MRI Study of Cavum Septi Pellucidi in Schizophrenia, Affective Disorder, and Schizotypal Personality Disorder
Abstract
OBJECTIVE: A cavum between the septi pellucidi may reflect neurodevelopmental anomalies in midline structures of the brain. The authors examined cavum septi pellucidi in subjects with schizophrenia, affective disorder, and schizotypal personality disorder and in normal subjects. METHOD: Thirty schizophrenic patients (15 chronic, 15 first-episode), 16 patients with affective disorder (first-episode), 21 patients with schizotypal personality disorder, and 46 normal subjects were evaluated with magnetic resonance imaging. Cavum septi pellucidi was assessed by counting the number of 1.5-mm slices containing cavum septi pellucidi. RESULTS: The presence or absence of cavum septi pellucidi did not differentiate among groups. However, the prevalence of abnormal cavum septi pellucidi (i.e., cavum septi pellucidi contained on four or more slices) was 30.4% for schizophrenic patients (36.4% for chronic, 25.0% for first-episode), 20.0% for patients with affective disorder, 18.8% for patients with schizotypal personality disorder, and 10.3% for normal subjects. When the authors used the Nopoulos et al. criteria for rating cavum septi pellucidi, which omitted borderline cases with cavum septi pellucidi on three slices, the prevalence of abnormal cavum septi pellucidi increased to 35.0% for schizophrenia (40.0% for chronic, 30.0% for first-episode), 25.0% for affective disorder, 27.3% for schizotypal personality disorder, and 13.0% for normal subjects. There was a statistically significant difference in ratings between schizophrenic and normal subjects. CONCLUSIONS: The results suggest that alterations in midline structures during the course of neurodevelopment may play a role in the pathogenesis of schizophrenia.
The cavum septi pellucidi is the space between the two leaflets of the septum pellucidum (1). Examples of “fused” and cavum septi pellucidi can be seen in figure 1 (fused in right panel, cavum in left panel [see black arrow]). In normal development it closes in 15% of humans within 1 month of birth, with 85% closure within 6 months of life (1, 2). This fusion likely results from rapid growth of the hippocampus and corpus callosum. Incomplete fusion results in the persistence of a cavum, which, in turn, reflects possible neurodevelopmental anomalies of these midline structures (1–3). Two cavum septi pellucidi anomalies have been described: “noncommunicating,” in which the walls of the cavum are intact and do not “communicate” with the lateral ventricular system, and “communicating,” in which the cavum opens to the lateral ventricular system. The latter is the result of later-developing internal hydrocephalus, or acquired/secondary cavum septi pellucidi due to blows to the head, as in, for example, professional and semiprofessional boxers (2, 4). Here, we focus on noncommunicating cavum septi pellucidi, since this is thought to be neurodevelopmental in origin.
Because abnormalities in temporal lobe structures, including the hippocampus, have been reported in schizophrenia (see references 4–9), and are thought by some to be at least partly neurodevelopmental in origin (see references 10–13), it would be important to evaluate more closely cavum septi pellucidi abnormalities in schizophrenia, including the relationship between cavum septi pellucidi and temporal lobe/clinical measures. We note that cavum septi pellucidi varies tremendously in normal adults, i.e., 2%–4% in early pneumoencephalographic studies (14, 15), 0.15%–28% in computerized tomography (CT) studies (16–18), 5.1%–58.7% in magnetic resonance (MR) studies (19–23), and 12%–31% in postmortem studies (2, 24, 25), suggesting that the criterion for “abnormal” versus “normal variant” is an important distinction (see references 22 and 25); i.e., small cavum septi pellucidi is likely a normal variant (2, 23, 24).
In the present study we evaluated temporal lobe structures and cavum septi pellucidi in chronic schizophrenic patients. We included patients with affective disorder as a contrast group because research suggests that they may have an underlying neurodevelopmental abnormality (26), reduced hippocampal volume (27), and possibly increased cavum septi pellucidi (21, 28). We also included subjects with schizotypal personality disorder because this disorder may be genetically linked to schizophrenia (29–31). Such a link is strongly supported by neurochemical (32) and attention/information processing studies (33). In addition, we included patients with schizotypal personality disorder because this disorder may provide important information not confounded by factors associated with schizophrenia, such as chronic illness and long-term medication. This study is the first to evaluate cavum septi pellucidi in schizotypal personality disorder, as well as the first to evaluate the relationship between cavum septi pellucidi and temporal lobe structures in chronic schizophrenic patients.
METHOD
Subjects
Subjects were 1) 15 male chronic schizophrenic patients and 15 male normal subjects; 2) 15 first-psychotic-episode schizophrenic patients (12 men and three women), 16 first-psychotic-episode patients with affective disorder (13 men and three women), and 18 normal subjects (16 men and two women); and 3) 21 male patients with schizotypal personality disorder and 13 male normal subjects.
Chronic schizophrenic and normal subjects were recruited from and tested at the Brockton Veterans Administration Medical Center, except for the MR imaging (MRI) scans, which were performed at Brigham and Women's Hospital. First-episode patients and normal subjects were recruited from and tested at the McLean/Harvard first psychosis project (34). Patients with schizotypal personality disorder and normal subjects were recruited from and tested at Massachusetts Mental Health Center, except for MRI scans, which were performed at Brigham and Women's Hospital. Patients with schizotypal personality disorder were recruited from a newspaper advertisement soliciting individuals who believed they had a sixth sense and who were anxious around people.
Chronic schizophrenic patients met DSM-III-R criteria for schizophrenia on the basis of interview with the Schedule for Affective Disorders and Schizophrenia—Lifetime Version (35) and information from psychiatric records. First-episode patients met DSM-III-R criteria for schizophrenia or affective disorder on the basis of interviews with the Structured Clinical Interview for DSM-III-R—Patient Version (36) and information from psychiatric records. DSM-III-R diagnoses of schizotypal personality disorder were based on interviews with the Structured Clinical Interview for DSM-III-R Personality Disorders (37). Interviews were conducted by a psychiatrist (C.C.D.), one of three psychologists (including M.E.S.), or a trained medical student. In addition, for more than 90% of the study group, one of us (R.W.M.) conducted a clinical interview. Diagnostic reliability is computed routinely every 6 months through use of a weighted kappa statistic (range=0.85 to higher than 0.95).
Subjects were 20–55 years old; had no history of ECT, neurologic illness, major head trauma, or alcohol and/or drug abuse within the past 5 years; and had no history of drug and/or alcohol dependence ever. Normal subjects were recruited through newspaper advertisement and were screened to exclude drug or alcohol abuse and neurologic and psychiatric illness in themselves or in their first-degree relatives. In addition, normal groups of subjects for patients with schizotypal personality disorder and first-episode patients received the Structured Clinical Interview for DSM-III-R—Non-Patient Edition (38) in order to rule out major psychopathology. All subjects were right-handed. After a complete description of the study to subjects, written informed consent was obtained.
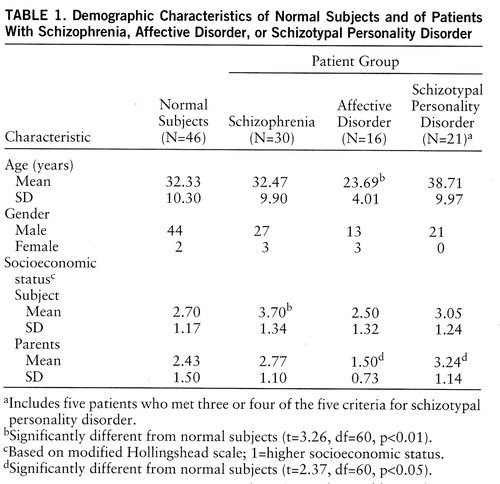
Demographic data for subjects are shown in table 1. There were no statistically significant differences in age or parental socioeconomic status between schizophrenic patients and normal subjects, but the socioeconomic status of schizophrenic patients was, as expected, significantly lower. For the first-episode schizophrenic patients, mean age and mean score for socioeconomic status and parental socioeconomic status did not differ from those of normal subjects; however, mean age for patients with first-episode affective disorder was significantly less than that of normal subjects. In addition, parental socioeconomic status of patients with first-episode affective disorder was higher than that of normal subjects, whereas parental socioeconomic status of patients with schizotypal personality disorder was lower than that of normal subjects. Of note, patients with first-episode affective disorder did not differ in age or parental socioeconomic status from first-episode schizophrenic patients, and patients with schizotypal personality disorder did not differ from schizophrenic patients in age or parental socioeconomic status.
Clinical Measures
Patients with chronic schizophrenia and schizotypal personality disorder were evaluated with the Scale for the Assessment of Positive Symptoms (SAPS) (39), the Scale for the Assessment of Negative Symptoms (SANS) (40), and the Thought Disorder Index (41). First-episode patients were evaluated with the Brief Psychiatric Rating Scale (BPRS) (42) and the Global Assessment Scale (GAS) (43).
MRI Scanning
MR images were acquired at Brigham and Women's Hospital for patients with chronic schizophrenia and schizotypal personality disorder and their normal comparison groups. MR images for first-episode patients and their normal comparison group were acquired at McLean Hospital. At both sites, the same 1.5-Tesla GE system was used, and acquisition parameters were as follows: TE=5 msec, TR=35 msec, one repetition, nutation angle=45°, field of view=24 cm, acquisition matrix=256×256×124, voxel dimensions=0.9375×0.9375×1.5 mm (see reference 5). These images, reformatted in the coronal plane, were 1.5-mm thick and consisted of 124 contiguous coronal slices.
Cavum Septi Pellucidi Measures
Number of slices containing cavum septi pellucidi. We used the same method for rating cavum septi pellucidi as Nopoulos et al. (23): 0=absence, 1=cavum seen in 1 slice of brain, 2=cavum seen in 2 slices, and so forth. The length of the cavum could thus be calculated by multiplying the number of slices containing cavum septi pellucidi by 1.5 mm.
Criteria for normal and abnormal cavum septi pellucidi. Cavum septi pellucidi on one–three slices was categorized as normal, and on four or more slices as abnormal. Our rationale was that cavum septi pellucidi contained on four or more slices (6 mm) is consistent with findings from postmortem data showing that abnormal cavum septi pellucidi has a length, on average, of 7.5 mm (44). Figure 1 provides an example of abnormal (left panel) and normal (right panel) cavum septi pellucidi. In a second classification of normal versus abnormal cavum septi pellucidi, we used the criteria of Nopoulos et al. (23), which classified one–two slices containing cavum septi pellucidi as small (normal) and four or more as large (abnormal) (0 slices and three slices were omitted; the latter was considered borderline).
Interrater reliability. Reliability was computed for the first 32 subjects by four independent raters (J.S.K., Y.H., I.A.F., and C.C.D.), blind to group membership, by using an intraclass correlation coefficient (r=0.97). The remaining subjects were rated by three independent raters (J.S.K., I.A.F., and Y.H.), also blind to group membership (r=0.93). In the small number of cases in which there was not complete agreement, ratings given by the majority of the raters were used for the analyses.
Volume measures. For the chronic schizophrenia data set only, temporal lobe regions of interest were manually outlined on a work station for each slice containing the region of interest (5). Voxels were then summed to calculate volumes. Relative volumes were calculated for anterior superior temporal gyrus, posterior superior temporal gyrus, parahippocampal gyrus, anterior hippocampus-amygdala complex, and posterior hippocampus. The average intraclass correlation coefficient for these volumes was 0.86 (5).
Data Analysis
We used chi-square test, or Fisher's exact test when expected cell sizes were less than five. Relationships between ratings of cavum septi pellucidi and volume of temporal lobe structures, as well as between cavum septi pellucidi and psychopathologic assessment scales, were assessed through use of Spearman's rho. A level of 0.05 was accepted as the level of significance.
RESULTS
Table 2 shows the presence of cavum septi pellucidi for all groups. The presence or absence of cavum septi pellucidi did not differentiate between groups. We used two approaches for categorizing the presence of cavum septi pellucidi as abnormal or normal. First, we categorized cavum septi pellucidi one–three slices as normal, and cavum septi pellucidi on four or more slices as abnormal. This rating leaves out 0 and is based on postmortem findings suggesting that the average length of abnormal cavum septi pellucidi is 7.5 mm (44) (see previous discussion). Using this rating scheme, we found that abnormal cavum septi pellucidi was present in 30.4% of schizophrenic patients, 20.0% of patients with affective disorder, 18.8% of patients with schizotypal personality disorder, and 10.3% of normal subjects (table 3). When the schizophrenic patients were subdivided further, 36.4% of the chronic and 25.0% of the first-episode patients showed abnormal cavum septi pellucidi. Schizophrenic patients showed more abnormal cavum septi pellucidi than did normal subjects (p=0.05, Fisher's exact test, one-tailed).
We next categorized cavum septi pellucidi as normal or abnormal on the basis of the criteria of Nopoulos et al. (23) (0 slices and three slices were excluded; the latter was considered borderline). Accordingly, abnormal cavum septi pellucidi was present in 35.0% of schizophrenic patients, 25.0% of patients with affective disorder, 27.3% of patients with schizotypal personality disorder, and 13.0% of normal subjects. When we subdivided the schizophrenic patients further, 40.0% of the chronic and 30.0% of the first-episode patients showed abnormal cavum septi pellucidi (schizophrenic patients differed from normal subjects; p=0.07, Fisher's exact test, one-tailed) (table 3).
Chronic schizophrenic patients showed negative correlations between the number of slices containing cavum septi pellucidi and relative volume of left (p=0.02) and right (p=0.02) hippocampus (figure 2). No other correlations between cavum septi pellucidi ratings and temporal lobe measures (i.e., amygdala, parahippocampal gyrus, superior temporal gyrus, or overall temporal lobe) were significant. Moreover, no correlations between temporal lobe volumes and cavum septi pellucidi reached statistical significance in normal subjects.
In chronic schizophrenic patients there were no significant correlations between cavum septi pellucidi ratings and scores on the SAPS (r=0.09, N=15, p=0.82), SANS (r=0.26, N=15, p=0.49), or Thought Disorder Index (r=0.08, N=15, p=0.80). In first-episode schizophrenic patients, BPRS and GAS scores showed no significant correlations with cavum septi pellucidi ratings (r=0.27, N=15, p=0.35 and r=–0.09, N=15, p=0.76, respectively). For patients with affective disorder, there also were no significant correlations between cavum septi pellucidi ratings and BPRS scores (r=–0.17, N=16, p=0.53) or GAS scores (r=0.46, N=16, p=0.07). For patients with schizotypal personality disorder, there were no significant correlations between cavum septi pellucidi ratings and scores on the SAPS (r=–0.13, N=21, p=0.62), SANS (r=–0.21, N=21, p=0.41), or Thought Disorder Index (r=0.11, N=21, p=0.69). Finally, we assessed the correlations between cavum septi pellucidi ratings and age (r=0.10, N=113, p=0.28), socioeconomic status (r=–0.05, N=113, p=0.61), and parental socioeconomic status (r=0.12, N=113, p=0.18); none was significant.
DISCUSSION
Eighty-five percent of normal subjects, 77% of schizophrenic patients, 63% of patients with affective disorder, and 76% of patients with schizotypal personality disorder showed some degree of cavum septi pellucidi. Thus, regardless of diagnosis, most subjects evinced cavum septi pellucidi. These rates are high compared to those of other studies that used CT or MRI (16, 19–23, 25), which have reported cavum septi pellucidi in 0.15% to 58.8% of schizophrenic patients. All of these studies, except that of Nopoulos et al. (23), used imaging techniques with less spatial resolution than in our study. The study by Nopoulos et al. was, in fact, the first to use 1.5-mm contiguous slices, and they reported cavum septi pellucidi in 58.7% of normal subjects and 58.8% of schizophrenic patients. Their figures are lower than the percentages reported in the current study (i.e., 85% of normal subjects, 77% of schizophrenic patients). This discrepancy may be due to the higher proportion of men in our study (see later discussion). It would appear, nonetheless, that the presence of cavum septi pellucidi, in and of itself, may have no pathological significance.
We next considered the rating of 6 mm or more of cavum septi pellucidi as abnormal (corresponding to cavum septi pellucidi on four or more slices). Using this criterion, we found that 30.4% of schizophrenic patients (36.4% of chronic and 25.0% of first-episode patients) and 10.3% of normal subjects showed abnormal cavum septi pellucidi. This finding is consistent with previous studies (19, 22, 23). Ratings of cavum septi pellucidi on four or more slices would therefore seem appropriate for classifying cavum septi pellucidi as abnormal, although it would, of course, need to be validated with a new group of subjects.
When we used the criteria of Nopoulos et al. (23), we noted an increase in abnormal cavum septi pellucidi: 35.0% of schizophrenic patients (40.0% of chronic and 30.0% of first-episode patients) and 13.0% of normal subjects had abnormal cavum septi pellucidi. Degreef et al. (25), in a postmortem study, reported abnormal cavum septi pellucidi in 32.1% of schizophrenic patients and 7.7% of normal subjects; these figures are similar to ours.
Eighteen percent of patients with schizotypal personality disorder showed abnormal cavum septi pellucidi on the basis of our criteria, in contrast to 27.3% on the basis of the Nopoulos et al. (23) criteria. These ratings were intermediate between those of schizophrenic and normal subjects; this finding is consistent with a study in our laboratory of neuropsychological performance in patients with schizotypal personality disorder that was intermediate between that of schizophrenic and normal subjects (45). It is also consistent with the report by Trestman et al. (46) of auditory P300 amplitude reduction in patients with schizotypal personality disorder that was intermediate between that of normal and schizophrenic subjects. These findings suggest that schizotypal personality disorder may be a milder form on a continuum of schizophrenia spectrum disorders.
Abnormal cavum septi pellucidi was evident in 20.0% of patients with first-episode affective disorder. It would, however, be premature to conclude that the occurrence of abnormal cavum septi pellucidi in affective disorder is not different from that in normal subjects, particularly in light of the fact that our group was small. Shioiri et al. (28), however, reported that cavum septi pellucidi in patients with bipolar disorder was not higher than that in normal subjects when small cavum septi pellucidi was excluded from the analysis; but the rate was higher than that in normal subjects when all ratings were included. Jurjus et al. (21) reported a lower frequency of cavum septi pellucidi in patients with affective disorder than in schizophrenic patients (25% versus 10%); there was no difference between patients with affective disorder and normal subjects, which they attributed to a gender effect (i.e., their study had an excess of male schizophrenic subjects). Others have also reported a higher rate of cavum septi pellucidi in male than in female subjects (16, 21–23, 25). Nopoulos et al. (23), for example, reported cavum septi pellucidi in 67% of male subjects, compared to 46% of female subjects. This gender effect may be evident in other neurodevelopmental abnormalities (11, 47). Eight of our group of 113 subjects were female: two were normal subjects, three first-episode schizophrenic patients, and three patients with affective disorder. One of the eight women, a schizophrenic patient, did show abnormal cavum septi pellucidi. More careful studies, with a larger cohort of female subjects, will be needed to address further the issue of gender effects.
The first report of an association between cavum septi pellucidi and psychosis was done through use of CT (16). This association was replicated by Mathew et al. (48) through use of MR, and there have been several MR studies of cavum septi pellucidi (reviewed in reference 49). More recently, Nopoulos et al. (50) reported an association between abnormal cavum septi pellucidi and left temporal lobe volume reduction in male schizophrenic patients. In their first study (23), six schizophrenic patients showed abnormal cavum septi pellucidi. This group, along with four new patients with abnormal cavum septi pellucidi, was the subject of the second study. In that study, 44 schizophrenic patients without cavum septi pellucidi were compared with 10 schizophrenic patients with abnormal cavum septi pellucidi. The schizophrenic patients with abnormal cavum septi pellucidi showed volume reduction only in the left temporal lobe, as well as a less severe decrease in total brain tissue, normal-sized frontal lobes, and pronounced asymmetry (right greater than left). In contrast, the schizophrenic patients with normal cavum septi pellucidi showed reduced volumes in overall brain and an increase in overall CSF. These investigators suggested that the pattern of structural abnormalities observed in schizophrenic patients with abnormal cavum septi pellucidi may reflect a regional, localized tissue reduction confined to the left temporal lobe in a subgroup of male schizophrenic patients.
In our study, we found an association between degree of cavum septi pellucidi and volume of hippocampus in male chronic schizophrenic patients. This finding is consistent with the hypothesis that growth of both the hippocampus and corpus callosum during fetal development results in a pushing together of the leaflets of septi pellucidi, which fuse; abnormalities in this process may lead to a lack of fusion (cavum septi pellucidi). Of note, the septum pellucidum is an important relay station with the hippocampus and hypothalamus, as well as being part of the limbic system that regulates emotion and motivational behavior (51, 52). Disturbances in this system may, therefore, lead to distortions of perception, cognition, and behavior seen in schizophrenia.
In conclusion, abnormal cavum septi pellucidi was confirmed in schizophrenia, and we noted an association between cavum septi pellucidi and hippocampal volume. These findings suggest that for at least a subgroup of patients, neurodevelopmental origins may be implicated in the pathogenesis of schizophrenia. A further study of patients with and without abnormal cavum septi pellucidi should help to clarify further the nature of these brain anomalies.
 |
 |
 |
Received April 24, 1997; revisions received Sept. 17 and Oct. 27, 1997; accepted Nov. 6, 1997. From the Clinical Neuroscience Division, Laboratory of Neuroscience, Department of Psychiatry, Brockton VA Medical Center and Harvard Medical School, Boston; the Brain Imaging Center and the Bipolar and Psychotic Disorders Program, McLean Hospital, Harvard Medical School, Belmont, Mass.; and the Surgical Planning Laboratory, MRI Division, Brigham and Women's Hospital, Department of Radiology, Harvard Medical School, Boston. Address reprint requests to Drs. McCarley or Shenton, Department of Psychiatry (116A), VA Medical Center-Brockton/West Roxbury, Harvard Medical School, 940 Belmont St., Brockton, MA 02401; [email protected]; [email protected] (e-mail). Supported in part by a Medical Research Service and a Schizophrenia Center grant through the Department of Veterans Affairs (Dr. McCarley) and by NIMH grants MH-40799 (Dr. McCarley), MH-01110 (Dr. Shenton), MH-50747 (Dr. Shenton), and MH-51948 (Dr. Tohen).

FIGURE 1. MR Images of Large Cavum Septi Pellucidi in Schizophrenic Patients (left panel) and No Cavum Septi Pellucidi in Normal Subjects (right panel)
aSame slice number on lower part of pictures. Lower parts of pictures show changes in cavum septi pellucidi in 1.5-mm serial slices containing cavum septi pellucidi.

FIGURE 2. Correlations Between Number of Slices With Cavum Septi Pellucidi and Relative Volumes of Lefta and Rightb Hippocampus in Chronic Schizophrenic Patients
ar=–0.61, N=15, p=0.02.
br=–0.60, N=15, p=0.02.
1 Sarwar M: The septum pellucidum: normal and abnormal. Am J Neuroradiol 1989; 10:989–1005Medline, Google Scholar
2 Shaw CM, Alvord EC: Cavum septi pellucidi et vergae: their normal and pathological state. Brain 1969; 92:213–224Crossref, Medline, Google Scholar
3 Rakic P, Yakovlev PI: Development of corpus callosum and cavum septi in man. J Comp Neurol 1968; 132:355–362Crossref, Google Scholar
4 Hoff AL, Neal C, Kushner M, DeLisi LE: Gender differences in corpus callosum size in first-episode schizophrenia. Biol Psychiatry 1994; 35:913–919Crossref, Medline, Google Scholar
5 Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
6 Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA: Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res 1990; 35:1–13Crossref, Medline, Google Scholar
7 Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyrus volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Link, Google Scholar
8 Rossi A, Stratta P, Mancini F, Gallucci M, Mattei P, Core L, Di Michele V, Casacchia M: Magnetic resonance imaging findings of amygdala-anterior hippocampus shrinkage in male patients with schizophrenia. Psychiatry Res 1994; 52:43–53Crossref, Medline, Google Scholar
9 Raine A, Harrison GN, Reynolds GP, Sheard C, Cooper JE, Medley I: Structural and functional characteristics of the corpus callosum in schizophrenics, psychiatric controls, and normal controls. Arch Gen Psychiatry 1990; 47:1060–1064Crossref, Medline, Google Scholar
10 Rossi A, Stratta P, Gallucci M, Passariello R, Casacchia M: Quantification of corpus callosum and ventricles in schizophrenia with nuclear magnetic resonance imaging: a pilot study. Am J Psychiatry 1989; 146:99–101Link, Google Scholar
11 Andreasen NC, Nasrallah HA, Dunn V, Olson SC, Grove WM, Ehrhardt JC, Coffman JA, Crossett JH: Structural abnormalities in the frontal system in schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry 1986; 43:136–144Crossref, Medline, Google Scholar
12 DeLisi LE, Lieberman J: Longitudinal perspective on the pathophysiology of schizophrenia: examining the neurodevelopmental versus neurodegenerative hypotheses. Schizophr Res 1991; 5:183–210Crossref, Medline, Google Scholar
13 Murray RM, O'Callaghan E, Castle J, Lewis SW: A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull 1992; 18:319–332Crossref, Medline, Google Scholar
14 Bonitz G: Zur klinisch-diagnostischen Bedeutung des erweiterten und kommunizierenden Cavum septi pellucidi. Nervenarzt 1969; 40:121–128Medline, Google Scholar
15 Sonntag I, Nadjimi M, Lajosi F, Fuchs G: Anlagebedingte Gehirnanomalien der Mittellinie. Nervenarzt 1971; 42:531–539Medline, Google Scholar
16 Lewis SW, Mezey GC: Clinical correlates of septum pellucidum cavities: an unusual association with psychosis. Psychol Med 1985; 15:43–54Crossref, Medline, Google Scholar
17 Nakano S, Hojo H, Kataoka K, Ramasak S: Age related incidence of cavum septi pellucidi and cavum vergae on CT scans of pediatric patients. J Comput Assist Tomogr 1981; 5:348–349Crossref, Medline, Google Scholar
18 Corsellis JN, Bruton CJ, Freeman-Browne D: The aftermath of boxing. Psychol Med 1973; 3:270–303Crossref, Medline, Google Scholar
19 Degreef G, Lantos G, Bogerts B, Ashtari M, Lieberman J: Abnormalities of the septum pellucidum on MR scans in first-episode schizophrenic patients. Am J Neuroradiol 1992; 13:835–840Medline, Google Scholar
20 Scott TF, Price TP, George MS, Brillman J, Rothfus W: Midline cerebral malformations and schizophrenia. J Neuropsychiatry Clin Neurosci 1993; 5:287–293Crossref, Medline, Google Scholar
21 Jurjus GJ, Nasrallah HA, Olson SC, Schwarzkopf SB: Cavum septum pellucidum in schizophrenia, affective disorder and healthy controls: a magnetic resonance imaging study. Psychol Med 1993; 23:319–322Crossref, Medline, Google Scholar
22 DeLisi LE, Hoff AL, Kushner M, Degreef G: Increased prevalence of cavum septum pellucidum in schizophrenia. Psychiatry Res Neuroimaging 1993; 50:193–199Crossref, Medline, Google Scholar
23 Nopoulos P, Swayze V, Flaum M, Ehrhardt JC, Yuh WT, Andreasen NC: Cavum septi pellucidi in normals and patients with schizophrenia as detected by magnetic resonance imaging. Biol Psychiatry 1997; 41:1102–1108Crossref, Medline, Google Scholar
24 Schwidde JT: Incidence of cavum septi pellucidi and cavum vergae in 1,032 human brains. Arch Neurol Psychiatry 1952; 67:625–633Crossref, Google Scholar
25 Degreef G, Bogerts B, Falkai P, Greve B, Lantos G, Ashtari M, Lieberman J: Increased prevalence of the cavum septum pellucidum in magnetic resonance scans and post-mortem brains of schizophrenic patients. Psychiatry Res Neuroimaging 1992; 45:1–13Crossref, Medline, Google Scholar
26 Nasrallah HA: Neurodevelopmental aspects of bipolar affective disorder. Biol Psychiatry 1991; 9:1–2Crossref, Google Scholar
27 Swayze VW II, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC: Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 1992; 31:221–240Crossref, Medline, Google Scholar
28 Shioiri T, Oshitani Y, Kato T, Murashita J, Hamakawa H, Inubushi T, Nagata T, Takahashi S: Prevalence of cavum septum pellucidum detected by MRI in patients with bipolar disorder, major depression and schizophrenia. Psychol Med 1996; 26:431–434Crossref, Medline, Google Scholar
29 Kendler KK, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D: The Roscommon family study, I: methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch Gen Psychiatry 1993; 50:527–540Crossref, Medline, Google Scholar
30 Kendler KK, McGuire M, Gruenberg AM, Spellman M, O'Hare A, Walsh D: The Roscommon family study, II: the risk of nonschizophrenic nonaffective psychoses in relatives. Arch Gen Psychiatry 1993; 50:645–652Crossref, Medline, Google Scholar
31 Kendler KK, Diehl SR: The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull 1993; 2:261–285Crossref, Google Scholar
32 Siever LJ: Schizophrenia spectrum personality disorder, in American Psychiatric Press Review of Psychiatry, vol 11. Edited by Tasman A, Riba M. Washington, DC, American Psychiatric Press, 1992, pp 25–42Google Scholar
33 Kremen WS, Seidman LJ, Pepple JR, Lyons MJ, Tsuang MT, Fa~raone SV: Neuropsychological risk indicators for schizophrenia: a review of family studies. Schizophr Bull 1994; 20:103–119Crossref, Medline, Google Scholar
34 Tohen M, Stol IA, Strakowski S, Faedda G, Maye RP, Goodwin D, Kolbrener M, Madigan A: The McLean first-episode psychosis project: six month recovery and recurrence outcome. Schizophr Bull 1992; 18:273–282Crossref, Medline, Google Scholar
35 Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia—Lifetime Version, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
36 Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version 1.0 (SCID-P). Washington, DC, American Psychiatric Press, 1990Google Scholar
37 Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Washington, DC, American Psychiatric Press, 1990Google Scholar
38 Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
39 Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
40 Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1981Google Scholar
41 Johnston MH, Holzman PS: Assessing Schizophrenic Thinking: A Clinical and Research Instrument for Measuring Thought Disorder. San Francisco, Jossey-Bass, 1979Google Scholar
42 Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
43 Endicott J, Spitzer RL, Fleiss JL, Cohen J: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33:766–771Crossref, Medline, Google Scholar
44 Shunk H: Congenital dilatations of the septi pellucidum. Radiology 1963; 81:610–618Crossref, Medline, Google Scholar
45 Voglmaier MM, Seidman LJ, Salisbury D, McCarley RW: Neu~ropsychological dysfunction in schizotypal personality disorder: a profile analysis. Biol Psychiatry 1997; 41:530–540Crossref, Medline, Google Scholar
46 Trestman RL, Horvath T, Kalus O, Peterson AE, Coccaro E, Mi~tropoulou V, Apter S, Davidson M, Siever LJ: Event-related potentials in schizotypal personality disorder. J Neuropsychiatry Clin Neurosci 1996; 8:33–40Crossref, Medline, Google Scholar
47 Flaum M, Arndt S, Andreasen NC: The role of gender in studies of ventricular enlargement in schizophrenia: a predominantly male effect. Am J Psychiatry 1990; 147:1327–1332Link, Google Scholar
48 Mathew RJ, Partain CL, Logan TP, Wilson WH: A study of the septum pellucidum and corpus callosum in schizophrenia with MR imaging. Acta Psychiatr Scand 1985; 72:414–421Crossref, Medline, Google Scholar
49 Shenton ME, Wible CG, McCarley RW: A review of magnetic resonance imaging studies of brain abnormalities in schizophrenia, in Brain Imaging in Clinical Psychiatry. Edited by Krishnan KRR, Doraiswamy PM. New York, Marcel Dekker, 1997, pp 297–380Google Scholar
50 Nopoulos P, Swayze V, Andreasen NC: Pattern of brain morphology in patients with schizophrenia and large cavum septi pellucidi. J Neuropsychiatry Clin Neurosci 1996; 8:147–152Crossref, Medline, Google Scholar
51 Andy OJ, Stephan H: The septum in the human brain. J Comp Neurol 1968; 133:383–410Crossref, Medline, Google Scholar
52 Raisman G: The connections of the septum. Brain 1966; 89:317–348Crossref, Medline, Google Scholar



