met full diagnostic criteria for the disorder, and few cognitive domains were evaluated. The few extant studies that examined cognitive performance in patients with clinically defined schizotypal personality disorder (10–12) have suggested deficit patterns similar to, but less severe than, those seen in schizophrenia. Similar results were reported in nonclinical “psychosis-prone” populations (3).
We previously studied the neuropsychological profile of schizotypal personality disorder by examining a wide range of cognitive functions in right-handed male subjects who met full DSM-IV criteria for schizotypal personality disorder and found significant deficits on measures of verbal learning and abstraction (13). These deficits were apparent against a background of general mild impairment in other cognitive domains. This profile was similar to that found in subjects with schizophrenia, albeit less severe, and we suggested it might reflect similar involvement of frontal and left temporal brain areas. As measured by the California Verbal Learning Test (14), verbal learning in patients with schizotypal personality disorder was characterized by a reduced number of words learned over five trials but not by an increased rate of forgetting. This is consistent with a disturbance in encoding or retrieval from memory but not storage of information. The learning deficit was further compounded by an impairment in semantic organization or “clustering” of the word list to facilitate learning, similar to that reported in first-degree relatives of patients with schizophrenia (15). In contrast, nonverbal learning skills were relatively preserved. Within the context of impaired performance on a measure of abstraction, it was unclear if our finding of a deficit in verbal learning reflected a primary deficit in language processes, in learning, or in concept formation and organization.
The purpose of the current study was to further examine the verbal learning deficit in DSM-IV-defined schizotypal personality disorder by evaluating selected components of verbal and nonverbal attention, learning, and memory. On the basis of results from our previous study, we hypothesized that 1) subjects with schizotypal personality disorder would show a general impairment on these measures relative to comparison subjects, 2) memory retention (i.e., rate of forgetting) would be relatively preserved, and 3) subjects with schizotypal personality disorder would show more impairment on verbal than nonverbal analogs of these various measures.
Method
Subjects
Subjects were 16 unmedicated right-handed men who met DSM-IV criteria for schizotypal personality disorder and 16 right-handed male comparison subjects matched for age and parental socioeconomic status (16). Because IQ, education, and personal socioeconomic status might be reduced in subjects with schizotypal personality disorder as a function of the disorder (the “matching fallacy” [17]), subject groups were not matched on these indices but rather on socioeconomic status of family of origin.
All subjects with schizotypal personality disorder were recruited from the general population through advertisements in local newspapers that asked for men who “believe they have ESP, clairvoyance, telepathy, or a ‘sixth-sense’; sense the presence of others when alone; think others can feel your emotions” (18), and who “are shy or uncomfortable around unfamiliar people or in close relationships.” The advertisement thus probed both positive and negative symptoms. Comparison subjects answered advertisements in the same newspapers recruiting healthy, right-handed men for a “brain imaging study” (all subjects had auditory event-related potentials measured; these results will be reported separately). All subjects spoke English as their first language, had normal or corrected-to-normal vision, and had normal hearing and normal upper limb function—basic requirements for optimal performance on the neuropsychological test battery. After complete description of the study to the subjects, written informed consent was obtained. All subjects were paid for their participation in the study.
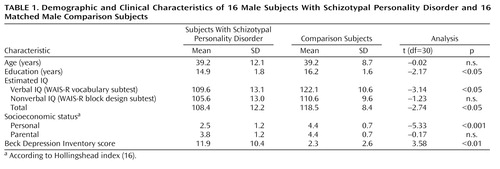
Demographic characteristics of the subject groups are summarized in Table 1. The groups did not differ significantly in age or parental socioeconomic status. The schizotypal personality disorder group had significantly fewer years of education, lower estimated IQ (19), and lower personal socioeconomic status than the comparison subjects. The groups did not differ on a nonverbal estimate of general ability. Because it was thought likely that symptoms of depression might be higher in the schizotypal personality disorder group, the Beck Depression Inventory (20) was administered to assess the effect of this variable on neuropsychological performance. As expected, subjects with schizotypal personality disorder reported significantly more depressive symptoms (mild levels) on the Beck Depression Inventory than did the comparison subjects. Comparison subjects were within normal limits on all clinical measures.
Procedures
Exclusion criteria
Subjects with less than a ninth-grade education, an estimated IQ < 80, a history of a diagnosed learning disorder or developmental disability, a previous head injury (with loss of consciousness or cognitive sequelae), a neurological disorder, a systemic illness, or who were taking psychotropic medications were excluded from the study. Subjects with schizotypal personality disorder were excluded if they had a history of bipolar disorder, psychotic disorder, or a current depressive disorder. Comparison subjects with any personal history of a DSM-IV axis I or II disorder or with a family history (first-degree relatives) of an axis I disorder were excluded from the study. All subjects were carefully screened for history of substance abuse with the Structured Clinical Interview for DSM-IV (SCID) (21). Comparison subjects with any history of substance abuse were excluded from the study. None of the subjects with schizotypal personality disorder had abused substances within the past 5 years or had any lifetime history of substance addiction.
Psychiatric assessment
Subjects were diagnosed using the SCID and the SCID for Axis II Personality Disorders (SCID-II) (22). One interviewer (M.M.V. or C.C.D.) administered the SCID and SCID-II to all schizotypal personality disorder and comparison subjects. All of the interviews were videotaped for reevaluation by a second examiner (L.J.S.). Interrater reliability for schizotypal personality disorder within our laboratory was 0.84 (kappa statistic), which was based on 24 subjects. All subjects with schizotypal personality disorder met full DSM-IV diagnostic criteria for the disorder. There was also considerable diagnostic overlap with other axis II personality disorders (paranoid, N=6; avoidant, N=2; obsessive-compulsive, N=2; borderline, N=4; and narcissistic, N=4), which is in accordance with other reports (23).
Neuropsychological Battery
The cognitive functions examined included various components of attention, learning, and memory that had comparable verbal and nonverbal counterparts (Table 2).
Short-term retention
Short-term retention deficits signify the rapid decay of a memory trace early in the process of learning. The Brown-Peterson technique (24), a distractor task designed to prevent rehearsal of information, requires subjects to count backward for various periods of time immediately after hearing a consonant trigram (e.g., TFL).
In the current study, the verbal score was made up of total consonant trigrams correctly recalled after 18 seconds of interference, a length of time when the distractor effect is most apparent (24). A similar nonverbal pattern recall task was developed (see reference 25); the nonverbal score included total design components correctly recalled after 18 seconds of interference.
Supraspan learning
In our previous study (13), subjects with schizotypal personality disorder performed normally on measures of attention span. Learning a string of digits that is greater than the immediate attention span provides a “stimulus overload” condition that is more sensitive to attention and learning deficits. The excess items are thought to serve as interference stimuli, so that recall represents the span plus what is retained (learned) despite interference. In the current study, the verbal supraspan test measure was the total score on the serial digit learning task (26). In this aurally presented task, the supraspan series of nine digits is repeated over 12 trials, and the final score reflects supraspan learning capacity. The nonverbal supraspan learning task measure was total score on the Corsi Block Tapping test (see reference 27). This measure includes tapping supraspan (span plus one) sequences on a board containing a spatial array of nine blocks. Without informing the subject, the same sequence is repeated every third trial for 12 trials, and the score reflects incidental learning of the recurring pattern.
Persistence
Persistence is a component of attention that involves sustained behavioral output over time. In this study, the verbal persistence measure included total words produced on the Controlled Oral Word Association Test (28). During this task, subjects are required to produce as many words as they can in 1 minute, beginning with each of the letters C, F, and L, excluding proper nouns, numbers, and the same word with a different suffix. The nonverbal persistence measure included total novel designs produced on the Design Fluency Test (29) during a 5-minute period. In this task, subjects are asked to “invent” as many different drawings as they can, which cannot be named and are not merely scribbles.
Learning
As in our previous study (13), the verbal learning function score was made up of the total words learned over five trials of the California Verbal Learning Test (14). The nonverbal learning task measure was total score on the Continuous Visual Memory Test (30). This task involves repeated exposure to seven random shapes (among distractors) over 112 trials. The total score reflects continuous recognition of the target and distractor items over 96 trials.
Memory retention
Selected subtests from the Wechsler Memory Scale—Revised (31) were used to measure percent memory retention (32). The verbal score was made up of the percentage of information retained from the logical memory stories of the Wechsler Memory Scale. This two-part test requires subjects to recall two paragraph stories immediately after hearing them (part 1) and after a 30-minute delay (part 2). Percentage of information retained was calculated as (part 2 performance/part 1 performance)× 100. The nonverbal score included percentage of information retained from the visual reproduction designs. This two-part task requires subjects to draw four geometric designs of increasing complexity immediately after seeing them (part 1) and after a 30-minute delay (part 2). Nonverbal memory retention was calculated as (part 2 performance/part 1 performance)× 100.
Data Analysis
In the current study, test scores of subjects with schizotypal personality disorder were standardized based on the means and standard deviations of the comparison group to help control for differences in psychometric properties (e.g., difficulty level, reliability) of the neuropsychological tests across domains (33). Differences between the two groups were evident in estimated IQ (19) and years of education (Table 1). Concerns have been raised about appropriate analysis of cognitive function in schizophrenia spectrum disorders because 1) performance on some cognitive tests might be weighted by education and IQ, and 2) education and IQ may be reduced as a result of the disorder. It is therefore unclear if these factors should be covaried from analyses in order to equate the groups and search for other areas of deficit, or if such a strategy only serves to remove the cognitive domain that is deficient and of interest. We chose to analyze the results both ways, to determine if specified cognitive deficits were present over and above any general deficit.
A related concern involves evaluation of selective cognitive deficits in the schizophrenia spectrum. Because these disorders may be characterized by a general reduction in ability, it is important to ensure that any apparent domain-specific impairments are not merely a reflection of a generalized deficit. This is done by covarying an index of general ability or IQ. However, our measure of estimated IQ (a composite of the WAIS-R [34] vocabulary and block design subtests) was weighted by verbal ability and education, both of which may be reduced in schizotypal personality disorder as a result of the disorder itself. We considered it appropriate, therefore, to separately calculate a verbal IQ estimate based on the WAIS-R vocabulary subtest and a nonverbal estimate of general ability based on the WAIS-R block design subtest (Table 1). There was no significant difference between the groups on the nonverbal IQ measure.
All analyses of test scores were executed using standard z scores based on the means and standard deviations of the comparison group. Repeated measures analyses of variance (ANOVA), multivariate option, with group (subjects with schizotypal personality disorder versus normal comparison subjects) as the between-subjects factor and cognitive test and test modality (verbal versus nonverbal) as the within-subject factors, were used to assess differences between profile shapes. This allowed for a comparison of between-group and within-group performance differences. This was followed by within-subject contrasts on each domain, in which the score for a particular function was contrasted with the mean of all functions in order to examine differences in individual cognitive domains and test scores. As above, analyses were repeated using verbal IQ, education, and Beck Depression Inventory scores as covariates to determine the effects of these on performance. Comparison analyses were run using nonverbal IQ as the covariate for general ability.
Results
Profile Analysis
The z score profiles for the subjects with schizotypal personality disorder are presented in Figure 1. Repeated measures ANOVA revealed a significant difference between groups (F=7.58, df=1, 11, p=0.02), indicating an overall reduction in test performance in subjects with schizotypal personality disorder. A test-by-modality interaction indicated a significant difference in profile shapes for verbal and nonverbal test analogs in the schizotypal personality disorder group (F=2.53, df=5, 70, p<0.05). Profile contrasts indicated that the performance of subjects with schizotypal personality disorder on the verbal measures of persistence, short-term retention, and learning were significantly different from that of comparison subjects (p<0.05). Performance on the nonverbal counterparts of these tests, and on the verbal and nonverbal measures of supraspan learning and memory retention, were not significantly different from normal. Within the schizotypal personality disorder group, subjects performed significantly more poorly on verbal than on nonverbal measures of persistence and learning (p<0.05). The performance of subjects with schizotypal personality disorder on verbal and nonverbal measures of short-term retention did not differ significantly.
In comparison analyses, when estimated nonverbal IQ was entered as the covariate, these results remained significant (F=5.45, df=1, 11, p<0.04). Adding years of education and Beck Depression Inventory scores as covariates resulted in reduced power (p<0.06). When verbal IQ was entered as the covariate, the results were no longer significant.
Discussion
In the current study, subjects with schizotypal personality disorder showed a mild general cognitive deficit as well as a more specific deficit on measures of verbal fluency, short-term verbal retention, and verbal learning. Nonverbal forms of these tests, simpler supraspan learning, and long-term retention of newly learned information were relatively preserved. The results are consistent with our previous findings of a significant verbal learning deficit in patients with schizotypal personality disorder (13). The findings are also supportive of the hypothesis that reduced performance on these tests is at least in part the result of a deficit in early processing (encoding) stages of verbal learning, rather than solely due to a primary deficit in organization or conceptualization.
Memory is a complex function that includes various components of attention, working memory, encoding, consolidation, storage, and retrieval (35). The current results suggest that the breakdown in verbal learning in patients with schizotypal personality disorder may occur in the early stages of verbal memory processing. Regarding attention processes, in our previous study (13), subjects with schizotypal personality disorder did not show a primary attention deficit. Similarly, subjects with schizotypal personality disorder in the current study did relatively well on simpler measures of supraspan learning, which involve repetition of discrete bits of information (digits, spatial location). However, as the tasks became more difficult—involving sustained behavioral output and interference or requiring further encoding of verbal information—a more specific deficit in verbal attention and memory processing became apparent. Subjects with schizotypal personality disorder showed significant deficits on verbal measures of persistence, short-term retention, and word list learning. In contrast, once information was stored, there was no specific deficit in memory retention. This replicates our previous results on the California Verbal Learning Test with two different measures of memory (the logical memory stories and visual reproduction design tasks from the Wechsler Memory Scale). In contrast, subjects did relatively well on the nonverbal versions of these measures of attention and memory.
There are several caveats to interpretation in this study. One possible limiting factor is that the verbal and nonverbal tests were not matched for level of difficulty or reliability. Without controlling for differences in these psychometric test properties, more difficult tests might be expected to show more of an effect, and less reliable tests might be expected to show less of an effect. In this study, we attempted to control for this by using standardized scores (33), and difficulty level may have been further mediated by covarying a nonverbal measure of general ability. It is true that the results were no longer significant when estimated IQ (19), a measure heavily weighted by vocabulary skills, was covaried from the analyses, but this is difficult to interpret. It is unclear if general deficits in verbal ability caused the reduced performance on certain verbal measures, or conversely, if inherent verbal learning deficits have resulted in deficient vocabulary.
The current results are consistent with the latter explanation or with a combination of these possibilities. A general verbal deficit alone would be expected to result in a general reduction in performance on all verbal tasks, but in the current study, verbal supraspan learning and verbal long-term memory retention skills were relatively preserved, even before IQ was taken into account. Thus, in male subjects with schizotypal personality disorder, a primary verbal learning deficit may account for reduced vocabulary, IQ scores, and verbal fluency. This raises another caveat for interpretation in that only male subjects were included in the current study to control for gender effects. Sex differences in neuropsychological test performance have been found in schizophrenic subjects and in the general population (36). It is unknown if the current results would differ in female subjects with schizotypal personality disorder.
Finally, the results of this study must be tempered by the small study group size and resultant reduction in power. Use of small study group sizes and multiple variables can result in a failure to produce significant and potentially meaningful results. In the current study, limited power resulted in the loss of significant findings when potentially confounding variables (e.g., years of education, Beck Depression Inventory score) were covaried from the analyses, and reduced power may have accounted for other negative findings.
The pattern of cognitive deficits apparent in subjects with schizotypal personality disorder in this study is consistent with hypotheses suggesting involvement of left hemisphere frontotemporal networks in schizophrenia and schizotypal personality disorder (e.g., references 6, 37). Deficits in auditory trigram recall have been associated with left-temporal hippocampal excision (38, 39) and frontal lobe dysfunction (40). Reduced performance on measures of verbal fluency has been associated with frontal lobe dysfunction, especially on the left (41). Finally, fewer words learned and reduced use of semantic clustering strategies by subjects who take the California Verbal Learning Test (14) have been associated with left temporal lobe dysfunction (42).
Finally, the results underscore the cognitive deficits and treatment needs of individuals with schizotypal personality disorder. Behaviorally, limitations in the early stages of verbal learning could result in problems in social, academic, and occupational performance (e.g., limited comprehension of verbal information, slow learning) and may eventually result in reduced vocabulary, verbal fluency, and IQ test scores. This may contribute to the low socioeconomic status of our subjects with schizotypal personality disorder. Selected primarily from the general population, this group has at least average intelligence and came from middle-class families, yet personal socioeconomic status was significantly lower than parental socioeconomic status, whereas this was not true in the comparison subject group. Further study of the cognitive, physiological, and structural correlates of brain function in schizotypal personality disorder populations is warranted to examine the cognitive and clinical features of this disorder, as well as to help shape our understanding of the pathophysiology of schizophrenia.
 |
 |
Received May 25, 1999; revision received Dec. 17, 1999; accepted Dec. 22, 1999. From the Department of Psychiatry, Harvard Medical School, Massachusetts Mental Health Center, Boston; the Department of Psychiatry, Brockton/West Roxbury VA Medical Center; and the Cambridge Hospital, Cambridge, Mass. Address reprint requests to Dr. McCarley, Brockton/West Roxbury VA Medical Center, Department of Psychiatry 116-A, 940 Belmont St., Brockton, MA 02401. Supported by NIMH grants MH-52807 (Dr. McCarley) and MH-01100 and MH-50747 (Dr. Shenton); a grant from the Medical Research Service of the Department of Veterans Affairs Schizophrenia Center (Dr. McCarley); and a Peter Livingston Research Fellowship and a Research and Education Fund Fellowship from the Department of Psychiatry, Harvard Medical School, Massachusetts Mental Health Center (Dr. Voglmaier).
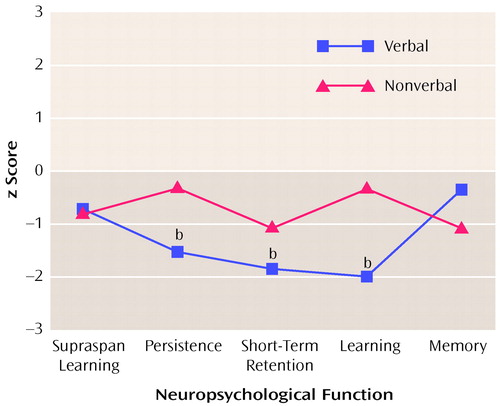
Figure 1. Verbal and Nonverbal Neuropsychological Performance of 16 Subjects With Schizotypal Personality Disorder Relative to That of Comparison Subjectsa
aPerformance of comparison subjects represented by z scores set at 0.
bSignificantly different from comparison subjects (p<0.05).
1. Kety SS, Rosenthal D, Wender PH, Schulsinger F, Jacobsen B: Mental illness in the biological and adoptive families of adopted individuals who have become schizophrenic: preliminary report based on psychiatric interviews, in Genetic Research in Psychiatry. Edited by Fieve RR, Rosenthal D, Brill H. Baltimore, Johns Hopkins University Press, 1975, pp 147–165Google Scholar
2. Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D: The Roscommon family study, I: methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch Gen Psychiatry 1993; 50:527–540Crossref, Medline, Google Scholar
3. Kremen WS, Seidman LJ, Pepple JR, Lyons MJ, Tsuang MT, Faraone SV: Contributions of neuropsychology toward identifying risk indicators for schizophrenia. Schizophr Bull 1994; 20:103–119Crossref, Medline, Google Scholar
4. Siever LJ: Schizophrenia spectrum personality disorders, in American Psychiatric Press Review of Psychiatry, vol 11. Edited by Tasman A, Riba M. Washington, DC, American Psychiatric Press, 1992, pp 25–42Google Scholar
5. Gur RC, Saykin AJ, Gur RE: Neuropsychological study of schizophrenia, in Schizophrenia Research: Advances in Neuropsychiatry and Psychopharmacology, vol 1. Edited by Tamminga CA, Schulz SC. New York, Raven Press, 1991, pp 153–162Google Scholar
6. Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O’Donnell B, Kimble M, Kikinis R, Jolesz FA: Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J Psychiatry 1993; 150:1849–1855Google Scholar
7. Saykin AJ, Gur RC, Gur RE, Mozley D, Mozley LH, Resnick SM, Kester B, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
8. Seidman LJ, Cassens GP, Kremen WP, Pepple JR: Neuropsychology of schizophrenia, in Clinical Syndromes in Adult Neuropsychology: The Practitioner’s Handbook. Edited by White RF. Amsterdam, Elsevier, 1992, pp 381–449Google Scholar
9. Seidman LJ, Talbot NL, Kalinowski AG, McCarley RW, Faraone SV, Kremen WS, Pepple JR, Tsuang MT: Neuropsychological probes of fronto-limbic dysfunction in schizophrenia: olfactory identification and Wisconsin Card Sorting Performance. Schizophr Res 1992; 6:55–65Crossref, Google Scholar
10. Thaker GK, Moran M, Lahti A, Adami H, Tamminga CA, Schulz SC: Pilot studies of schizotypal subjects, in Schizophrenia Research: Advances in Neuropsychiatry and Psychopharmacology, vol I. Edited by Tamminga CA, Schulz SC. New York, Raven Press, 1991, pp 201–208Google Scholar
11. Condray R, Steinhauer SR: Schizotypal personality disorder in individuals with and without schizophrenic relatives: similarities and contrasts in neurocognitive and clinical functioning. Schizophr Res 1992; 7:33–41Crossref, Medline, Google Scholar
12. Condray R, Steinhauer SR, Goldstein G: Language comprehension in schizophrenics and their brothers. Biol Psychiatry 1992; 32:790–802Crossref, Medline, Google Scholar
13. Voglmaier MM, Seidman LJ, Salisbury D, McCarley RW: Neuropsychological dysfunction in schizotypal personality disorder: a profile analysis. Biol Psychiatry 1997; 41:530–540Crossref, Medline, Google Scholar
14. Delis DC, Kramer JH, Kaplan E, Ober BA: California Verbal Learning Test Manual—Research Edition. San Diego, Psychological Corp, 1987Google Scholar
15. Lyons MJ, Toomey R, Seidman LJ, Kremen WS, Faraone SV, Tsuang MT: Verbal learning and memory in relatives of schizophrenics: preliminary findings. Biol Psychiatry 1995; 37:750–753Crossref, Medline, Google Scholar
16. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
17. Meehl PE: Nuisance variables and the ex post facto design, in Minnesota Studies of the Philosophy of Science. Edited by Radner M, Winoker S. Minneapolis, University of Minnesota Press, 1970, pp 373–402Google Scholar
18. Lyons MJ, Merla ME, Young L, Kremen WS: Impaired neuropsychological functioning in symptomatic volunteers with schizotypy: preliminary findings. Biol Psychiatry 1991; 30:424–426Crossref, Medline, Google Scholar
19. Brooker BH, Cyr JJ: Tables for clinicians to use to convert WAIS-R short forms. J Clin Psychol 1986; 42:983–986Crossref, Google Scholar
20. Beck AT: The Beck Depression Inventory. Philadelphia, Center for Cognitive Therapy, 1978Google Scholar
21. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
22. First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin L: Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II): User’s Guide. Washington, DC, American Psychiatric Press, 1997Google Scholar
23. Oldham JM, Skodol AE, Kellman HD, Hyler SE, Rosnick L, Davies M: Diagnosis of DSM-III-R personality disorders by two structured interviews: patterns of comorbidity. Am J Psychiatry 1992; 149:213–220Link, Google Scholar
24. Peterson LR: Short-term memory. Sci Am 1966; 215:90–95Crossref, Medline, Google Scholar
25. Caine ED, Ebert MH, Weingartner H: An outline for the analysis of dementia. Neurology 1977; 27:1087–1092Google Scholar
26. Benton AL, de Hamsher K, Spreen O: Contributions to Neuropsychological Assessment. Iowa City, University of Iowa, 1983Google Scholar
27. Milner B: Interhemispheric differences in the localization of psychological processes in man. Br Med Bull 1971; 27:272–277Crossref, Medline, Google Scholar
28. Benton AL, de Hamsher K: Multilingual Aphasia Examination. Iowa City, University of Iowa, 1976Google Scholar
29. Jones-Gotman M, Milner B: Design fluency: the invention of nonsense drawings after focal cortical lesions. Neuropsychologia 1977; 15:653–674Crossref, Medline, Google Scholar
30. Trahan DE, Larrabee GJ: Continuous Visual Memory Test Professional Manual. Odessa, Fla, Psychological Assessment Resources, 1988Google Scholar
31. Wechsler D: Wechsler Memory Scale—Revised Manual. San Diego, Psychological Corp, 1987Google Scholar
32. Seidman LJ, Stone WS, Jones R, Harrison RH, Mirsky A: Comparative effects of schizophrenia and temporal lobe epilepsy on memory. J Int Neuropsychol Soc 1998; 4:342–352Crossref, Medline, Google Scholar
33. Chapman LJ, Chapman JP: Strategies for resolving the heterogeneity of schizophrenics and their relatives using cognitive measures. J Abnorm Psychol 1989; 98:357–366Crossref, Medline, Google Scholar
34. Wechsler D: Wechsler Adult Intelligence Scale—Revised Manual. Cleveland, Psychological Corp, 1981Google Scholar
35. Lezak MD: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
36. Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, Tsuang MT: Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry 1998; 155:1358–1364Google Scholar
37. Weinberger DR, Berman KF, Suddath R, Torrey EF: Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 1992; 149:890–897Link, Google Scholar
38. Milner B: Memory and medial temporal regions of the brain, in Biology of Memory. Edited by Pribram KH, Broadbent DE. New York, Academic Press, 1970, pp 29–50Google Scholar
39. Milner B: Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg 1972; 19:421–446Crossref, Medline, Google Scholar
40. Kapur N: Pattern of verbal memory deficits in patients with bifrontal pathology and patients with third ventricle lesions, in Practical Aspects of Memory: Current Research and Issues, vol 2. New York, John Wiley & Sons, 1988, pp 10–15Google Scholar
41. Miceli G, Caltagirone C, Gainotti G, Masullo C, Silveri MC: Neuropsychological correlates of localized cerebral lesions in non-aphasic brain-damaged patients. J Clin Neuropsychol 1981; 3:53–63Crossref, Medline, Google Scholar
42. Crosson B, Novak TA, Trenery MR, Craig PL: Differentiation of verbal memory deficits in blunt head injury using the recognition trial of the California Verbal Learning Tests: an exploratory study. Clin Neuropsychol 1989; 3:29–44Crossref, Google Scholar



