The Hippocampal Formation in Schizophrenia
Abstract
The hippocampal formation is one of the most extensively studied regions of the brain, with well-described anatomy and basic physiology; moreover, aspects of human memory mediated by the hippocampus are well characterized. In schizophrenia, alterations in hippocampal anatomy, perfusion, and activation are consistently reported; impairments in declarative memory function, especially in the flexible use of event memories (e.g., in the service of memory-based inference), are common. Postmortem molecular changes suggest a selective reduction in glutamate transmission in the dentate gyrus and in its efferent fibers, the mossy fiber pathway. A reduction in dentate gyrus glutamatergic output and in its information processing functions could generate two co-occurring outcomes in the hippocampus: 1) a change in homeostatic plasticity processes in cornu ammonis 3 (CA3), accompanied by increased activity due to reduced afferent stimulation from the dentate gyrus onto CA3 neurons, a process that could increase the pattern completion functions of CA3, and 2) the loss of mnemonic functions specific to the dentate gyrus, namely pattern separation, a change that could increase the prevalence of illusory pattern completion and reduce discrimination between present and past experiences in memory. The resulting increase in “runaway” CA3-mediated pattern completion could result in cognitive “mistakes,” generating psychotic associations and resulting in memories with psychotic content. Tests of this model could result in novel approaches to the treatment of psychosis and declarative memory alterations and in novel animal preparations for basic schizophrenia research.
The unique anatomy of the hippocampus distinguishes it in animal and human brains (Figure 1). Its role came abruptly to the attention of the larger scientific world in the early 1950s when Henry Molaison, at age 27, had a bilateral medial temporal lobe resection to treat an intractable seizure disorder. While the operation successfully brought his seizures under control, it left Molaison without the ability to make new declarative memories (1, 2). While his amnesia was particularly dense because of the extensive nature of his resection, conditions typically involving less extensive hippocampal damage, such as medial temporal lobe stroke or hippocampal sclerosis (3), were subsequently also associated with declarative memory loss. These untoward human conditions complement data from animal models (4, 5) that demonstrate the dependence of declarative memory—memory for events and facts—on the hippocampus proper and surrounding medial temporal lobe cortex. These observations have motivated several generations of scientists to examine the role of the hippocampus in memory (5–16); this study has advanced knowledge to support the idea that the hippo-campus has a discrete role in normal mnemonic function and demonstrates pathology in many human diseases of memory.
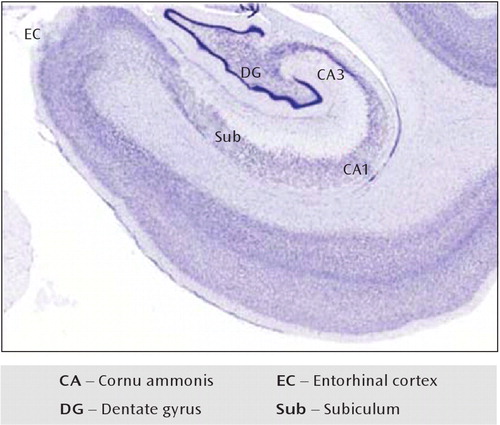
FIGURE 1. Tissue From a Human Hippocampus, With Nissl Staininga
aThe medial temporal lobe includes the entorhinal cortex, which serves to channel information from regions of the parahippocampal gyrus into the hippocampus proper through the perforant pathway, projecting to the dentate gyrus; the granule cell mossy fiber pathway from dentate gyrus projects to pyramidal neurons of CA3, and CA3 pyramidal neurons project to CA1 via the Schäffer collateral pathway. The subiculum is the output region of the hippocampus.
The clinical implications of hippocampal damage were initially examined in populations demonstrating frank memory impairments, such as individuals with Alzheimer's disease (17), normal aging (18), or epilepsy (19). More recently, the presence of hippocampal dysfunction has become apparent in some psychiatric conditions, including depression (20), posttraumatic stress disorder (21), drug abuse (22), and now schizophrenia (23–25). In schizophrenia, the association between dysfunction of the hippocampus and manifestations of the illness has become convincing, with evidence of altered function developed in many laboratories (26–30). The substantial growth in fundamental knowledge of normal hippocampal anatomy and physiology has invited novel formulations of hippocampal dysfunction in schizophrenia. In this article, we review the literature documenting hippocampal impairment in schizophrenia, and we propose how known structural and molecular changes may give rise to functional deficits in the syndrome.
The Hippocampal Formation: A Structure Specialized for Conjunctive Memory Formation and Pattern Completion
The integrity of the hippocampal formation is important for declarative memory but not for other kinds of memory (e.g., skill and habit memory, classical conditioning, and priming) (1, 13, 14, 31). The hippocampus is responsible for 1) the fast binding of inputs from multiple neocortical regions (conjunctive encoding), wherein the array of features that constitute an event are bound into an integrated, but flexibly addressable, memory trace (11, 12, 15), and 2) the subsequent reinstatement (retrieval) of previously learned input patterns (12, 32–34). At retrieval, conjunctive representations may permit associative recognition, inferential reasoning, and event recollection through pattern completion mechanisms that result in retrieval of an extended representation from partial input. The anatomy of the hippo-campus exquisitely complements its function (35).
The medial temporal lobe consists of the hippocampal formation—the cornu ammonis 1–3 (CA1–3), dentate gyrus, and subiculum—and the surrounding perirhinal, parahippocampal, and entorhinal cortex; its component structures are arranged hierarchically and topographically (35–37) (Figure 2). The hippocampal formation is a convergence zone, wherein unimodal and polymodal neo-cortical outputs ultimately come together. Specifically, the outputs from polymodal association and sensory neo-cortex provide the dominant projections to the perirhinal and parahippocampal cortex, which in turn project to the entorhinal cortex. The primary projections to the hippo-campus come from the entorhinal cortex 1) through the perforant pathway to the dentate gyrus and 2) through direct projections to hippocampal subfields CA3 and CA1. The perforant path is the first synapse in the hippocampal formation's largely unidirectional trisynaptic pathway: the entorhinal cortex (layer II) to the dentate gyrus to CA3 to CA1 (39, 40). It is through this hierarchical, successively organized representational system that information from the neocortex converges on the hippocampus, wherein it is rapidly bound in the form of a conjunctive representation—that is, a flexible representation that captures the co-occurrence of the multiple features that constitute an event. While each anatomic region in this hierarchy likely plays a different role in information processing and mnemonic function, their collective computations and interactions enable declarative memory (37, 41–43).
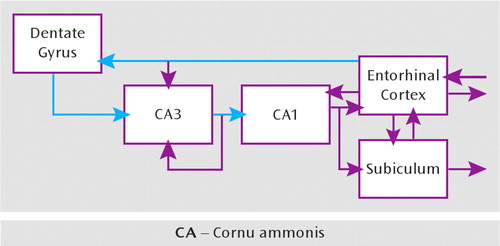
FIGURE 2. Connectivity Within the Hippocampusa
aConnectivity is characterized by the distinctive one-way excitatory projection from the entorhinal cortex to the dentate gyrus to CA3 to CA1, called the trisynaptic pathway (blue). In addition, the entorhinal cortex also projects to CA3 and CA1 directly and independently. CA3 has a rich recurrent collateral network that strongly connects the CA3 pyramidal neurons with each other and is believed to participate in the memory functions of the hippocampus. Figure adapted from reference 38 by permission of the authors and John Wiley and Sons.
Within the hippocampus, pyramidal layers are densely packed with glutamate-containing excitatory neurons. Inhibitory interneurons containing γ-aminobutyric acid (GABA) lie within the polymorphic layer and send their processes to modulate excitatory cell firing. The excitatory glutamate projections within the hippocampus have a low firing threshold, endowing the structure with a great capacity for plasticity, advantaging learning and memory functions. CA3 has extensive networks of recurrent collateral projections, connections that are thought to be the anatomic substrate for the conjunctive encoding and pattern completion processes central to declarative memory (12, 31). Moreover, the dentate gyrus and, to a lesser extent, CA3 are thought to play a fundamental role in mediating pattern separation, wherein novel events that are similar to, but not exactly the same as, past events are established as unique (i.e., pattern-separated) hippocampal representations (38, 44). We next briefiy discuss each of these computations.
Conjunctive Encoding
The neocortex consists of multiple processing regions that represent specific classes of features, including stimulus attributes, spatial configurations, and domains of meaning. During the early stages of event processing, external inputs and internal thoughts give rise to multiple neocortical representations that code for the event's features; the specific neocortical structures recruited during event processing are a function of the features of the episode and the allocation of attention. The outputs from these neocortical regions project to the medial temporal lobe, ultimately converging on the hippocampus, which rapidly forms a conjunctive trace that captures the relations between the event features. In this manner, episodic encoding requires convergent functions of posterior neocortical and frontoparietal networks (which interact to support the representational processing of events in a goal-directed manner) and regions of the medial temporal lobe (which are responsible for forming durable mnemonic representations of the features [items] of the event and for creating a bound representation in which the event's features are linked together) (45–48).
By definition, one-shot learning is necessary for episodic memory (11, 12, 31, 49), which enables organisms to later recognize previously encountered stimuli and to later recollect details of specific past events (5, 8, 11, 13, 31). Given the architecture of the intrahippocampal subfields, attention has focused on CA3 and its interactions with the entorhinal cortex, the dentate gyrus, and CA1. In particular, conjunctive encoding is thought to depend on the widespread collateral connections within CA3, which constitute a powerful autoassociative learning mechanism that allows for the rapid binding of co-occurring event inputs distributed to multiple CA3 neurons. Supportive evidence for the critical role of CA3 comes from studies of mice with deletion of N-methyl-D-amino (NMDA) receptor subunit 1 (NR1) restricted to CA3 cells (CA3-NR1 knockout mice), which demonstrate impaired learning on tasks that require the rapid acquisition of conjunctive, or relational, information (50–52).
In humans, understanding of medial temporal lobe function has partially come from relating the neural responses triggered by an event (e.g., event-related potentials or blood-oxygen-level-dependent [BOLD] activation shown by functional magnetic resonance imaging [fMRI]) to the memory behavior arising from the event (e.g., memory formation and subsequent remembering) (53–56). For example, encoding activation, as detected by fMRI, occurs in response to novel stimuli in the hippo-campus and surrounding perirhinal, parahippocampal, and entorhinal cortex. Moreover, later discrimination between novel and previously encountered items depends at least partially on the strength of the encoded memory, which varies in a continuous manner and seems to underlie the subjective perception of stimulus familiarity (Figure 3) (58–63). The hippocampus, perirhinal cortex, and parahippocampal cortex are hypothesized to support complementary forms of learning during novel stimulus processing; whereas the perirhinal cortex is more active while processing novel items that are subsequently recognized than while processing items that are subsequently forgotten, the hippocampus proper is more active while processing items about which specific contextual details of the item's encounter (e.g., source memory) can be later recollected (59, 63). These findings complement the data on the CA3-NR1 knockout mice, as well as other evidence, in implicating hippocampal subfields as differentially critical for conjunctive encoding.
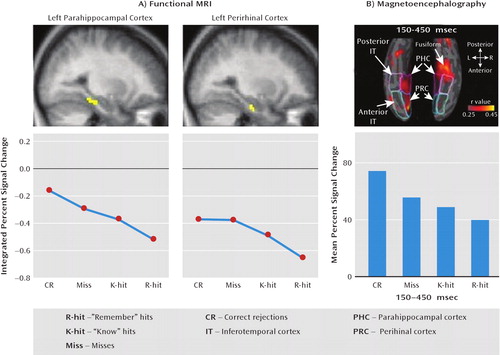
FIGURE 3. Suppression of Activation in the Medial Temporal Lobe Cortex With Repetition of Stimulia
aSuppression varied in a graded manner according to the strength of the memory of the perceived item. Part A: fMRI revealed parametrically decreasing parahippocampal and perirhinal activation, with activation monotonically declining across memory probes, from those perceived as novel to those perceived as having been previously encountered. Part B: magnetoencephalography revealed a similar graded relationship between perceived memory strength and activation in medial temporal lobe cortical areas, with this effect occurring 150–450 msec after stimulus onset. Figure adapted from reference 57 by permission of Elsevier.
Pattern Completion and Pattern Separation
Hippocampal-dependent conjunctive representations are thought to separately code the features or items of an event, maintaining the compositionality of the elemental representations and organizing them in terms of their relations to one another (12, 31, 64, 65). Critically, the compositional nature of conjunctions allows for reactivation of such representations from partial input (pattern completion) (11, 12, 33), a process thought to underlie event recollection. From the complementary learning systems perspective (12, 15), the hippocampus differentially supports the formation and subsequent retrieval of item-item and item-context conjunctions, rather than memory for individual event features (items) per se (11, 57, 58, 66, 67).
At retrieval, conjunctive representations may permit associative recognition (e.g., recognizing which two stimuli had previously co-occurred), inferential reasoning (e.g., deciding that A goes with C because each had previously independently co-occurred with B), and recollection (e.g., source memory) through pattern completion mechanisms that result in retrieval of an extended representation from partial input. Pattern completion may critically depend on mechanisms in CA3, CA1, and the subiculum and on their interactions. For example, CA3-NR1 knockout mice demonstrate impaired retrieval when cued by a partial set of inputs, as evidenced by a failure to reactivate encoding patterns in CA1 (68). Pattern completion in CA3 is thought to be triggered by inputs arriving from the entorhinal cortex, such that when these inputs are sufficiently similar to part of a previously encoded event, the input can serve to reactivate the stored conjunctive pattern (38).
Pattern completion can be viewed as a hippocampal attractor state that is favored because of prior event encoding. A challenge for an effective memory system is to be able to 1) pattern complete when the input was indeed part of a prior event that should be recollected, on the one hand, and 2) establish a distinct memory representation when the input is similar to, but different from, the past, on the other hand. Because the entorhinal cortex projects both to the dentate gyrus and to CA3, it is thought that the dentate gyrus may enable this latter critical function— namely, it is thought that the pathway from the entorhinal cortex to the dentate gyrus to CA3 differentially supports the pattern separation of similar events, such that their representations in CA3 are distinct (38). The architecture of the dentate gyrus as well as the nature of its projections to CA3 appear well suited on computational principles to support pattern separation (38), and electrophysiological data on animals (44) and fMRI data on humans (69) have yielded initial support for the putative role of the dentate gyrus in establishing pattern-separated hippocampal representations.
Schizophrenia and the Hippocampal Formation
The anatomy of schizophrenia involves multiple cerebral regions, including the medial temporal lobe. Cognitive functions mediated by the medial temporal lobe— notably declarative memory (30)—are compromised in individuals with the illness and in relatives at genetic risk. Support for hippocampal involvement in the illness, once suggestive (70, 71), is now compelling (23).
Schizophrenia Phenomenology
Schizophrenia can be conceptualized as an illness of component symptom complexes that are largely independent core phenotypes, each with its own clinical manifestations, pathophysiology, and risk genes (72), an orientation predicted by the early formulations of Kraepelin and Bleuler. Two critical symptom components important to the focus of this article are psychosis and cognitive dysfunction, especially in memory dimensions. Psychosis has always been the defining feature of schizophrenia (73) and is its most florid manifestation (74). While most clinicians today rarely see psychotic symptoms in their full manifestation, the historical reports of psychotic illness (75) and first-person accounts (76, 77) provide a stark reminder of its impact on function. Cognitive dysfunction in schizophrenia has been considered a distinct complex only recently; its specific dimensions have been extensively examined (78–83). Individuals with schizophrenia show a generalized compromise in cognitive performance, with certain categories of cognition showing particular impairment, including visual and verbal declarative memory, working memory, and processing speed (80, 82–88).
Declarative memory is one of the most consistently impaired functions in schizophrenia (26, 83, 85, 89–91). Abnormal performance on memory tasks that depend on conjunctive representations has been repeatedly reported, including 1) impairments in the flexible (inferential) use of learned knowledge (92–94) and 2) greater deficits in recall relative to item recognition (95), memory for the source or context of an experience relative to item memory (96), and recognition based on the recollection of event details relative to perceived item familiarity (97). Alterations in declarative memory performance are associated with reductions in hippocampal volume and functional activation during memory tasks (98, 99). In persons with ultra-high risk for developing psychosis, it is the verbal memory index (because of lower logical memory scores) that identifies those who go on to develop psychosis (100). It is also the case that unaffected relatives show poorer memory performance than comparison subjects on a range of memory tests (101).
Hippocampal Characteristics in Schizophrenia
Early speculations about changes in the hippocampal formation among people with this illness were based on behavioral evidence (70) and anatomy (102, 103). Functional alterations (104), reports of cellular and molecular pathology (105–108), and sensitivity to antipsychotic drugs (109) subsequently converged to confirm these early speculations, consistent with the extensive evidence of declarative memory dysfunction.
Anatomy.
Hippocampal size is reduced bilaterally in schizophrenia (110–113), with volumetric reductions found more often for the hippocampus than for any other brain region (114, 115). Hippocampal volume reduction is seen as early as the first psychotic episode (116, 117) and has been reported to progress to some degree with the illness (118, 119). The volume alteration appears to be independent of the actions of antipsychotic drugs (120) and may be greater in probands without a family history of schizophrenia (121). It has been detected in nonpsychotic siblings of schizophrenia pro-bands (122), in persons at risk for schizophrenia (123, 124), as well as in people with psychotic bipolar disorder (125). Studies of hippocampal shape have detected regional abnormalities of contour in affected persons (126) and in well siblings of schizophrenic probands (122). These observations are consistent with a hippocampal alteration that is modestly progressive over the course of illness, is present in unaffected family members, and may be more severe in the face of low genetic load.
Perfusion.
Increases in basal cerebral perfusion in the hippocampus were identified in early, lower-resolution imaging studies (127–131). More recent data (109, 132–135), including cerebral blood volume measures (136), similarly indicate that basal perfusion is elevated in the medial temporal lobe, particularly in medication-free individuals with schizophrenia, and further demonstrate that perfusion is partially “normalized” by antipsychotic treatment (109). The increase in regional perfusion may correlate with the magnitude of psychosis in medication-free patients (137).
Task-associated activation.
Functional MRI BOLD activation patterns in the medial temporal lobe are abnormal in schizophrenia patients performing declarative memory tasks, often with dysfunction present in the left hemisphere (97, 99, 132, 138–145). In particular, a reduction in hippocampal activation has been observed during tasks assessing verbal memory with temporal context, shallow and deep word encoding, transitive inference with overlapping patterns, relationships between visual stimuli, arbitrary pair encoding, and word pair novelty. As such, BOLD hippocampal activations during conjunctive memory tasks (those previously linked with activation of the medial temporal lobe in healthy individuals) are reduced in patient groups compared with healthy subjects (92, 138, 140, 145, 146). In addition, there is a blunting in schizophrenia of the increase in posterior hippocampal activation induced by smooth pursuit eye movement (147); the nicotine-induced reduction in fMRI BOLD activity seen in a healthy comparison hippocampus, which is thought to be associated with activation of the hippocampal α7 cholinergic receptor (148), is similarly blunted in schizophrenia (149). This observation supports a previous finding of reduced nicotine receptors in the postmortem schizophrenia hippocampus (150) and extends these molecular observations to function. At a network level, examinations of interactions between the medial temporal lobe and the prefrontal cortex have consistently found alterations in connectivity between these two regions in schizophrenia (130, 133, 151), raising questions about the interdependence of their respective pathologies. Taken as a whole, fMRI BOLD activation studies consistently implicate alterations in functional activation in the hippocampus in schizophrenia, especially during tasks dependent on conjunctive memory.
Neurochemistry and histology.
The imaging studies we have reviewed here that show changes in in vivo hippo-campal activity are consistent with a body of postmortem schizophrenia data showing cellular and molecular tissue abnormalities in the medial temporal lobe (106). While the study of synaptic plasticity markers (152–154), proteins associated with putative risk genes (155, 156), glutamate receptors and their intracellular signaling markers (105, 157–159), and other proteins associated with glutamate transmission (160, 161) has broadened the literature on hippocampal abnormalities in schizophrenia, it has not resulted in evidence of a unified molecular pathology. On the other hand, several findings from human tissue studies have implicated hippocampal subfields, especially the dentate gyrus, in schizophrenia; these make a cogent case for regional glutamatergic pathology within the medial temporal lobe, with differential impairment localized to the dentate gyrus. In particular, Reif et al. (162, 163) reported a reduction in Ki-67, a marker of adult neurogenesis in the dentate gyrus in schizophrenia, suggesting that the generation of new neurons in the dentate gyrus may be reduced in the illness, and such a reduction is plausibly associated with changes in the schizophrenia risk gene DISC-1 (164) or NRG1 (165, 166). Intriguingly, recent data suggest that intact neuro-genesis in the dentate gyrus is critical for effective pattern separation (167), raising the possibility that reduced neurogenesis in schizophrenia could result in an imbalance between pattern completion and pattern separation (a point to which we will return later). Kolomeets, Uranova, and colleagues, using electron microscopy (168, 169), described a reduction in the number of synapses of dentate gyrus mossy fibers onto CA3 pyramidal neuronal spines in postmortem schizophrenia tissue, a finding supporting a reduction in transmission efficiency of mossy fiber synapses from the dentate gyrus onto CA3 neurons. In addition, NR1 mRNA—the obligate subunit of the NMDA receptor—is selectively reduced within the hippocampus in the dentate gyrus (105, 157, 159, 170) (Figure 4). Altar et al. (171) isolated dentate gyrus granule cells for microarray analysis and reported reduced gene expression coding for proteins involved in metabolism in the dentate gyrus. Along with findings of other molecular changes in the dentate gyrus in schizophrenia (172, 173), extant postmortem data make a cogent case for the den-tate gyrus being a prominent hippocampal site of unique molecular pathology.
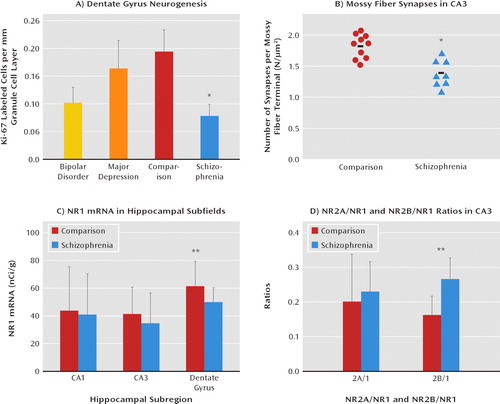
FIGURE 4. Evidence of Selective Decrease in Excitatory Transmission From the Dentate Gyrus to CA3 in Schizophreniaa
aPart A: evidence of a decrease in neurogenesis in schizophrenia in relation to comparison postmortem dentate gyrus tissue was reported by Reif et al. (163) (adapted by permission of Nature Publishing Group). Part B: a reduction in the innervations of CA3 by the dentate gyrus mossy fibers was found by Kolomeets et al. (168) (adapted by permission of John Wiley and Sons.). Part C: a decrease in N-methyl-D-aspartate (NMDA) receptor subunit 1 (NR1) mRNA in the dentate gyrus was reported by Gao et al. (105). Part D: increase in CA3 NR2B subunit composition of the NMDA receptor was observed by one of us (C.A.T., unpublished data).
*p<0.01. **p<0.05.
As noted earlier, the dentate is the gateway structure of the trisynaptic pathway and is thus positioned to critically influence the downstream function of the hippocampus proper, especially CA3. Initial evidence revealing differential functions of the hippocampal subfields—revealed by electrophysiology (174), focal lesions in rodents (175), and regionally selective genetically manipulated animals (175–178)—suggests that distinct behavioral syndromes may accompany dysfunction of each subfield (179).
A Model of Hippocampal Dysfunction in Schizophrenia
What could be a parsimonious model of hippocampal dysfunction in the illness? The studies just reviewed suggest specific pathological features of the hippocampus in schizophrenia: 1) a consistent, albeit small, reduction in hippocampal volume, 2) an increase in hippocampal basal perfusion, 3) an activation deficit during declarative memory tasks that depend on conjunctive representations (possibly related to elevated basal activity), and 4) a reduction in dentate gyrus neurogenesis and efferent excitatory signaling from dentate gyrus granule cells. A number of these in vivo alterations correlate with symptoms of the illness (180, 181) and thus appear functionally relevant. Meanwhile, the conceptualization of schizophrenia is evolving, moving away from categorical distinctions and toward a dimensional component symptom formulation (72), encouraging a model separating psychosis and cognitive pathology. The genetic etiologies of schizophrenia are multiple and complex (182–187), and the neurochemical pathways implicated in symptom formation have become more varied (24, 28, 188–190) yet no more certain. At the same time, cognitive neuroscience is providing a rich foundational literature within which to understand the functional neurobiology of schizophrenia through learning and memory models. We propose a formulation of hippocampal processes in schizophrenia, guided by models of learning and memory.
The proposed model is based on evidence of a significant, but localized, reduction in glutamatergic transmission within the dentate gyrus and in its efferent pathways (105, 157, 159, 163, 168, 170, 173), an idea consistent with a subfieldspecific, hypoglutamatergic state in the medial temporal lobe in schizophrenia (191–193). We suggest that the den-tate gyrus, situated at the proximal end of the trisynaptic pathway, may generate two co-occurring outcomes consequent to a reduction in its excitatory efferent transmission: first, it may alter the plasticity characteristics of its target region, CA3, lowering the threshold in that subfield for long-term potentiation; second, it may reduce the functional contribution of the dentate-to-CA3 pathway to hippocampal memory computations, diminishing dentate-mediated pattern separation and promoting CA3-mediated pattern completion (Figure 5). The processes that mediate both of these classes of outcomes have been previously studied in human, animal, and tissue systems. Thus, markers of both may be tested in living humans, in postmortem brain tissue, and in simplified animal models, by focusing on molecular markers of long-term potentiation in CA3 tissue, reduced glutamate transmission in the dentate gyrus, and reduced pattern separation mnemonic function, in vivo.
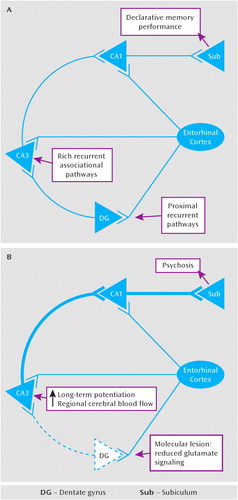
FIGURE 5. Simplified Hippocampal Circuit Subserving Declarative Memorya
aThis circuit (part A) is well studied and is the basis for the model of psychosis as a disorder of learning and memory. We propose that in psychosis (part B), reduced glutamatergic transmission in the dentate gyrus is the basis for reduced pattern separation function in schizophrenia and, furthermore, serves to generate an increase in long-term potentiation in CA3 and greater pattern completion function, including the production of psychotic thoughts and the encoding of the psychotic productions as normal memory.
Examining Plasticity Characteristics in Hippocampal Subfields
Long-term potentiation is a process of synaptic reorganization, triggered by NMDA receptor (NMDAR) activation, accompanied by increases in postsynaptic Ca2+ concentrations, resulting in activity-dependent synaptic strengthening; it is thought to represent the cellular basis of long-term memory (194–196). Long-term potentiation is known to adapt as a function of the prior activity level of the synapse (197, 198). Homeostatic plasticity mechanisms occur over time to modify the overall level of excitability of synapses in target tissue while preserving previous patterns of synaptic strengthening. In animal models, if incoming sensory inputs to a brain region are reduced, the threshold for development of long-term potentiation falls and lower levels of sensory input generate more long-term potentiation (199–201). The schizophrenia model we propose here suggests that the reduction in the mossy fiber activity onto CA3 neurons will generate plasticity changes in CA3, reducing the threshold for long-term potentiation and augmenting it. The molecular events mediating and expressing long-term potentiation have been extensively characterized and are commonly quantified as markers of activity-dependent tissue plasticity. Molecular changes in tissue marking increased long-term potentiation include increases in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) trafficking into the synaptic membrane (202, 203), facilitated by increases in transmembrane AMPAR-regulatory proteins (204, 205) and elevation of postsynaptic signaling molecules such as calcium/calmodulin-dependent protein kinase II, mitogen-activating protein kinase, and postsynaptic membrane-associated guanylate kinases, such as PSD-95 and SAP-102 (194, 195). NMDARs participate in the expression of long-term potentiation by increasing the proportion of their receptors containing NR2B subunits, possibly, associated with palmitoylation of the NR2B subunit (206). Long-term maintenance of long-term potentiation with synaptic strengthening is accompanied by structural remodeling of the synapse, including enlargement of existing spines and an increase in new dendritic spines (207). These use-dependent plasticity changes occur in a highly dynamic manner, mediated by phosphorylation of the signaling proteins, largely in dendrites that are spatially and temporally compartmentalized (195, 204, 208).
We postulate that in schizophrenia exaggerated plasticity changes associated with long-term potentiation will be found within CA3 and can result in increased neuronal excitability, along with increased cerebral perfusion, in the CA3 subfield (209, 210). The heightened sensitivity in CA3 would be magnified both by a strengthened pathway from the entorhinal cortex to CA3 (see the following) and by the extensive excitatory collateral architecture in CA3. Functionally, heightened CA3 activity could generate exaggerated pattern completion memory functions (38, 44) and enhance the production of incorrect or illogical associations, including psychotic experiences, which would then produce memories with psychotic content (211). On the basis of the well-studied molecular characteristics of long-term potentiation and tissue plasticity in laboratory preparations, these molecules can be targeted in human postmortem tissue to evaluate regional plasticity alterations in the hippocampus. To validate this proposed model, markers of increased plasticity in CA3 need to be identified in human hippocampal tissue. Results that would confirm this model include findings of an increase in NR2B- versus NR2A-containing NMDARs, an increase in phosphorylation of the GluR1 subunit of the AMPAR (indicating an increase in AMPARs at the synapse), an increase in PSD-95 and/or SAP-102 in postsynaptic membrane, increases in dendritic spines, and—in vivo—subfield-specific increases in cerebral perfusion measures. At the same time, the proposed model would predict reduced glutamate transmission markers in the dentate gyrus; findings including reduced NR1, decreases in new granule cells, alterations in the presynaptic dynamics of glutamate release (reduced probability of release), or increases in glutamate uptake by astrocytes would serve to confirm the model. This model does not specify a unitary etiology for glutamatergic reductions in the dentate gyrus; rather, it is a pathophysiology that could be associated with a family of genetic and environmental factors each of which reduces glutamatergic transmission within and efferent from the dentate gyrus onto CA3 neurons, including the possibilities of reduced neurogenesis (162, 163), risk gene effects in the hippocampus with functional alleles of DISC1 (164) or NRG1 (165, 166), or alterations in inhibitory GABA modulation of granule cells (152, 212). Failure to find evidence of heightened neuronal activity in CA3 or of reduced glutamate signaling in the dentate gyrus would progressively falsify the model.
Diminished Pattern Separation
The role of the hippocampus in learning and memory includes two necessary but opposing functions: 1) pattern separation at initial memory storage (to render stored memory patterns distinct from each other and avoid “spurious blending” [38]) and 2) pattern completion at memory recall (to recover a full, or more complete, memory from a partial cue). Unique characteristics of the dentate-to-CA3 connection (38, 44) optimize the ability of this pathway to foster pattern separation, including its strong but sparse afferents to CA3. The effect of reduced neurogenesis and/or reduced glutamate transmission in the dentate gyrus in schizophrenia could disadvantage pattern separation and sensitize CA3 for pattern completion functions, as CA3 would become differentially driven by direct entorhinal cortex inputs, rather than by the dentate gyrus inputs that foster orthogonalization of hippocampal representations of similar but distinct events. The relative shift toward pattern completion could plausibly advantage inappropriate associations, generate false or illogical memories, and create a susceptibility to psychosis. The known hippocampal hyperperfusion in schizophrenia may refiect this hypothesized increase in pattern completion under circumstances in which pattern separation would prevail in the healthy brain (i.e., during a novel experience, the schizophrenic hippocampus may erroneously retrieve a memory of a past event rather than appropriately encode a new distinct memory representation), a form of hippocampal prediction error.
Data suggest that hippocampal prediction errors serve to enhance hippocampal long-term potentiation (213) and fMRI BOLD signal (214), which may contribute to hippocampal hyperperfusion and advantage inappropriate associations. Collectively, these changes may also generally hinder accurate declarative memory encoding and retrieval.
Experimental tests of the proposed pattern separation deficit in schizophrenia are possible with fMRI; finding deficits in pattern separation in individuals with schizophrenia would serve to strengthen the model. Specifically, high-resolution fMRI of human hippocampal subfield activation during an incidental stimulus repetition paradigm has recently revealed that signal from voxels inclusive of the dentate gyrus differentiates between repeated stimuli and novel stimuli that are similar to previously encountered stimuli (suggesting pattern separation), whereas CA1 shows a generalized response to these two classes of stimuli (suggesting pattern completion) (69, 215). Our model predicts that individuals with schizophrenia will fail to show a pattern separation bias in voxels inclusive of the dentate gyrus and that the degree to which this occurs should correlate with the magnitude of behavioral impairments in conjunctive memory expression and hippocampal hyperperfusion. Second, to the extent that schizophrenia is associated with a bias toward “runaway” pattern completion, owing to the hypothesized disproportionate influence of entorhinal cortex inputs and recurrent collaterals on CA3 (relative to dentate gyrus inputs), individuals with the illness should more frequently experience hippocampal prediction errors, wherein CA3 outputs to CA1 (i.e., predictions) deviate from the direct cortical inputs from the entorhinal cortex to CA1 (i.e., sensory reality) (213). Standard-resolution fMRI data indicate that hippocampal BOLD signal increases when experience-dependent conjunctive predictions are violated (216, 217), and our model predicts that the magnitude of these hippocampal mismatch or prediction error indices should be enhanced in individuals with the illness, again correlating with other behavioral and physiological measures of declarative memory and hippocampal dysfunction. A failure to find these types of alterations in medial temporal lobe function would progressively falsify the model.
Clinical Correlates
Certain clinical characteristics of psychotic thought in schizophrenia are consistent with this model. Individuals perceive their psychotic processes as normal thought; John Nash said that his delusional thoughts “come into my mind just like my other thoughts, so I have no option but to believe them” in response to his colleagues' questions challenging his unusual thought content (77). Psychotic productions in affected individuals are rarely continuously new but are to some extent recurring, e.g., patients can have the same and/or related delusions, the same and/or related set of hallucinations, indicating their status as memories (211). Moreover, psychotic experiences are often of high salience, including episodic memories of danger, negative criticism, or excessive grandiosity, increasing their strength; this is consistent with the known enhancing effect of emotional stress on memory and on GluR1 phosphorylation (218–220).
While this model is developed here as a hypothesis for schizophrenia, it could also be evaluated as a more general hypothesis of psychosis. The proposed model could account for the elevated incidence of psychotic symptoms in diseases known to be associated with hippocampal pathology, such as Alzheimer's dementia (221), depression (222), and many forms of epilepsy (223), supporting the concept of psychosis as a dimension. Moreover, it would provide a mechanism for the frequent experience of psychotic constructs in normal persons with no psychiatric diagnosis, where prevalence figures approach 8.5% (224). A model of psychosis like the one proposed here is a syndromal model, analogous to congestive heart failure in cardiac disease, where etiologies are multiple, can be complex, and could include overlapping genetic and environmental risks.
Implications for Treatment, Current and Future
Although data that clarify the effect of antipsychotic drugs on the function of the medial temporal lobe remain remarkably incomplete, they suggest that at least some of the actions of antipsychotics could be mediated within the medial temporal lobe. D1 and D2 dopamine receptors are localized, each with a distinctive distribution, throughout the medial temporal lobe; manipulating signaling at D1 or D2 receptors alters medial temporal lobe function and memory performance in experimental paradigms (225–228). Inferential memory deficits in schizophrenia are improved with antipsychotic drug treatment (229). Thus, the available data document a role for dopamine in medial temporal lobe plasticity, even if with inadequate specificity. Focused examination could answer these questions further.
In the future, if the proposed model of psychosis is supported, it would have additional implications for treatment. Based on the model proposed, novel treatments could be directed toward reversing glutamatergic insufficiency in the dentate gyrus and/or changing the unopposed increase of long-term potentiation in CA3. Treatments could be syndromal, acting like digitalis in congestive heart failure. These treatments would be most effective in acute psychosis during early prodrome periods and possibly also during early years of illness, when plasticity mechanisms are most accessible. The possibility exists that known antipsychotic drugs, with actions mediated through serotonin and dopa-mine receptors, deliver a component of their therapeutic action through enhancing dentate gyrus glutamate transmission or through modulating long-term potentiation in CA3 (228, 230, 231). Further, basal perfusion measures in the hippocampus in individuals with psychotic illnesses might provide a sensitive biomarker for testing novel antipsychotic mechanisms. If the model is further verified, then approaches to either increase neural transmission in the dentate gyrus or to reduce transmission in CA3 would be predicted to reduce the formation of new delusions and hallucinations and to promote the deconstruction of delusional memories.
Summary
The proposed dentate gyrus glutamate insufficiency model suggests that the initial generation of psychotic associations may occur in a supersensitive, entorhinal cortex-driven CA3 subfield between normal mental constructs that are mistakenly associated and then are committed to memory, some with psychotic content. Another clinical feature consistent with this model, and with alterations in homeostatic plasticity, is the observation that antipsychotic treatment does not immediately and fully resolve psychotic symptoms (232). This modulated symptom response to pharmacological intervention is consistent with a homeostatic plasticity process. Other models of the involvement of hippocampal dysfunction in schizophrenia have also been proposed (213, 233–236) as a means to guide hypothesis-testing study of hippocampal pathophysiology. Models of pathophysiology in schizophrenia, to the extent they are supported in future study, will have direct and testable implications for relevant animal models of the illness and novel treatment approaches in schizophrenia.
1. : Loss of recent memory after bilateral hippocampal lesions (1957). J Neuropsychiatry Clin Neurosci 2000; 12:103–113Crossref, Medline, Google Scholar
2. : Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg 1972; 19:421–446Crossref, Medline, Google Scholar
3. : Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci 1996; 16:5233–5255Crossref, Medline, Google Scholar
4. : Neurotoxic lesions of perirhinal cortex impair visual recognition memory in rhesus monkeys. Neuroreport 2001; 12:1913–1917Crossref, Medline, Google Scholar
5. : The medial temporal lobe. Annu Rev Neurosci 2004; 27:279–306Crossref, Medline, Google Scholar
6. : Lasting consequences of bilateral medial temporal lobectomy: clinical course and experimental findings in HM. Semin Neurol 1984; 4:249–259Crossref, Google Scholar
7. : Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev 2001; 108:311–345Crossref, Medline, Google Scholar
8. : A cortical-hippocampal system for declarative memory. Neuroscience 2000; 1:41–50Medline, Google Scholar
9. : Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus 2001; 11:551–568Crossref, Medline, Google Scholar
10. : Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 1999; 9:7–24Crossref, Medline, Google Scholar
11. : Memory, Amnesia, and the Hippo-campal System. Cambridge, Mass, MIT Press, 1993Google Scholar
12. : Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 1995; 102:419–457Crossref, Medline, Google Scholar
13. : Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 1992; 99:195–231; correction, 1992; 99:582Crossref, Medline, Google Scholar
14. : Cognitive neuroscience of human memory. Annu Rev Psychol 1998; 49:87–115Crossref, Medline, Google Scholar
15. : Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev 2003; 110:611–646Crossref, Medline, Google Scholar
16. : The medial temporal lobe and memory, in Neurobiology of Learning and Memory, 2nd ed. Edited by Kesner RPMartinez JL. New York, Elsevier, 2007, pp 305–337Crossref, Google Scholar
17. : Dementia of the Alzheimer type. Annu Rev Neurosci 1980; 3:77–95Crossref, Medline, Google Scholar
18. : The hippocampus: organizational patterns in health and senescence. Mech Ageing Dev 1979; 9:89–102Crossref, Medline, Google Scholar
19. : Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus 1991; 1:41–66Crossref, Medline, Google Scholar
20. : Toxic effects of depression on brain function: impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am J Psychiatry 2008; 165:731–739Link, Google Scholar
21. : Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus 2008; 18:729–736Crossref, Medline, Google Scholar
22. : Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci 2007; 64:2120–2132Crossref, Medline, Google Scholar
23. : Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 2001; 11:520–528Crossref, Medline, Google Scholar
24. : Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry 2006; 63:630–638Crossref, Medline, Google Scholar
25. : Hippo-campal function, declarative memory, and schizophrenia: anatomic and functional neuroimaging considerations. Curr Neurol Neurosci Rep 2005; 5:249–256Crossref, Medline, Google Scholar
26. : The cognitive neuro-science of memory function and dysfunction in schizophrenia. Biol Psychiatry 2008; 64:18–25Crossref, Medline, Google Scholar
27. : The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev 2009; 34:468–486Crossref, Medline, Google Scholar
28. : Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 2008; 31:234–242Crossref, Medline, Google Scholar
29. : Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotox Res 2008; 14:97–104Crossref, Medline, Google Scholar
30. : The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol 2005; 1:321–353Crossref, Medline, Google Scholar
31. : From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford, UK, Oxford University Press, 2001Google Scholar
32. : Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature 1999; 400:671–675Crossref, Medline, Google Scholar
33. : Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci 1971; 262:23–81Crossref, Medline, Google Scholar
34. : Neuroanatomy of memory. Annu Rev Neurosci 1993; 16:547–563Crossref, Medline, Google Scholar
35. : Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus 2000; 10:420–430Crossref, Medline, Google Scholar
36. : The Human Hippocampus. New York, Springer, 1998Crossref, Google Scholar
37. : MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol 1998; 19:659–671Medline, Google Scholar
38. : Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus 1994; 4:661–682Crossref, Medline, Google Scholar
39. : Anatomical organization of the parahippocampal-hippocampal network. Ann NY Acad Sci 2000; 9:1–24Google Scholar
40. : The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 1989; 31:571–591Crossref, Medline, Google Scholar
41. : Hippocampal formation, in The Human Nervous System. Edited by Paxinos G. San Diego, Academic Press, 1990, pp 711–755Crossref, Google Scholar
42. : Perirhinal and parahippocampal cortices of the macaque monkey: cytoarchitectonic and chemoarchitectonic organization. J Comp Neurol 2003; 463:67–91Crossref, Medline, Google Scholar
43. : Postnatal development of the primate hippocampal formation. Dev Neurosci 2007; 29:179–192Crossref, Medline, Google Scholar
44. : Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem 2007; 14:745–757Crossref, Medline, Google Scholar
45. : Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem 2009; 91:139–154Crossref, Medline, Google Scholar
46. : Making memories: brain activity that predicts how well visual experience will be remembered. Science 1998; 281:1185–1187Crossref, Medline, Google Scholar
47. : Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 1998; 281:1188–1191Crossref, Medline, Google Scholar
48. : The cognitive neuroscience of remembering. Nat Rev Neurosci 2001; 2:624–634Crossref, Medline, Google Scholar
49. : Elements of Episodic Memory. New York, Oxford University Press, 1983Google Scholar
50. : Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 2002; 297:211–218Crossref, Medline, Google Scholar
51. : Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 2003; 38:305–315Crossref, Medline, Google Scholar
52. : The role of CA3 hippocampal NMDA receptors in paired associate learning. J Neurosci 2006; 26:908–915Crossref, Medline, Google Scholar
53. : Observing the transformation of experience into memory. Trends Cogn Sci 2002; 6:93–102Crossref, Medline, Google Scholar
54. : When encoding yields remembering: insights from event-related neuroimaging. Philos Trans R Soc Lond B Biol Sci 1999; 354:1307–1324Crossref, Medline, Google Scholar
55. : Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 2007; 13:280–291Crossref, Medline, Google Scholar
56. : Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 2006; 16:693–700Crossref, Medline, Google Scholar
57. : Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron 2005; 47:751–761Crossref, Medline, Google Scholar
58. : Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2001; 2:51–61Crossref, Medline, Google Scholar
59. : Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA 2003; 100:2157–2162Crossref, Medline, Google Scholar
60. : Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus 2004; 14:919–930Crossref, Medline, Google Scholar
61. : Human recognition memory: a cognitive neuroscience perspective. Trends Cogn Sci 2003; 7:313–319Crossref, Medline, Google Scholar
62. : Associative memory and the me-dial temporal lobes. Trends Cogn Sci 2007; 11:126–135Crossref, Medline, Google Scholar
63. : Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 2004; 42:2–13Crossref, Medline, Google Scholar
64. : Functional organization of the hippocampal memory system. Proc Natl Acad Sci USA 1996; 93:13500–13507Crossref, Medline, Google Scholar
65. : Transitivity, fiexibility, conjunctive representations, and the hippocampus, II: a computational analysis. Hippocampus 2003; 13:299–312Crossref, Google Scholar
66. : Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci 2000; 3:1149–1152Crossref, Medline, Google Scholar
67. : The neural system that mediates familiarity memory. Hippocampus 2006; 16:504–520Crossref, Medline, Google Scholar
68. : Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 2002; 297:211–218Crossref, Medline, Google Scholar
69. : Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 2008; 319:1640–1642Crossref, Medline, Google Scholar
70. : An anatomy of schizophrenia? Arch Gen Psychiatry 1973; 29:177–189Crossref, Medline, Google Scholar
71. : Auditory hallucinations and cognitive impairment in a patient with a lesion restricted to the hippocampus. Schizophr Res 2003; 64:87–89Crossref, Medline, Google Scholar
72. : Medicine: what are the right targets for psychopharmacology? Science 2003; 299:350–351Crossref, Medline, Google Scholar
73. : Schizophrenia. N Engl J Med 1994; 330:681–690Crossref, Medline, Google Scholar
74. : Phenotype of schizophrenia: a review and formulation. Mol Psychiatry 2004; 10:27–39Crossref, Google Scholar
75. : Development in somatic treatments: introduction. Am J Psychiatry 1994; 151(June suppl):216–219Link, Google Scholar
76. : The Center Cannot Hold: My Journey Through Madness. New York, Hyperion, 2007Google Scholar
77. : A Beautiful Mind. New York, Simon & Schuster, 1998Google Scholar
78. : Information processing and attention dysfunctions in schizophrenia. Schizophr Bull 1993; 19:233–259Crossref, Medline, Google Scholar
79. : Computerized neurocognitive scanning, II: the profile of schizophrenia. Neuropsychopharmacology 2001; 25:777–788Crossref, Medline, Google Scholar
80. : General and specific cognitive deficits in schizophrenia. Biol Psychiatry 2004; 55:826–833Crossref, Medline, Google Scholar
81. : Review of cognition and brain structure in schizophrenia: profiles, longitudinal course, and effects of treatment. Psychiatr Clin North Am 2004; 27:1–18, viiCrossref, Medline, Google Scholar
82. : Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998; 12:426–445Crossref, Medline, Google Scholar
83. : Identification of separable cognitive factors in schizophrenia. Schizophr Res 2004; 72:29–39Crossref, Medline, Google Scholar
84. : Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry 1994; 51:124–131Crossref, Medline, Google Scholar
85. : Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
86. : Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000; 26:119–136Crossref, Medline, Google Scholar
87. : The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet 2000; 67:369–382Crossref, Medline, Google Scholar
88. : Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 2001; 98:6917–6922Crossref, Medline, Google Scholar
89. : Eye tracking, attention, and schizotypal symptoms in nonpsychotic relatives of patients with schizophrenia. Arch Gen Psychiatry 1997; 54:169–176Crossref, Medline, Google Scholar
90. : CNTRICS final task selection: long-term memory. Schizophr Bull 2009; 35:197–212Crossref, Medline, Google Scholar
91. : Working and strategic memory deficits in schizophrenia. Neuropsychology 1998; 12:278–288Crossref, Medline, Google Scholar
92. : Fronto-hippocampal function during temporal context monitoring in schizophrenia. Biol Psychiatry 2006; 60:1268–1277Crossref, Medline, Google Scholar
93. : Learning and generalization in schizophrenia: effects of disease and antipsychotic drug treatment. Biol Psychiatry 2010; 67:926–932Crossref, Medline, Google Scholar
94. : Transitive inference in schizophrenia: impairments in relational memory organization. Schizophr Res 2004; 68:235–247Crossref, Medline, Google Scholar
95. : Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry 1999; 156:1358–1366Abstract, Google Scholar
96. : Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Arch Gen Psychiatry 1999; 56:639–644Crossref, Medline, Google Scholar
97. : Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry 2003; 53:48–55Crossref, Medline, Google Scholar
98. : Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry 1993; 50:190–197Crossref, Medline, Google Scholar
99. : Impaired hippocampal function during the detection of novel words in schizophrenia. Biol Psychiatry 2004; 55:668–675Crossref, Medline, Google Scholar
100. : Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry 2005; 162:71–78Link, Google Scholar
101. : Declarative memory in unaffected adult relatives of patients with schizophrenia: a systematic review and meta-analysis. Schizophr Res 2005; 78:13–26Crossref, Medline, Google Scholar
102. : Basal ganglia and limbic system pathology in schizophrenia: a morphometric study of brain volume and shrinkage. Arch Gen Psychiatry 1985; 42:784–791Crossref, Medline, Google Scholar
103. : Disorientation of the hippocampal pyramidal cell and its processes in schizophrenic patients (letter). Biol Psychiatry 1981; 16:101–102Google Scholar
104. : Deconstructing psychosis with human brain imaging. Schizophr Bull 2007; 33:921–931Crossref, Medline, Google Scholar
105. : Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry 2000; 157:1141–1149Link, Google Scholar
106. : The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004; 174:151–162Crossref, Medline, Google Scholar
107. : Altered hippocampal muscarinic M4, but not M1, receptor expression from subjects with schizophrenia. Biol Psychiatry 2007; 61:1161–1170Crossref, Medline, Google Scholar
108. : First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry 2006; 11:118–119Crossref, Medline, Google Scholar
109. : Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus 2001; 11:543–550Crossref, Medline, Google Scholar
110. : Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res 1990; 35:1–13Crossref, Medline, Google Scholar
111. : Temporal lobe pathology in schizophrenia: a quantitative MRI study. Am J Psychiatry 1989; 146:464–472Link, Google Scholar
112. : Confirmation of reduced temporal limbic structure volume on MRI in male patients with schizophrenia. Psychiatry Res 1996; 67:135–143Crossref, Medline, Google Scholar
113. : Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophr Res 1995; 17:47–58Crossref, Medline, Google Scholar
114. : Brain volume in first-episode schizophrenia: systematic review and meta-analysis of MRI studies. Br J Psychiatry 2006; 188:510–518Crossref, Medline, Google Scholar
115. : Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005; 162:2233–2245Link, Google Scholar
116. : Smaller anterior hippocampal formation volume in anti-psychotic-naive patients with first-episode schizophrenia. Am J Psychiatry 2003; 160:2190–2197Link, Google Scholar
117. : Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage 2004; 21:1563–1575Crossref, Medline, Google Scholar
118. : Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a MRI study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry 2006; 63:139–149Crossref, Medline, Google Scholar
119. : Duration of illness and treatment effects on hippocampal volume in male patients with schizophrenia. Br J Psychiatry 2005; 186:26–31Crossref, Medline, Google Scholar
120. : A longitudinal study on the effects of typical versus atypical antipsychotic drugs on hippocampal volume in schizophrenia. Schizophr Res 2007; 94:288–292Crossref, Medline, Google Scholar
121. : Hippocampus and amygdala volumes in schizophrenia and other psychoses in the Northern Finland 1966 birth cohort. Schizophr Res 2005; 75:283–294Crossref, Medline, Google Scholar
122. : Hippo-campal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry 2003; 54:1234–1240Crossref, Medline, Google Scholar
123. : Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry 2001; 49:811–823Crossref, Medline, Google Scholar
124. : Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry 2004; 61:346–353Crossref, Medline, Google Scholar
125. : Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry 2005; 57:633–639Crossref, Medline, Google Scholar
126. : Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry 2002; 159:2000–2006Link, Google Scholar
127. : Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar
128. : Temporal lobe metabolic differences in medication-free outpatients with schizophrenia via the PET-600. Neuropsychopharmacology 1996; 15:541–554Crossref, Medline, Google Scholar
129. : Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992; 160:179–186Crossref, Medline, Google Scholar
130. : The missing link: a failure of fronto-hippocampal integration in schizophrenia. Nat Neurosci 1998; 1:266–267Crossref, Medline, Google Scholar
131. : Left hemisphere dysfunction and left hemisphere over-activation in schizophrenia. J Abnorm Psychol 1978; 87:226–238Crossref, Medline, Google Scholar
132. : Impaired recruitment of the hippocampus dur-ing conscious recollection in schizophrenia. Nat Neurosci 1998; 1:318–323Crossref, Medline, Google Scholar
133. : Prefrontal atrophy in first episodes of schizophrenia associated with limbic metabolic hyperactivity. J Psychiatr Res 2005; 39:117–127Crossref, Medline, Google Scholar
134. : Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. Am J Psychiatry 2005; 162:1840–1848Link, Google Scholar
135. : Resting neural activity distinguishes subgroups of schizophrenia patients. Biol Psychiatry 2004; 56:931–937Crossref, Medline, Google Scholar
136. : Hippocampal hyperactivity is associated with positive symptoms (abstract). Schizophr Bull 2009; 35(suppl 1):160Google Scholar
137. : Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology 2006; 31:221–230Crossref, Medline, Google Scholar
138. : Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch Gen Psychiatry 2007; 64:999–1014Crossref, Medline, Google Scholar
139. : Elaborative verbal encoding and altered anterior parahippocampal activation in adolescents and young adults at genetic risk for schizophrenia using FMRI. Biol Psychiatry 2007; 61:564–574Crossref, Medline, Google Scholar
140. : Hippocampal activation during transitive inference in humans. Hippo-campus 2004; 14:153–162Crossref, Medline, Google Scholar
141. : Functional abnormalities of medial temporal cortex during novel picture learning among patients with chronic schizophrenia. Schizophr Res 2002; 59:187–198Crossref, Google Scholar
142. : Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. Am J Psychiatry 2003; 160:1305–1312Link, Google Scholar
143. : Hippocampal dysfunction during episodic memory encoding in patients with schizophrenia—an fMRI study. Schizophr Res 2003; 64:83–85Crossref, Medline, Google Scholar
144. : Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry 2005; 57:1011–1019Crossref, Medline, Google Scholar
145. : The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry 2006; 63:356–365Crossref, Medline, Google Scholar
146. : Dissociation between medial temporal lobe and basal ganglia memory systems in schizophrenia. Schizophr Res 2005; 77:321–328Crossref, Medline, Google Scholar
147. : Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry 2004; 161:315–321Link, Google Scholar
148. : Effects of nicotine on hippocampal and cingulate activity during smooth pursuit eye movement in schizophrenia. Biol Psychiatry 2006; 59:754–761Crossref, Medline, Google Scholar
149. : fMRI of response to nicotine during a smooth pursuit eye movement task in schizophrenia. Am J Psychiatry 2005; 162:391–393; correction, 162:832Link, Google Scholar
150. : Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 1995; 38:22–33Crossref, Medline, Google Scholar
151. : Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 2005; 62:379–386Crossref, Medline, Google Scholar
152. : Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA 2007; 104:10164–10169Crossref, Medline, Google Scholar
153. : Hippocampal complexin proteins and cognitive dysfunction in schizophrenia. Arch Gen Psychiatry 2005; 62:263–272Crossref, Medline, Google Scholar
154. : Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr Res 2005; 73:159–172Crossref, Medline, Google Scholar
155. : Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet 2007; 16:129–141Crossref, Medline, Google Scholar
156. : Decreased hippocampal expression of the susceptibility gene PPP3CC and other calcineurin subunits in schizophrenia. Biol Psychiatry 2005; 57:702–710Crossref, Medline, Google Scholar
157. : Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neurore-port 2001; 12:2971–2974Crossref, Medline, Google Scholar
158. : Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippo-campus 2001; 11:569–577Medline, Google Scholar
159. : Decreased expression of mRNAs encoding non-NMDA glutamate receptors GluR1 and GluR2 in medial temporal lobe neurons in schizophrenia. Mol Brain Res 1995; 29:211–223Crossref, Medline, Google Scholar
160. : Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry 2000; 48:381–388Crossref, Medline, Google Scholar
161. : Serine race-mase protein expression in cortex and hippocampus in schizophrenia. Neuroreport 2006; 17:1181–1185Crossref, Medline, Google Scholar
162. : Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur Arch Psychiatry Clin Neurosci 2007; 257:290–299Crossref, Medline, Google Scholar
163. : Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 2006; 11:514–522Crossref, Medline, Google Scholar
164. : Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 2007; 130:1146–1158Crossref, Medline, Google Scholar
165. : Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the CNS. Proc Natl Acad Sci USA 2009; 106:4507–4512Crossref, Medline, Google Scholar
166. : The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron 2007; 54:583–597Crossref, Medline, Google Scholar
167. : A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009; 325:210–213Crossref, Medline, Google Scholar
168. : Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse 2007; 61:615–621Crossref, Medline, Google Scholar
169. : Ultrastructural alterations in hippocampal mossy fiber syn-apses in schizophrenia: a post-mortem morphometric study. Synapse 2005; 57:47–55Crossref, Medline, Google Scholar
170. : Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex, and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res 1997; 751:217–231Crossref, Medline, Google Scholar
171. : Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry 2005; 58:85–96Crossref, Medline, Google Scholar
172. : Molecular abnormalities of the hippocampus in severe psychiatric illness: post-mortem findings from the Stanley Neuro-pathology Consortium. Mol Psychiatry 2004; 9:609–620Crossref, Medline, Google Scholar
173. : Increased frequency of den-tate granule cells with basal dendrites in the hippocampal formation of schizophrenics. Psychiatry Res 2003; 122:89–97Crossref, Medline, Google Scholar
174. : Hippocampal network dynamics constrain the time lag between pyramidal cells across modified environments. J Neurosci 2008; 28:13448–13456Crossref, Medline, Google Scholar
175. : Dorsal hippocampus, CA3, and CA1 lesions disrupt temporal sequence completion. Behav Neurosci 2008; 122:9–15Crossref, Medline, Google Scholar
176. : Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 2007; 317:94–99Crossref, Medline, Google Scholar
177. : CA3 NMDA receptors are required for experience-dependent shifts in hippocampal activity. Hippocampus 2007; 17:1003–1011Crossref, Medline, Google Scholar
178. : Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 2008; 319:1260–1264Crossref, Medline, Google Scholar
179. : Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol 2009; 39:24–36Crossref, Medline, Google Scholar
180. : Event-related potential abnormalities correlate with structural brain alterations and clinical features in patients with chronic schizophrenia. Schizophr Res 1994; 11:259–271Crossref, Medline, Google Scholar
181. : Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. Am J Psychiatry 2002; 159:217–226Link, Google Scholar
182. : The genetic deconstruction of psychosis. Schizophr Bull 2007; 33:905–911Crossref, Medline, Google Scholar
183. : Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 2009; 460:753–757Crossref, Medline, Google Scholar
184. : Common variants conferring risk of schizophrenia. Nature 2009; 460:744–747Crossref, Medline, Google Scholar
185. : Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460:748–752Crossref, Medline, Google Scholar
186. : Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 2008; 320:539–543Crossref, Medline, Google Scholar
187. : Genetics of clinical features and sub-types of schizophrenia: a review of the recent literature. Curr Psychiatry Rep 2008; 10:164–170Crossref, Medline, Google Scholar
188. : Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol 2006; 63:1372–1376Crossref, Medline, Google Scholar
189. : A dopaminergic deficit hypothesis of schizophrenia: the path to discovery. Dialogues Clin Neurosci 2006; 8:137–142Medline, Google Scholar
190. : Six degrees of separation: on the prior probability that schizophrenia susceptibility genes converge on synapses, glutamate and NMDA receptors. Mol Psychiatry 2006; 11:981–983Crossref, Medline, Google Scholar
191. : Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991; 148:1301–1308Link, Google Scholar
192. : Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol 1998; 12:21–36Crossref, Medline, Google Scholar
193. : Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol 2006; 26:365–384Crossref, Medline, Google Scholar
194. : Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008; 33:18–41Crossref, Medline, Google Scholar
195. : Understanding synapses: past, present, and future. Neuron 2008; 60:469–476Crossref, Medline, Google Scholar
196. : Learning induces long-term potentiation in the hippocampus. Science 2006; 313:1093–1097Crossref, Medline, Google Scholar
197. : Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci 1982; 2:32–48Crossref, Medline, Google Scholar
198. : Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci 2003; 358:649–655Crossref, Medline, Google Scholar
199. : Obligatory role of NR2A for meta-plasticity in visual cortex. Neuron 2007; 53:495–502Crossref, Medline, Google Scholar
200. : Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology 2007; 52:200–214Crossref, Medline, Google Scholar
201. : Bidirectional modifications of visual acuity induced by monocular deprivation in juvenile and adult rats. J Neurosci 2006; 26:7368–7374Crossref, Medline, Google Scholar
202. : A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE 2006; 2006(356):re11Crossref, Medline, Google Scholar
203. : Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 2002; 25:578–588Crossref, Medline, Google Scholar
204. : The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 2007; 23:613–643Crossref, Medline, Google Scholar
205. : S-Nitrosylation of stargazin regulates surface expression of AMPA-glutamate neurotransmitter receptors. Proc Natl Acad Sci USA 2009; 106:16440–16445Crossref, Medline, Google Scholar
206. : Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron 2009; 64:213–226Crossref, Medline, Google Scholar
207. : Coordinated changes in dendritic arborization and synaptic strength during neural circuit development. Neuron 2009; 61:71–84Crossref, Medline, Google Scholar
208. : Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron 2008; 60:788–802Crossref, Medline, Google Scholar
209. : Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 1996; 19:126–130Crossref, Medline, Google Scholar
210. : LTP and LTD: an embarrassment of riches. Neuron 2004; 44:5–21Crossref, Medline, Google Scholar
211. : Why do delusions persist? Front Hum Neurosci 2009; 3:12Crossref, Medline, Google Scholar
212. : Fast synaptic subcortical control of hippo-campal circuits. Science 2009; 326:449–453Crossref, Medline, Google Scholar
213. : The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 2005; 46:703–713Crossref, Medline, Google Scholar
214. : Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron 2008; 60:378–389Crossref, Medline, Google Scholar
215. : Imaging the human medial temporal lobe with high-resolution fMRI. Neuron 2010; 65: 298–308Crossref, Medline, Google Scholar
216. : Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci 2007; 27:8517–8524Crossref, Medline, Google Scholar
217. : An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol 2006; 4:e424Crossref, Medline, Google Scholar
218. : Synaptic AMPA receptor plasticity and behavior. Neuron 2009; 61:340–350Crossref, Medline, Google Scholar
219. : Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 2007; 131:160–173Crossref, Medline, Google Scholar
220. : Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol 1997; 77:3013–3020Crossref, Medline, Google Scholar
221. : Genetic variation in the G720/G30 gene locus (DAOA) influences the occurrence of psychotic symptoms in patients with Alzheimer's disease. J Alzheimers Dis 2009; 18:953–960Crossref, Medline, Google Scholar
222. : Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry 2008; 165:872–880Link, Google Scholar
223. : Schizophrenia and epilepsy: is there a shared susceptibility? Neurosci Res 2009; 63:227–235Crossref, Medline, Google Scholar
224. : The structure of the extended psychosis phenotype in early adolescence—a cross-sample replication. Schizophr Bull 2009; Dec 31 [Epub ahead of print]Medline, Google Scholar
225. : Dopamine D2 receptors in the rat, monkey and the postmortem human hippocampus: an autoradiographic study using the novel D2-selective ligand iodine-125-NCQ 298. Neurosci Lett 1991; 125:12–14Crossref, Medline, Google Scholar
226. : PET mapping of extrastriatal D2-like dopamine receptors in the human brain using an anatomic standardization technique and 11C FLB 457. NeuroImage 1999; 10:666–674Crossref, Medline, Google Scholar
227. : Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci 2006; 26:7723–7729Crossref, Medline, Google Scholar
228. : D1/D5 dopamine receptors inhibit depotentiation at CA1 synapses via cAMP-dependent mechanism. J Neurosci 1998; 18:1270–1279Crossref, Medline, Google Scholar
229. : Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet 1997; 349:1730–1734Crossref, Medline, Google Scholar
230. : Memory and frontal lobe functions: possible relations with dopamine D2 receptors in the hippocampus. NeuroImage 2007; 34:1643–1649Crossref, Medline, Google Scholar
231. : D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci 1996; 16:7478–7486Crossref, Medline, Google Scholar
232. : Changes in psychopathology and dyskinesia after neuroleptic withdrawal in a double-blind design. Schizophr Res 1993; 10:267–271Crossref, Medline, Google Scholar
233. : Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 2002; 42:165–179Crossref, Medline, Google Scholar
234. : Hippocampal abnormalities and memory deficits: new evidence of a strong patho-physiological link in schizophrenia. Brain Res Rev 2007; 54:92–112Crossref, Medline, Google Scholar
235. : Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 2007; 27:11424–11430Crossref, Medline, Google Scholar
236. : Circuitry-based gene expression profiles in GABA cells of the trisynaptic pathway in schizophrenics versus bipolars. Proc Natl Acad Sci USA 2008; 105:20935–20940Crossref, Medline, Google Scholar



