Ionotropic Glutamate Receptors and Expression of N-Methyl-d-Aspartate Receptor Subunits in Subregions of Human Hippocampus: Effects of Schizophrenia
Abstract
OBJECTIVE: Multiple quantifiable biologic abnormalities have been localized to the hippocampus in schizophrenia. Alterations in glutamate-mediated transmission at N-methyl-d-aspartic acid (NMDA)-sensitive receptors in hippocampus have been implicated in the pathophysiology of the illness. The authors tested the hypothesis that glutamatergic transmission within and efferent from hippocampus is altered in schizophrenia.METHOD: The authors analyzed postmortem hippocampal tissue from individuals with schizophrenia and from healthy individuals. The tissue samples had been collected by two brain tissue banks, one in Maryland and the other in Melbourne, Australia. lonotropic receptor binding for the NMDA, kainate, and 3H-amino-3-hydroxy-5-methylisoxazol-4-propionate (AMPA) receptors was quantified by using usual radioligand techniques. In situ hybridization autoradiography was used to quantify mRNA for the NMDA receptor subunits NR1, NR2A, and NR2B.RESULTS: Ligand binding to the ionotropic glutamate receptors (NMDA, kainate, and AMPA) did not differ significantly overall or in any subregion between the schizophrenia tissue and the healthy comparison tissue. The only exception was AMPA receptor binding in hippocampal subregion CA2, which was slightly but significantly less in schizophrenia. However, the level of mRNA for the NMDA receptor subunits NR1 and NR2B was significantly different between groups; in several hippocampal subregions, the level of NR1 mRNA was lower and the level of NR2B mRNA higher in schizophrenia.CONCLUSIONS: Because the NR1 subunit of the NMDA receptor is critical to full receptor activity, a reduction of NR1 in hippocampus in schizophrenia suggests a functional impairment in glutamatergic transmission at the NMDA receptor, resulting in reduced glutamatergic transmission within and possibly efferent from the hippocampus in schizophrenia. This defect could underlie a hypoglutamatergic state in regions of limbic cortex, consistent with published results from other lines of research in schizophrenia.
Several lines of evidence, including in vivo human imaging results and postmortem tissue findings, suggest abnormalities of human hippocampal structure and function in schizophrenia. In vivo, hippocampal size in persons with schizophrenia is reduced bilaterally, albeit mildly, especially in anterior regions (1–5). Structural and histologic abnormalities in postmortem hippocampal tissue from affected persons have been repeatedly reported (6–11), although some of these findings have not been consistently replicated (12). Moreover, in vivo functional studies of persons with schizophrenia have directly demonstrated an alteration in neuronal activity in the limbic system and/or parahippocampal gyrus, as measured by positron emission tomography and [18F]fluorodeoxyglucose or regional cerebral blood flow techniques (13–17). In addition, considerable evidence of compromised cognitive function, especially in short-term memory and attention, exists in schizophrenia (18–20); these dysfunctions may represent the behavioral correlates of hippocampal pathology.
Studies from our laboratory using an in vivo animal preparation of psychosis with phencyclidine (PCP) support this focus. Because PCP and its congener ketamine induce psychotic-like phenomena in normal persons (21–23) and psychosis exacerbation in schizophrenia (21, 24), PCP’s actions in animals have been studied to shed light on the mechanisms of psychosis, and even of schizophrenia, in humans (25). Changes in neuronal activity and immediate early gene activation/repression in rat brain after PCP occur predominantly in limbic regions, including hippocampus (26, 27). Moreover, an increase in N-methyl-d-aspartic acid (NMDA)-sensitive glutamate receptor binding occurs exclusively in rat hippocampus 12–24 hours after PCP administration (28, 29). The selective PCP agonist MK801 also demonstrates these actions. One parsimonious explanation for these PCP-induced neurochemical changes in rat hippocampus is that PCP administration causes inhibition at the NMDA-sensitive glutamate receptor in the trisynaptic perforant pathway, and the effect is additive within the hippocampal projection fields and becomes greatest in the Schaffer collateral terminal field of CA1. This inhibition could result in a functionally significant reduction in glutamatergic output from the hippocampal cortex to other limbic regions, including but not limited to an effect on the anterior cingulate cortex. We have speculated that this reduction in excitatory output from hippocampus and reduced afferent cingulate stimulation is associated with psychosis across psychotic diagnoses, whether the psychotic symptoms are induced by PCP or associated with schizophrenic psychosis (25).
These findings, along with additional data (25, 30), encouraged a hypothesis of reduced glutamatergic output from hippocampus and insufficient afferent activation of the anterior cingulate cortex as a mechanism for human psychosis and for aspects of schizophrenia, especially positive psychotic symptoms and possibly cognitive dysfunction. To test this hypothesis further, we collected postmortem brain tissue from persons with schizophrenia and a healthy comparison group and measured ionotropic glutamate receptor binding and NMDA receptor subunit composition in hippocampus, looking for evidence of a regional endogenous alteration in glutamate-mediated neurotransmission in schizophrenia.
Method
Materials
3H-Glutamic acid (54.1 Ci/mmol), 3H-amino-3-hydroxy-5-methylisoxazol-4-propionate (AMPA) (56.6 Ci/mmol), and 3H kainic acid (58 Ci/mmol) were obtained from New England Nuclear (Boston). NMDA, AMPA, kainate, quisqualate, and potassium thiocyanate were purchased from Sigma (St. Louis). Deoxyadenosine 5′-a-(35S) thiotriphosphate trimethylammonium salt (35S-dATP) (1000 Ci/mmol) was obtained from Amersham (Piscataway, N.J.).
Postmortem Human Tissue
Postmortem human brain tissue was analyzed in three different tissue sets, one from the Maryland Brain Collection of the Maryland Psychiatric Research Center, Baltimore, and two from the Melbourne Brain Collection of the Mental Health Research Institute, Parkville, Victoria, Australia. The tissue from the Maryland Brain Collection was collected from 12 normal subjects and 16 subjects with schizophrenia. The tissue from the Melbourne Brain Collection was collected from 19 normal subjects and 19 subjects with schizophrenia. The normal and schizophrenia groups within each sample were matched as closely as possible on age, sex, and postmortem interval. (For donors whose deaths were witnessed, the postmortem interval was the time between death and autopsy. For others, the postmortem interval was halfway between the time the donor was found dead and the time the donor was last seen alive.) Tissue was rapidly frozen to –70°C and stored until required. The tissue storage time at –70°C before assay did not differ between the schizophrenia group and the normal comparison group. In addition, in the tissue sets from the Melbourne Brain Collection, the pH of the brain tissue was measured, as described previously (31), and did not differ between the two groups. Coronal tissue slices were cut approximately 2 cm thick at a mid-anterior level of the hippocampus, and the hippocampus was identified and blocked. Blocks posterior to the fimbria but anterior to the anterior-posterior midpoint of the hippocampus were used, obtained similarly in the schizophrenia tissue and the comparison tissue in each tissue set. However, neither landmarks nor histologic characteristics were used to standardize the anterior-posterior position. Regions in the hippocampal tissue that were measured are identified in Figure 1. Blocks were covered with mounting media (Lipshaw, Detroit) to reduce desiccation and stored at –70°C until further processing. Sections were cut at 20 μm using a cryostat, thaw-mounted onto Probe-On microscope slides (Fisher, Pittsburgh), and stored at –70°C until further processing. For tissue from Melbourne Brain Collection, frozen 20 μm sections were cut in Australia from hippocampal tissue blocks and placed on Probe-On slides. The slides were placed in a slide box with desiccant and air freighted to the United States in excess dry ice.
After the collection of tissue, two experienced research clinicians carried out an extensive review of the case histories of the subjects with a provisional diagnosis of schizophrenia by using a structured instrument (32). Diagnoses of schizophrenia were confirmed by consensus of the clinicians according to DSM-III-R criteria. Demographic characteristics associated with the tissue are presented in Table 1. The most recent doses of antipsychotic drugs recorded in the case histories were noted and equivalence calculated (33).
Chronic Administration of Antipsychotic Drugs in Rats
Because antipsychotic treatment is nearly ubiquitous in persons with schizophrenia, we tested whether the density of glutamate receptors in schizophrenia could be confounded by effects of antipsychotic treatment on the receptors. We measured the effects of chronic administration of haloperidol on the density of glutamate receptors and NMDA subunits in rat brain.
We treated Sprague Dawley rats (Charles River Laboratories, Inc., Wilmington, MA,) with water containing haloperidol or no drug for 6 months and compared ionotropic glutamate receptor density in hippocampus in both groups of rats to suggest any change associated with chronic antipsychotic treatment in the measures we assessed in human tissue. The rats were given drinking water containing either no drug or haloperidol (34) and housed in 12-hour light/dark conditions with ad libitum food and water. Haloperidol was dissolved in 10% glacial acetic acid. The solution was diluted with distilled water and the pH was adjusted to 5.5–6.0 by using 10 N sodium hydroxide to give a stock solution of 0.25 mg/ml. This solution was further diluted with distilled water to 0.025 mg/ml every 7 days. The dose of haloperidol in the drinking water was modified according to the measured daily water intake of the rats. Trunk blood was collected at the time of sacrifice at 6 months, and the plasma was frozen at –20°C until analysis. Haloperidol was quantified by using a modification of the method of Bianchetti and Morsel in which chlorohaloperidol was used as the internal standard and reduced haloperidol was simultaneously determined. Quantification of haloperidol was done in the laboratory of Thomas Cooper (Nathan S. Kline Institute, Orangeburg, N.Y.).
Rats were sacrificed by decapitation at the end of the period of chronic drug administration. The brains were rapidly removed, frozen by immersion in –40°C isopentane, mounted using embedding matrix, and stored at –80°C. Coronal sections (20 μm thick) were cut in a cryostat from several coronal levels, thaw-mounted onto Probe-On microscope slides, and dried at room temperature under an airflow. The slides were kept desiccated at –80°C until they were used in the binding experiments.
Receptor Autoradiography
All receptor and message analysis was carried out in the Maryland Psychiatric Research Center laboratory. For glutamate receptor binding, all sections were initially washed for 30 minutes and preincubated for 10 minutes in appropriate buffer at 0–4°C to eliminate endogenous glutamate. For NMDA receptor binding, slides were incubated for 45 minutes at 0–4°C with 100 nM 3H-glutamate in the presence of 2.5 μM quisqualate and 1 μM kainate in 50 mM Tris acetate buffer at pH 7.0. For nonspecific binding, 500 μM NMDA was used. For AMPA receptor binding, slides were incubated for 45 minutes at 0–4°C with 37 μM 3H AMPA in the presence of 2.5 mM CaCl2 and 100 mM potassium thiocyanate in 50 mM Tris HCl buffer at pH 7.2. For nonspecific binding, 1 mM glutamate was used. For kainate receptor binding, slides were incubated for 30 minutes at 0–4°C with 50 nM 3H-kainate in 50 mM Tris citrate buffer at pH 7.0. For nonspecific binding, 100 μM kainate was used. All sections were rinsed, then blown dry under a stream of warm air. Dried sections were placed in X-ray cassettes along with 3H standards (Amersham) for 5 days (AMPA) or 2 weeks (NMDA and kainate) at 4°C. Quantitative analysis of autoradiograms were performed by using a Compaq computer-based densitometer and image analyzer (Loats Inc., Westminster, Md.).
In Situ Hybridization
For quantification of the NMDA receptor subunit NR1, the following oligo probe sequence was used: 5′-GCCATCTGCCACCAGGTGCACCTCGTAGGTGAAGTTCATGGTCCGTGCCAGCTTGATGAGCAGGTC-3′ (35).
For NR2A, the oligo probe sequence was 5′-AGAAGGCCCGTGGGAGCTTTCCCTTTGGCTAAGTTTC-3′ (36).
For NR2B, the oligo probe sequence was 5′-GGGCCTCCTGGCTCTCTGCCATCGGCTAGGCACCTGTTGTAACCC-3′ (36).
In situ hybridization experiments, including probe labeling, hybridization, and posthybridization washing, were performed as previously described (37). Briefly, the labeled probes were prepared with 35S dATP and terminal transferase (United States Biochemical Corp., Cleveland). The sections were hybridized overnight at 37°C; the hybridization buffer consisted of 50% deionized formamide, 4 saline sodium citrate buffer, 1 Denhardt’s solution, 10% dextran sulfate, 250 μg/ml yeast tRNA, 500 μg/ml single-strand salmon DNA, and 100 mM dithiothreitol. After the overnight hybridization, the sections were rinsed four times for 15 minutes each in 2 saline sodium citrate buffer containing 50% formamide at 46°C, two times for 30 minutes each in 1 saline sodium citrate buffer at room temperature, and then 2 minutes each in 70%, 90%, and 100% ethanol, and air dried. For autoradiography, sections were apposed to Hyperfilm Bmax (Amersham) along with 14C microscales standards at 4°C and exposed for 2 weeks. Films were developed using Kodak D-19. Films of the sections were digitized and their optical densities read in anatomical regions of interest by using quantitative densitometry on an Inquiry System (Loats, Inc.).
Statistical Analysis
Analysis of variance (ANOVA) was used to analyze demographic characteristics associated with the three sets of postmortem tissue samples. Analysis of variance was used to analyze the relationship between tissue set (Maryland Brain Collection, Melbourne Brain Collection 1, Melbourne Brain Collection 2) and diagnosis (normal or schizophrenia) for each dependent variable (level of NR1, NMDA, and kainate) for which data were available from all three tissue sets. For variables with data for two tissue sets (AMPA), a two-by-two ANOVA was used. Independent t tests were used for variables with data for one tissue set (NR2A, NR2B) and to compare data for rats that were given haloperidol or no drug. Significant differences by tissue set were found for postmortem interval (F=41.32, df=2, 63, p=0.0001) and gender (F=15.83, df=2, 63, p=0.0001) (Table 1). However, no diagnosis or interaction effects with diagnosis were present.
Results
Glutamate Receptor Binding
NMDA-sensitive glutamate receptor binding did not differ by diagnosis (schizophrenia tissue versus normal comparison tissue) in any subregions of the hippocampal or parahippocampal cortex where it was assessed (Table 2). The three tissue sets differed in the level of NMDA-sensitive glutamate receptor binding in entorhinal cortex (F=4.47, df=1, 24, p=0.05), but there was no interaction of tissue set with site or diagnosis. Representative autoradiograms for NMDA receptor binding for schizophrenia tissue and normal comparison tissue are shown in Figure 1 (panels E and F).
Kainate receptor binding did not differ by diagnosis in hippocampus (Table 2). The three sets of tissue samples differed in the level of kainate receptor binding in CA1 (F=41.48, df=1, 45, p=0.0001), CA2 (F=27.25, df=1, 37, p=0.0001), CA3 (F=20.51, df=1, 39, p=0.0001), dentate gyrus (F=9.90, df=1, 38, p=0.003), and entorhinal cortex (F=9.45, df=1, 43, p=0.004), but there was no interaction of tissue set with site and diagnosis nor of tissue set and site. Unlike previous studies (38, 39), our analysis did not show a lower level of kainate binding in CA2 in schizophrenia than in normal comparison tissue (F=1.38, df=1, 37, p=0.25). Representative autoradiograms for kainate receptor binding are shown in Figure 1 (panels C and D).
The level of AMPA receptor binding was similar in the schizophrenia tissue and the normal comparison tissue in all subregions analyzed, except for a small but significant difference in CA2, where there was less AMPA receptor binding in schizophrenia (F=5.62, df=1, 25, p=0.03). The three sets of tissue samples differed in the level of AMPA receptor binding in CA1 (F=36.75, df=1, 30, p=0.0001), CA2 (F=64.33, df=1, 25, p=0.001), CA3 (F=33.45, df=1, 27, p=0.0001), dentate gyrus (F=30.98, df=1, 26, p=0.0001), and entorhinal cortex (F=55.79, df=1, 27, p=0.0001), but there were no interactions of tissue set with any other variable (Table 2). Representative autoradiographs for AMPA receptor binding are shown in Figure 1 (panels A and B).
Glutamate Receptor Binding and Chronic Antipsychotic Administration
NMDA, kainate, and AMPA receptor binding in hippocampal subregions of rat brain were similar in rats that received the typical antipsychotic haloperidol for 6 months and in control rats (Table 3). The mean plasma level of haloperidol when rats received 1.5 mg/kg/day of the drug was 6.8 ng/ml (SD=1.1) in trunk blood at sacrifice in our laboratory (34).
NMDA Subunit mRNA
The level of mRNA for the NR1 subunit was significantly lower in the dentate gyrus in the schizophrenia tissue than in the normal comparison tissue (F=4.86, df=1, 42, p=0.03) (Table 4, Figure 2). In addition, the level of NR1 mRNA in CA3was 25.2% lower in the schizophrenia tissue than in the normal comparison tissue, although the difference was not significant (F=3.35, df=1, 39, p=0.07). The three sets of tissue samples differed in the level of NR1 mRNA in CA1 (F=5.92, df=1, 49, p=0.02), but there were no interactions of tissue set with diagnosis. In all tissue sets, any differences in level of NR1 mRNA by diagnosis, even if not significant, showed lower levels in schizophrenia tissue.
The level of mRNA for the NR2A subunit showed no differences between the schizophrenia tissue and the normal comparison tissue. However, the level of NR2A mRNA in CA2 was 20% lower in the schizophrenia tissue than in the normal comparison tissue, although the difference was not significant (t=0.52, df=10, p=0.62) (Table 4). For this analysis, only tissue from the Maryland Brain Collection was available.
The level of mRNA for the NR2B subunit in CA2 was 39.6% higher in the schizophrenia tissue than in the normal comparison tissue, a significant difference (t=4.00, df=10, p=0.003) (Table 4, Figure 2). In CA3 the level of NR2B mRNA was 19.2% higher in schizophrenia tissue, although the difference was not significant (t=2.11, df=10, p=0.06). For this analysis, only tissue from the Maryland Brain Collection was available. The differences in NR2B mRNA between schizophrenia tissue and normal comparison tissue characteristically showed a higher level in schizophrenia; the nonsignificant differences in NR2A mRNA also showed a higher level in schizophrenia, but the difference in NR1 mRNA showed a lower level in schizophrenia.
Discussion
The hippocampus has been a consistent focus of biologic study in schizophrenia, yielding several replicable observations of abnormality. This emphasis has been encouraged by observations of behavioral disruptions in the illness that suggest hippocampal dysfunction (40, 41). The behavioral actions of PCP in healthy humans and in schizophrenia have implicated the blockade of glutamate transmission at the NMDA receptor in psychosis (22, 24 , 25, 30, 42). Moreover, specific anatomic data exist (reviewed below) that support hippocampal pathology related to glutamate in the illness.
Several alterations in neurochemical measures of glutamatergic and related transmitter function in the postmortem hippocampus and/or the related dorsoventral temporal cortex in schizophrenia have been identified. Because the glutamate system has several different receptor families, receptor configurations, transmitters/modulators, and modulating receptor sites, the task of focusing a search for a pathologic site involving glutamate neurotransmission has been challenging. Although there appears to be no difference in the density of hippocampal NMDA glutamate receptors in schizophrenia and in normal tissue (39, 43, 44), a lower level of kainate binding, particularly in CA2, has been found (38, 39, 45), but not consistently (46). Lower levels of non-NMDA receptor binding (39) and lower concentrations of non-NMDA receptor mRNA (47) have both been reported in CA3 in schizophrenia. Moreover, lower levels of the glutamate receptor subunit mRNAs for GluR2, KA2 and GluR6 (kainate and AMPA subunits) have been found selectively in hippocampus in schizophrenia (48), and a higher flip/flop isoform ratio has been identified for AMPA receptors (49). However, in one study, the level of GluR1–GluR7 mRNA subunits appeared no different in schizophrenia than in normal tissue in all brain regions evaluated (50), whereas another study found a lower level of GluR1 mRNA in the parahippocampal gyrus and a lower level of GluR2 mRNA in several hippocampal subregions in schizophrenia (51). These results suggest possible, but inconsistently found, differences in the kainate and AMPA receptor complexes in schizophrenia.
A higher level of binding to NR1/NR2B receptors has been reported in superior temporal cortex in schizophrenia (52). The level of aspartate binding, putatively to presynaptic elements, has been reported to be either higher (46) or no different (45) in temporal cortex in schizophrenia than in normal tissue. Reports of lower levels of hippocampal synaptophysin are consistent with a lower density of synapses in hippocampus in schizophrenia (53). Some evidence supports higher glutamate concentrations within temporal cortex, but not in hippocampus, in schizophrenia (54). Release of glutamate and release of γ-aminobutyric acid (GABA) may be lower in schizophrenia tissue (55). Differences in GABAA receptor density, in GABA release, and in glutamate-related transmitters and their enzymes in hippocampus have been reported in the illness (30, 45, 56). Often, more pronounced or significant differences have been found within the left compared to the right hemisphere of the temporal cortex (57). Although these findings taken together do not support a single, simple interpretation of glutamatergic involvement in schizophrenia, they do indicate potential instability in ionotropic glutamate transmission in hippocampus in this illness. The data reported here fit into a growing published literature on the complexity of glutamatergic transmission in brain, its distinctive regional characteristics, and our still evolving understanding of the primary elements of the system (58).
In the study reported here, the hippocampal block dissections were not entirely standardized with respect to their position along the anterior-posterior axis; thus, the pathologic findings could not be localized along the long axis of the hippocampus with any precision. On the basis of segregated afferent and efferent pathways and the now suggested anterior-posterior hippocampal differences in schizophrenia (59), it has become apparent that this localization may be important. Consequently, we have begun a new examination of the whole hippocampus in schizophrenia using a specialized tissue collection technique to check if these reported abnormalities are regionally localized within hippocampus.
The hypothesis underlying the study reported here was based on the possibility that PCP-induced psychosis and schizophrenic psychosis might share a common biologic mechanism. The mechanism of PCP-induced psychosis, inferred from animal studies done in this laboratory (27, 28), is reduced glutamatergic transmission at the NMDA receptor within the trisynaptic hippocampal glutamatergic pathway (25). These findings suggest the simplistic idea that schizophrenia tissue would evidence the same up-regulation of the NMDA receptor (as a marker of glutamatergic transmission at those hippocampal synapses) as the rat PCP preparation (28). The rat studies of chronic haloperidol administration showed no difference in these parameters with antidopaminergic actions, suggesting that findings for NMDA receptors could be distinguished from the effects of chronic drug treatment.
The initial hypothesis, that we would find NMDA receptor up-regulation in hippocampus, particularly in CA1or CA3, secondary to an internal disruption of hippocampal glutamatergic transmission, was not confirmed by this study. Using levels of receptor density as an index of an alteration in glutamate mediated transmission in hippocampus, the results suggest no differences between the schizophrenia tissue and the normal comparison tissue. These results are consistent with reports from other laboratories (39, 43, 44).
Subsequent analysis of the expression of NMDA receptor subunits was indicated to fully examine the a priori hypothesis. The NR1receptor subunit of the multimeric NMDA receptor is the critical subunit for the ionophore function of gating calcium ions. The NR1subunit has multiple isoforms that have distinct pharmacologic properties (60); consequently considerable diversity exists among brain NMDA receptors. The NR2 family of NMDA subunits, which are also found in hippocampus, have four members: NR2A, NR2B, NR2C, and NR2D. The NMDA receptor on hippocampal pyramidal cells is thought to be composed of NR1, NR2A, and NR2B subunits, with the NR2A and NR2B subunits present in only moderate concentrations. The NR2C and NR2D subunits are expressed at the lowest levels in hippocampus and are thought to combine with the NR1 subunit located preferentially on the hippocampal interneurons. Experimentally constituted receptors, lacking the NR1 subunit, fail to generate a functioning NMDA ionophore that can gate ionic calcium flow (36). NMDA receptors composed of NR1, NR2A, and NR2B subunits demonstrate significant pharmacologic, functional, and anatomic differences from NMDA receptors composed of NR1, NR2C, and NR2D subunits (60). For example, in recombinant NMDA receptor studies in vitro, the NR1-NR2A-NR2B receptors increased the elevation of calcium that is modulated by protein kinase C and mediated by NMDA receptors, whereas the NR1-NR2C-NR2D receptors decreased this intracellular calcium measure (62). In this paper, we have evaluated the subunit message that purportedly directly modulates hippocampal pyramidal neuronal activity, i.e., the mRNA of the NR1, NR2A, and NR2B subunits.
A lower level of NR1 expression and a higher level of NR2A or NR2B expression, as reported here, are consistent with the idea that the composition of at least some of the hippocampal NMDA receptors—those that reside on pyramidal neurons—may be abnormal in schizophrenia. A linkage analysis of schizophrenia in an African tribal family suggested that alterations in the NMDA receptor may provide a genetic predisposition to the illness (63), although this observation needs further replication. Molecular techniques have provided mouse models of regionally reduced NR1-composed NMDA receptors using antisense (64), adenovirus (65), or customized recombination systems (66). These mouse preparations show behavioral alterations in the whole animal (67). Such animal models may provide critical preclinical systems for understanding the biologic consequences of lower levels of NR1. Indeed, transgenic mice with a lower than normal expression of the NR1 NMDA receptor subunit have some characteristics similar to those observed in pharmacologically induced animal models of schizophrenia (68).
An NMDA receptor lacking the NR1 subunit would be unable to gate calcium and thus would not be fully functional, but it could demonstrate full ligand binding. One might tentatively suggest, based on this evidence, that several subregions of the hippocampus may have a lower number of functioning NMDA receptors in schizophrenia, compared with normal tissue, and may thus be “hypoglutamatergic.” This altered receptor mechanism would reduce NMDA-mediated neural transmission not through receptor blockade but by abnormal receptor composition and consequently abnormal function. The outcome of this difference in receptor composition would be reduced transmission at the NMDA receptor. Perhaps any mechanism that compromises hippocampal NMDA-sensitive glutamate-mediated neurotransmission in an analogous fashion would produce psychosis. These speculations would be further supported by the demonstration of lower levels of NR1 protein and higher levels of NR2A and NR2B protein in hippocampal subregions in schizophrenia and by documentation of NR2C and NR2D levels, efforts that are now ongoing in our laboratory.
These early studies suggest an abnormality of the composition of the NMDA-sensitive glutamate receptor in hippocampus in schizophrenia. Preliminary data from other laboratories show NMDA subunit alterations in thalamus (69) and in superior temporal cortex (52) in schizophrenia, suggesting that such differences in subunits might not be limited to hippocampus. Moreover, higher levels of NR2B subunit protein in hippocampus in schizophrenia has recently been reported from another laboratory, suggesting that higher levels of NR2B mRNA will translate into higher levels of NR2B protein (70). Although the interpretation of the data remains tentative, it is consistent with considerable evidence documenting glutamate-related abnormalities of limbic cortex and pathology in hippocampus in schizophrenia.
 |
 |
 |
 |
Received July 26, 1999; revision received Dec. 15, 1999; accepted Dec. 22, 1999. From the Maryland Psychiatric Research Center, University of Maryland School of Medicine; and the Mental Health Research Institute, Parkville, Victoria, Australia. Address reprint requests to Dr. Tamminga, Maryland Psychiatric Research Center, University of Maryland, Box 21247, Baltimore, MD 21228; ctamming@ mprc.umaryland.edu (e-mail).
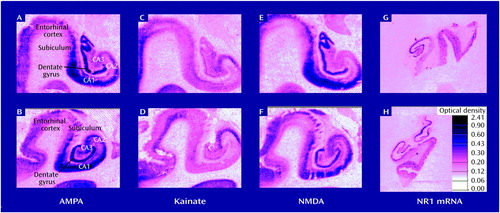
Figure 1. Autoradiograms of Postmortem Brain Sections Showing NMDA, Kainate, and AMPA Receptor Binding and Level of mRNA for NMDA Subunit NR1 in Hippocampal Subregions of Normal Comparison Subjects and Subjects With Schizophreniaa
aPanels A, C, E, and G: tissue from normal comparison subjects; panels B, D, F, and H: tissue from subjects with schizophrenia.
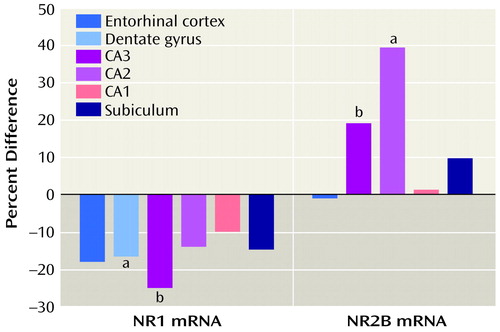
Figure 2. Percentage Difference From Normal Comparison Levels of mRNA for NMDA Receptor Subunits NR1 and NR2B in Hippocampal Subregions of Subjects With Schizophrenia
aSignificant difference between level in schizophrenia and normal level (p<0.05).
bNonsignificant difference between level in schizophrenia and normal level (p<0.10).
1. Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA: Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res 1990; 35:1–13Crossref, Medline, Google Scholar
2. Bogerts B, Falkai P, Haupts M, Greve B, Ernst S, Tapernon-Franz U, Heinzmann U: Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics: initial results from a new brain collection. Schizophr Res 1990; 3:295–301Crossref, Medline, Google Scholar
3. Becker T, Elmer K, Schneider F, Schneider M, Grodd W, Bartels M, Heckers S, Beckmann H: Confirmation of reduced temporal limbic structure volume on magnetic resonance imaging in male patients with schizophrenia. Psychiatry Res 1996; 67:135–143Crossref, Medline, Google Scholar
4. Bilder RM, Bogerts B, Ashtari M, Wu H, Alvir JM, Jody D, Reiter G, Bell L, Lieberman JA: Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophr Res 1995; 17:47–58Crossref, Medline, Google Scholar
5. Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR Jr, Weinberger DR: Temporal lobe pathology in schizophrenia: a quantitative magnetic resonance imaging study. Am J Psychiatry 1989; 146:464–472Link, Google Scholar
6. Kovelman JA, Scheibel AB: A neurohistological correlate of schizophrenia. Biol Psychiatry 1984; 19:1601–1621Google Scholar
7. Heckers S, Heinsen H, Geiger B, Beckmann H: Hippocampal neuron number in schizophrenia. Arch Gen Psychiatry 1991; 48:1002–1008Google Scholar
8. Jeste DV, Lohr JB: Hippocampal pathologic findings in schizophrenia: a morphometric study. Arch Gen Psychiatry 1989; 46:1019–1024Google Scholar
9. Falkai P, Bogerts B: Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 1986; 236:154–161Crossref, Medline, Google Scholar
10. Benes FM, Kwok EW, Vincent SL, Todtenkopf MS: A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry 1998; 44:88–97Crossref, Medline, Google Scholar
11. Benes FM, Sorensen I, Bird ED: Reduced neuronal size in posterior hippocampus of schizophrenic patients. Schizophr Bull 1991; 17:597–608Crossref, Medline, Google Scholar
12. Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE: The hippocampus and para-hippocampus in schizophrenic, suicide, and control brains. Arch Gen Psychiatry 1990; 47:1029–1034Google Scholar
13. Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT: Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar
14. Nordahl TE, Kusubov N, Carter C, Salamat S, Cummings AM, O’Shora-Celaya L, Eberling J, Robertson L, Huesman RH, Jagust W, Budinger TF: Temporal lobe metabolic differences in medication-free outpatients with schizophrenia via the PET-600. Neuropsychopharmacology 1996; 15:541–554Crossref, Medline, Google Scholar
15. Haznedar MM, Buchsbaum MS, Luu C, Hazlett EA, Siegel BV Jr, Lohr J, Wu J, Haier RJ, Bunney WE Jr: Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry 1997; 154:682–684Link, Google Scholar
16. Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM: Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1998; 1:318–323Crossref, Medline, Google Scholar
17. Fletcher P: The missing link: a failure of fronto-hippocampal integration in schizophrenia (comment). Nat Neurosci 1998; 1:266–267Crossref, Medline, Google Scholar
18. Gruzelier J, Seymour K, Wilson L, Jolley A, Hirsch S: Impairments on neuropsychologic tests of temporohippocampal and frontohippocampal functions and word fluency in remitting schizophrenia and affective disorders. Arch Gen Psychiatry 1988; 45:623–629Crossref, Medline, Google Scholar
19. Venables PH: Hippocampal function and schizophrenia: experimental psychological evidence. Ann NY Acad Sci 1992; 658:111–127Crossref, Medline, Google Scholar
20. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Google Scholar
21. Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R: Study of a new schizophrenomimetic drug: serenyl. Arch Neurol Psychiatry 1959; 71:363–369Crossref, Google Scholar
22. Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner D, Heninger GR, Bowers MB, Charney DS: Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51:199–214Crossref, Medline, Google Scholar
23. Pearlson GD: Psychiatric and medical syndromes associated with phencyclidine (PCP) abuse. Johns Hopkins Med J 1981; 148:25–33Medline, Google Scholar
24. Lahti AC, Koffel B, LaPorte D, Tamminga CA: Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995; 13:9–19Crossref, Medline, Google Scholar
25. Tamminga CA: Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol 1998; 12:21–36Crossref, Medline, Google Scholar
26. Gao XM, Shirakawa O, Du F, Tamminga CA: Delayed regional metabolic actions of phencyclidine. Eur J Pharmacol 1993; 241:7–15Crossref, Medline, Google Scholar
27. Gao XM, Hashimoto T, Tamminga CA: Phencyclidine (PCP) and dizocilpine (MK801) exert time-dependent effects on the expression of immediate early genes in rat brain. Synapse 1998; 29:14–28Crossref, Medline, Google Scholar
28. Gao XM, Tamminga CA: MK801 induces late regional increases in NMDA and kainate receptor binding in rat brain. J Neural Transm Gen Sect 1995; 101:105–113Crossref, Medline, Google Scholar
29. Gao XM, Tamminga CA: Phencyclidine produces changes in NMDA and kainate receptor binding in rat hippocampus over a 48-hour time course. Synapse 1996; 23:274–279Crossref, Medline, Google Scholar
30. Tsai G, Passani LA, Slusher BS, Carter R, Baer L, Kleinman JE, Coyle JT: Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry 1995; 52:829–836Crossref, Medline, Google Scholar
31. Kingsbury AE, Foster OJ, Nisbet AP, Cairns N, Bray L, Eve DJ, Lees AJ, Marsden CD: Tissue pH as an indicator of mRNA preservation in human postmortem brain. Brain Res Mol Brain Res 1995; 28:311–318Crossref, Medline, Google Scholar
32. Hill C, Keks NA, Roberts S, Opeskin K, Dean B, MacKinnon A, Copolov D: Problem of diagnosis in postmortem brain studies of schizophrenia. Am J Psychiatry 1996; 153:533–537Link, Google Scholar
33. Foster P: Neuroleptic equivalence. Pharmaceutical J 1998; 243:431–432Google Scholar
34. Gao XM, Hashimoto T, Cooper TB, Tamminga CA: The dose-response characteristics of rat oral dyskinesias with chronic haloperidol or clozapine administration. J Neural Transm 1997; 104:97–104Crossref, Medline, Google Scholar
35. Bockers TM, Zimmer M, Muller A, Bergmann M, Brose N, Kreutz MR: Expression of the NMDA R1 in selected human brain regions. Neuroreport 1994; 5:965–969Crossref, Medline, Google Scholar
36. Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH: Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994; 12:529–540Crossref, Medline, Google Scholar
37. Gao XM, Tamminga CA: An increase in NMDA-sensitive [ 3H]glutamate and [3H]kainate binding in hippocampus 24 hours after PCP. Neurosci Lett 1994; 174:149–153Crossref, Medline, Google Scholar
38. Kerwin RW, Patel S, Meldrum B, Czudek C, Reynolds GP: Asymmetrical loss of glutamate receptor subtype in left hippocampus in schizophrenia. Lancet 1988; 1:583–584Crossref, Medline, Google Scholar
39. Kerwin R, Patel S, Meldrum B: Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain postmortem. Neuroscience 1990; 39:25–32Crossref, Medline, Google Scholar
40. Stevens JR: An anatomy of schizophrenia? Arch Gen Psychiatry 1973; 29:177–189Google Scholar
41. Gray JA: Dopamine release in the nucleus accumbens: the perspective from aberrations of consciousness in schizophrenia. Neuropsychologia 1995; 33:1143–1153Google Scholar
42. Javitt DC, Zukin SR: The role of excitatory amino acids in neuropsychiatric illness. J Neuropsychiatry Clin Neurosci 1990; 2:44–52Crossref, Medline, Google Scholar
43. Kornhuber J, Mack-Burkhardt F, Riederer P, Hebenstreit GF, Reynolds GP, Andrews HB, Beckmann H: [3H] MK-801 binding items in postmortem brain regions of schizophrenic patients. J Neural Transm 1989; 77:231–236Crossref, Medline, Google Scholar
44. Ishimaru M, Kurumaji A, Toru M: NMDA-associated glycine binding site increases in schizophrenic brains (letter). Biol Psychiatry 1992; 32:379–381Crossref, Medline, Google Scholar
45. Simpson MD, Slater P, Royston MC, Deakin JF: Regionally selective deficits in uptake sites for glutamate and gamma-aminobutyric acid in the basal ganglia in schizophrenia. Psychiatry Res 1992; 42:273–282Crossref, Medline, Google Scholar
46. Deakin JF, Slater P, Simpson MD, Gilchrist AC, Skan WJ, Royston MC, Reynolds GP, Cross AJ: Frontal cortical and left temporal glutamatergic dysfunction in schizophrenia. J Neurochem 1989; 52:1781–1786Google Scholar
47. Harrison PJ, Kerwin RW, McLaughlin D: Decreased hippocampal expression of a glutamate receptor gene in schizophrenia. Lancet 1991; 337:450–452Crossref, Medline, Google Scholar
48. Porter RH, Eastwood SL, Harrison PJ: Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex, and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res 1997; 751:217–231Crossref, Medline, Google Scholar
49. Eastwood SL, Burnet PW, Harrison PJ: GluR2 glutamate receptor subunit flip and flop isoforms are decreased in the hippocampal formation in schizophrenia: a reverse transcriptase-polymerase chain reaction (RT-PCR) study. Brain Res Mol Brain Res 1997; 44:92–98Crossref, Medline, Google Scholar
50. Breese CR, Freedman R, Leonard SS: Glutamate receptor subtype expression in human postmortem brain tissue from schizophrenics and alcohol abusers. Brain Res 1995; 674:82–90Crossref, Medline, Google Scholar
51. Reynolds, Czudek C, Andrews HB: Deficit and hemispheric asymmetry of GABA uptake sites in the hippocampus in schizophrenia. Biol Psychiatry 1990; 27:1038–1044Google Scholar
52. Grimwood S, Slater P, Deakin JFW, Hutson PH: NR2B-containing NMDA receptors are up-regulated in temporal cortex in schizophrenia. Neuroreport 1999; 10:461–465Crossref, Medline, Google Scholar
53. Eastwood SL, Harrison PJ: Decreased synaptophysin in the medial temporal lobe in schizophrenia demonstrated using immunoautoradiography. Neuroscience 1995; 69:339–343Crossref, Medline, Google Scholar
54. Kutay FZ, Pogun S, Hariri NI, Peker G, Erlacin S: Free amino acid level determinations in normal and schizophrenic brain. Prog Neuropsychopharmacol Biol Psychiatry 1989; 13:119–126Crossref, Medline, Google Scholar
55. Sherman AD, Hegwood TS, Baruah S, Waziri R: Deficient NMDA-mediated glutamate release from synaptosomes of schizophrenics. Biol Psychiatry 1991; 30:1191–1198Google Scholar
56. Simpson MDC, Slater P, Deakin JFW, Royston MC, Skan WJ: Reduced GABA uptake sites in the temporal lobe in schizophrenia. Neurosci Lett 1989; 107:211–215Crossref, Medline, Google Scholar
57. Zaidel DW, Esiri MM, Harrison PJ: The hippocampus in schizophrenia: lateralized increase in neuronal density and altered cytoarchitectural asymmetry. Psychol Med 1997; 27:703–713Crossref, Medline, Google Scholar
58. Wolosker H, Blackshaw S, Snyder SII: Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci USA 1999; 96:13409–13414Google Scholar
59. Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI: Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci USA 1998; 95:11406–11411Google Scholar
60. Tanaka M, Caudle RM, Benoliel R, Finegold AA, Iadorola MJ: Cellular neuroanatomy of NMDA NR1 splice variants. Abstracts of the Society for Neuroscience 1998; 24:340Google Scholar
61. Brimecombe JC, Boeckman FA, Aizenman E: Functional consequences of NR2 subunit composition in single recombinant N-methyl-d-aspartate receptors. Proc Natl Acad Sci USA 1997; 94:11019–11024Google Scholar
62. Grant ER, Bacskai BJ, Anegawa NJ, Pleasure DE, Lynch DR: Opposing contributions of NR1 and NR2 to protein kinase C modulation of NMDA receptors. J Neurochem 1998; 71:1471–1481Google Scholar
63. Riley BP, Tahir E, Rajagopalan S, Mogudi-Carter M, Faure S, Weissenbach J, Jenkins T, Williamson R: A linkage study of the N-methyl-d-aspartate receptor subunit gene loci and schizophrenia in southern African Bantu-speaking families. Psychiatr Genet 1997; 7:57–74Crossref, Medline, Google Scholar
64. Roberts EB, Meredith MA, Ramoa AS: Suppression of NMDA receptor function using antisense DNA block ocular dominance plasticity while preserving visual responses. J Neurophysiol 1998; 80:1021–1032Google Scholar
65. Kammesheidt A, Kato K, Ito K, Sumikawa K: Adenovirus-mediated NMDA receptor knockouts in the rat hippocampal CA1 region. Neuroreport 1997; 8:635–638Crossref, Medline, Google Scholar
66. Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S: Subregion- and cell type-restricted gene knockout in mouse brain. Cell 1996; 87:1317–1326Google Scholar
67. McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA: Impaired hippocampal representation of space in CA1-specific NMDA R1 knockout mice. Cell 1996; 87:1339–1349Google Scholar
68. Mohn AR, Gainetdinov RR, Caron MG, Koller BH: Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 1999; 98:427–436Crossref, Medline, Google Scholar
69. Meador-Woodruff JH, Ibrahim HM, Richardson-Burns SM, Hogg AJ Jr, Haroutunian V, Davis KL, Watson SJ Jr: Neurochemical anatomy of the thalamus in schizophrenia: glutamate-specific abnormalities. Schizophr Res 1999; 36:74Google Scholar
70. Pearson BJ: Expression of the NR2B subunit of the NMDA receptor is increased in postmortem hippocampus of schizophrenics. Schizophr Res 1999; 36:75Google Scholar



