Levels-of-Processing Effect on Frontotemporal Function in Schizophrenia During Word Encoding and Recognition
Abstract
OBJECTIVE: Patients with schizophrenia improve episodic memory accuracy when given organizational strategies through levels-of-processing paradigms. This study tested if improvement is accompanied by normalized frontotemporal function. METHOD: Event-related blood-oxygen-level-dependent functional magnetic resonance imaging (fMRI) was used to measure activation during shallow (perceptual) and deep (semantic) word encoding and recognition in 14 patients with schizophrenia and 14 healthy comparison subjects. RESULTS: Despite slower and less accurate overall word classification, the patients showed normal levels-of-processing effects, with faster and more accurate recognition of deeply processed words. These effects were accompanied by left ventrolateral prefrontal activation during encoding in both groups, although the thalamus, hippocampus, and lingual gyrus were overactivated in the patients. During word recognition, the patients showed overactivation in the left frontal pole and had a less robust right prefrontal response. CONCLUSIONS: Evidence of normal levels-of-processing effects and left prefrontal activation suggests that patients with schizophrenia can form and maintain semantic representations when they are provided with organizational cues and can improve their word encoding and retrieval. Areas of overactivation suggest residual inefficiencies. Nevertheless, the effect of teaching organizational strategies on episodic memory and brain function is a worthwhile topic for future interventional studies.
Individuals with schizophrenia have selectively impaired verbal episodic memory (1) against a background of more generalized cognitive dysfunction (see reference 2 for exception). Memory impairments appear to be due more to encoding and retrieval deficits than to long-term storage problems (3–5). Less consistent impairments on recognition than on free recall have led some researchers to stress the importance of encoding over retrieval (6, 7), although a recent meta-analysis (8) found moderate effects on recognition and large effects on free recall, suggesting that both stages of memory processing are disrupted. It is important to determine the stage of memory impairment in schizophrenia, as this information can help target pharmacological and behavioral interventions. An inherent limitation of neuropsychological testing is that encoding cannot be independently assessed because retrieval is required to obtain performance indices.
This limitation can be overcome by neuroimaging, which can be used to assess brain function during both stages of task performance. In previous positron emission tomography (9) and functional magnetic resonance imaging studies (10), we found that right anterior prefrontal activation during recognition was equal in patients and comparison subjects, suggesting that associated episodic retrieval mechanisms were relatively intact. In contrast, there was prominent left prefrontal underactivation and bilateral temporal-limbic overactivation during encoding and retrieval. Patients’ self-reports suggested that these frontotemporal abnormalities might have been due to differences during encoding, with patients relying on rote rehearsal rather than on more effective semantic organizational strategies (10). To explore the role of semantic processing, encoding strategies were directly manipulated in a recent behavioral study (11), in which we found that patients showed the same benefit as healthy comparison subjects from semantic versus perceptual encoding (i.e., levels-of-processing effect) on recognition speed and accuracy. This study demonstrated that providing a semantic strategy improves patients’ performance. The purpose of the current study was to determine if providing this strategy also normalizes brain function during both stages of memory processing, or if there are residual abnormalities that cannot be explained by encoding strategies.
In the current study, we again employed a levels-of-processing technique. In this paradigm, Craik and Lockhart (12) found that as information processing moves from shallow perceptual processing to more elaborative semantic-associative encoding, the strength of the memory trace increases. This increase results in a levels-of-processing effect in which retrieval is better when it follows deep processing. Because this paradigm provides participants with explicit encoding instructions, the need to generate organizational strategies is reduced. Although the paradigm can be used with verbal and nonverbal stimuli, the current study used words, and participants were required to determine if the words were presented in uppercase or lowercase letters (shallow) or if words were concrete or abstract (deep). Functional neuroimaging in healthy volunteers has demonstrated that deep versus shallow word processing is accompanied by increased prefrontal and hippocampal activation, with left ventrolateral prefrontal effects during encoding and right anterior prefrontal activation during retrieval (13–15).
Imaging of episodic memory in schizophrenia has revealed abnormalities in many of these same prefrontal and hippocampal regions (16). Levels-of-processing paradigms are well suited to test the hypothesis that frontotemporal abnormalities during memory performance may be due to patients’ failure to adopt a semantic encoding strategy. However, relatively few experiments have been performed. Heckers and colleagues (17, 18) asked patients to study word lists under shallow and deep conditions and measured blood flow during cued-recall word-stem completion. Although recall was lower in patients, both groups benefited similarly from deep encoding. Between-group comparisons revealed greater right hippocampal activation in the comparison subjects and greater prefrontal activation in the patients. Hippocampal response was blunted because of patient overactivation during baseline and shallow retrieval. A subsequent functional magnetic resonance imaging (fMRI) study examined participants during encoding and tested recognition outside the magnet (19). Although both groups in this study benefited equally from deep processing, the patients showed decreased left prefrontal and increased left superior temporal activation for deep versus shallow words. Thus, there is evidence of both under- and overactivation of temporal-limbic and prefrontal cortex regions depending on stage of processing, and no study has examined encoding and retrieval in the same group of subjects.
The subjects in the current study underwent fMRI during word encoding and recognition in a levels-of-processing paradigm. As noted previously, when we examined this paradigm behaviorally (11), we found unimpaired levels-of-processing effects on recognition accuracy, suggesting that semantic processing was sufficiently intact for patients to benefit from organizational cues. This background provided support for our first hypothesis that left ventrolateral prefrontal activation to deep versus shallow words is unimpaired in patients during word encoding. Based on previous findings (9–11), we also hypothesized that patients do not show impairments in the right anterior prefrontal cortex during retrieval of words that had undergone deep versus shallow processing. If these hypotheses were supported and residual differences in temporal-limbic function during encoding or recognition were found, these results would suggest the presence of other information processing abnormalities that are not due to top-down frontally mediated organizational processes.
Method
Participants
Participants were 14 patients with schizophrenia (two female) and 14 healthy comparison subjects (one female) from the Schizophrenia Research Center at the University of Pennsylvania. One comparison subject who developed a psychiatric illness, one noncompliant patient, and one patient with excessive movement were excluded. Groups did not differ in age (comparison subjects: mean=31.4 years, SD=6.5; patients: mean=35.1 years, SD=8.0), education (comparison subjects: mean=14.1 years, SD=1.8; patients: mean=13.3 years, SD=2.7), parental education (comparison subjects: mean=14.3 years, SD=2.4; patients: mean=13.3 years, SD=2.9), or reading level, as measured by the National Adult Reading Test (20) (comparison subjects: mean level=29.9 years, SD=9.2; patients: mean level=27.9 years, SD=7.5). All comparison subjects and all but two patients were right-handed. The comparison subjects underwent a standard evaluation (21), including administration of the Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (22), and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (23). They had no history of illness affecting brain function or any major psychiatric illness in first-degree relatives.
The psychiatric evaluation for patients included clinical assessment; structured interview (24); history obtained from family, care providers, and records; and scales for measuring symptoms and functioning administered by investigators trained to a criterion reliability of 0.90 (intraclass correlation). All patients had a DSM-IV diagnosis of schizophrenia established in a consensus conference based on all information available and had no history of any other disorder or event that might affect brain function. Patients were mildly to moderately ill according to Scale for the Assessment of Negative Symptoms (SANS) (25) (mean score=27.4, SD=15.6, range=2–62), Scale for the Assessment of Positive Symptoms (SAPS) (26) (mean score=18.1, SD=13.4, range=0–48), and Brief Psychiatric Rating Scale (BPRS) (27) (mean score=30.5, SD=8.0, range=18–43). All patients were receiving medication; three received typical antipsychotics, 10 received atypical antipsychotics, and one received both typical and atypical antipsychotics. Average daily doses were 415.0 mg/day in chlorpromazine equivalents (SD=85, range=160–500) of typical antipsychotics and 20.9 mg/day in olanzapine equivalents (SD=1.4, range=5.0–45.0) of atypical antipsychotics. No patient was receiving anticholinergics. The patients’ mean age at illness onset was 20.6 years (SD=3.1), and the mean duration of illness was 14.6 years (SD=9.4). After complete description of the study to the subjects, written informed consent was obtained.
Tasks
Images were acquired during four conditions: word encoding, letter N-back, word recognition, and source monitoring. N-back and source monitoring results will be reported separately. Encoding and recognition tasks utilized a previously described levels-of-processing paradigm (11). Words were randomly assigned to four shallow and four deep blocks of 10 words each. During shallow encoding, participants made a left button press for uppercase letters and right button press for lowercase letters. During deep encoding, a left button press was made for concrete words and right button press was made for abstract words. Shallow and deep blocks were alternated in pseudorandom order with no more than two block repetitions in a row. Words were presented for 3 seconds each with “jittered” interstimulus intervals of 0–9 seconds to permit event-related analysis. Visual fixation cross-hairs appeared between stimuli. Instructions (e.g., “press if the word is concrete or abstract”) appeared for 6 seconds, followed by 6 seconds of cross-hairs during block transitions. Abbreviated instructions remained visible (Figure 1) to reduce working memory demands. Images from the 12-second instruction periods were not included in the analysis. Task time was 12.5 minutes.
For recognition, 40 target stimuli (20 uppercase, 20 lowercase) from the encoding task were randomly chosen (20 shallow, 20 deep) and mixed with 20 novel stimuli (10 uppercase, 10 lowercase) matched for length, frequency, and concreteness. The 40 remaining targets were reserved for source monitoring. Words were presented for 3 seconds with “jittered” interstimulus intervals of 0–12 seconds. Participants were instructed to press a left button if the word was a previous target (“old”) and a right button if it had not been presented (“new”) and to guess if unsure. Abbreviated response instructions remained visible, and fixation cross-hairs appeared between stimuli. Total task time was 6.5 minutes.
Tasks were triggered by the scanner and coupled to image acquisition by using Power Laboratory (28) on a Macintosh computer (Apple, Cupertino, Calif.). Stimuli were rear-projected to the center of the visual field by using a Power Lite 7300 video projector (Epson America, Long Beach, Calif.) and viewed through a mirror mounted on the head coil. Responses were recorded with a nonferromagnetic keypad (FORP, Current Design Inc., Philadelphia). Practice was given, and all participants understood the instructions and use of the keypad.
Image Acquisition
Data were acquired on a clinically approved 3-T Siemens Trio Scanner (Siemens USA, Malvern, Pa.). A 5-minute magnetization-prepared, rapid acquisition gradient echo image was acquired for anatomic overlays of functional data and spatial normalization (29). fMRI was acquired with blood-oxygenation-level-dependent (BOLD) imaging (30) by using a 36-slice whole-brain, single-shot gradient-echo, echo-planar sequence (TR/TE=3000/30 msec, field of view=240 mm, matrix=64×64, slice thickness/gap=3/0 mm).
Data Analysis
Percentage of correct responses and median reaction time (in milliseconds) were calculated to index classification accuracy and speed. Because the sampling distribution of a proportion does not satisfy normality assumptions, arcsine transformation (31) was applied to percentage scores. During recognition, responses were scored as correct “old” responses to targets, correct “new” responses to novels, incorrect “new” responses to targets, and incorrect “old” responses to novels. Recognition discriminability and response bias were calculated according to the Two-High Threshold Theory (32). Recognition discriminability assesses recognition accuracy, and response bias was categorized as liberal or conservative (response bias >0.5=“liberal” bias; response bias <0.5=“conservative” bias). Reaction time for correct responses was used to index psychomotor speed. Group differences were tested by entering response bias and recognition discriminability into separate two (patient, comparison subject) by two (shallow, deep) multivariate analyses of variance (Proc GLM) (SAS Institute, Cary, N.C.) (33) with repeated measures for the second factor. Analysis of variance was used to decompose any significant interactions and test component recognition scores for group differences.
fMRI data were preprocessed in SPM2 (Wellcome Department of Cognitive Neurology, University College, London) and MEDx (version 3.43) (Sensor Systems, Inc., Sterling, Va.) by using standard procedures (10). In SPM2, images were motion-corrected to the median image with trilinear interpolation. The resulting translational motion parameters did not differ between groups (F=0.81, df=1, 26, p=0.37) or tasks (F=0.13, df=1, 26, p=0.72). In MEDx, images were selectively scaled (34) to the mean of voxels not significantly correlated (p<0.01) with the task, band-pass filtered (0.016–0.125 Hz), and spatially smoothed (8 mm full width at half maximum, isotropic).
Subject-level statistical analyses were performed by using the general linear model in SPM2. Condition events were modeled with a canonical hemodynamic response function. For encoding, there were four event types: correct shallow, correct deep, interblock instructions, and errors. For recognition, there were four event types: correct shallow, correct deep, correct novel, and errors. Contrast maps were obtained through linear contrasts of event types.
Statistical analysis was completed for each individual in their own stereotaxic space, and contrast maps were Talairach-transformed in two steps by using MEDx. First, a least-squares surface registration algorithm (35) was used to coregister the median image to the structural image. Coregistration parameters were applied to individuals’ contrast maps. Second, a nonlinear transformation into Talairach space was performed based on commissural landmarks identified by a trained investigator.
Group-level random-effects analyses were performed in SPM2 for within- and between-group comparisons. Within-group analyses were accomplished by entering whole brain contrasts for comparison subjects and patients separately into one-sample t tests. Between-group analyses were completed with inclusive masks in SPM2 to restrict contrasts to voxels with above-threshold responses for either group. The resulting contrast maps were entered into two-sample t tests. SPM{T} maps were transformed to unit normal distribution SPM{Z}. Significance thresholds were based on spatial extent (k) and peak height (u). We used a height threshold corresponding to an uncorrected p value of 0.005, which required a minimum of eight voxels in a cluster, resulting in corrected probability of p<0.05 (36).
Results
Performance
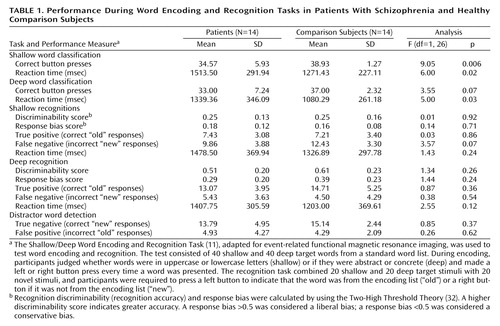
As previously noted (11), patients were slower and less accurate than comparison subjects in classifying words during encoding (F=5.99, df=1, 26, p<0.05). There was also a main effect of level-of-processing (F=29.63, df=1, 26, p<0.0001) and interactions between test variable and levels-of-processing (F=29.58, df=1, 26, p<0.0001) and test variable and diagnosis (F=6.0, df=1, 28, p<0.05). Across groups, healthy participants were more accurate (F=8.67, df=1, 26, p<0.05) but slower (F=29.61, df=1, 26, p<0.0001) in performing shallow versus deep word classification tasks. As Table 1 shows, although patients were slower during both conditions, differences in classification accuracy were significant for shallow encoding and approached significance for deep encoding. Thus, the patients showed normal levels-of-processing effects on word classification speed and accuracy but were slower and less accurate than the comparison subjects in word classification.
Recognition results replicated previous findings of unimpaired levels-of-processing effects. Both groups had better discriminability after deep encoding (F=79.65, df=1, 26, p<0.0001), with no effect of diagnosis (F=0.69, df=1, 26, p=0.41) or any interaction of diagnosis with levels-of-processing (F=1.59, df=1, 26, p=0.22). When response bias was examined, main effects were found for levels-of-processing (F=36.38, df=1, 26, p<0.0001), and no main effects (F=0.53, df=1, 26, p=0.47) or interactions (F=3.81, df=1, 26, p=0.06) were found for diagnosis. Our previous finding of a more conservative bias in patients after deep encoding was not replicated. As Table 1 shows, no group differences were found in component recognition scores.
Given that a growing literature suggests that atypical antipsychotics may have beneficial effects on cognition (37), we also performed an exploratory analysis to determine if performance differences existed between patients receiving typical (N=3) versus atypical (N=11) antipsychotic medication. Two-sample t tests were performed to detect any medication subgroup differences in the performance measures reported in Table 1. Tests were also performed on demographic and clinical variables. The analyses revealed that the patients who were taking typical antipsychotics performed worse during deep word classification and deep recognition tasks. Compared to the patients taking atypical antipsychotics, the patients taking typical antipsychotics had slower classification speed (t=3.61, df=11, p<0.005), lower recognition discriminability (t=–4.26, df=11, p<0.005), fewer true positive responses (t=–3.22, df=11, p<0.05), more false negative responses (t=3.76, df=11, p<0.005), and slower reaction times during deep recognition (t=3.09, df=11, p<0.01). There were no demographic (age, education, parental education) or clinical differences (duration of illness, BPRS total score, SAPS total score) between medication subgroups except that the group receiving typical antipsychotics had more severe negative symptoms, as measured by the SANS (t=3.77, df=9.9, p<0.005). Of these results, only the differences in deep recognition discriminability and reaction time survived a Bonferroni correction for multiple comparisons.
Task-Related BOLD Change
Word encoding
Table 2 presents results for correct deep minus shallow word classification. As Figure 2 (upper panel) shows, both groups activated the left inferior frontal gyrus. Activation was restricted to the ventrolateral prefrontal cortex in the comparison subjects (Brodmann’s area 47), but in the patients it extended from Broca’s area (Brodmann’s area 44) to the ventrolateral prefrontal cortex (Brodmann’s area 47) (peak voxel-level coordinates: x=–52, y=22, z=–0; z score=3.86). The comparison subjects also activated the frontal pole (Brodmann’s area 10) and insula. In the patients, additional activation was seen in the temporal-limbic and posterior regions, including left parahippocampal (Brodmann’s area 36) and superior temporal gyri (Brodmann’s area 22), right caudate tail, and cerebellum.
Between-group contrasts did not reveal any areas of greater activation in the healthy participants. Areas of greater activation in the patients included the left thalamus and lingual gyrus (Brodmann’s area 19) (Figure 2, lower panel). Greater thalamic activation extended to the hippocampus (peak voxel-level coordinates: x=–32, y=–42, z=–4; z score=3.37).
Word recognition
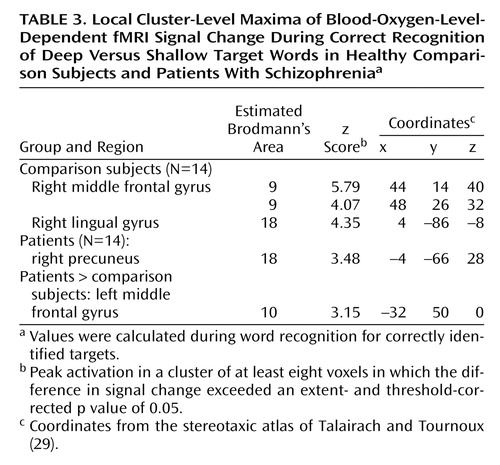
Table 3 presents results for successful recognition of deep versus shallow targets. As Figure 3 (upper panel) shows, the right dorsolateral prefrontal (Brodmann’s area 9) and visual association (Brodmann’s area 18) areas were activated in the healthy comparison subjects. Although the visual association regions (Brodmann’s area 18) were also activated in the patients, the right dorsolateral prefrontal cortex was not activated in the patients. However, between-group contrasts did not reveal any areas of greater activation in the comparison subjects. Reanalysis at a lower threshold (p<0.1, corrected) confirmed that task-related activity could be visualized in the right dorsolateral prefrontal cortex of the patients (peak voxel-level coordinates: x=56, y=22, z=8; z score=2.54; Brodmann’s area 45/46) at a less conservative threshold. In between-group contrasts, there were no areas of greater activation in the comparison subjects, but the patients showed greater activation in the left frontal pole (Brodmann’s area 10) (Figure 3).
Discussion
The current results supported our first hypothesis and showed that when organizational demands are reduced, patients can engage the left ventrolateral prefrontal cortex (Brodmann’s area 47) during semantic versus perceptual encoding and produce a normal levels-of-processing effect on word recognition. As reported previously (11), words were recognized more quickly and accurately after deep processing in both groups. Although no differences were found between groups in left ventrolateral prefrontal cortex function, the activation patterns were not identical, and our second hypothesis was not fully supported. During encoding, the patients were slower and less accurate in classifying words, and they showed left hemispheric overactivation in the thalamus, hippocampus, and lingual gyrus. During recognition, activity in the right prefrontal cortex was above threshold only for the comparison subjects, although no significant difference between groups was found when the results for the two groups were directly contrasted. The patients also showed overactivation of the left frontal pole during retrieval. These results indicate that patients can engage the left prefrontal cortex during semantic processing and argue against a static lesion model of hypofrontality (38). However, in the patients, residual patterns of temporal-limbic overactivation during encoding and less robust right anterior prefrontal activation during retrieval suggest the presence of other information processing difficulties during both stages of memory processing that are independent of top-down frontally mediated semantic organizational control.
The left ventrolateral prefrontal cortex was activated during encoding in both groups. This region is consistently associated with depth of processing (39). As noted by Kubicki and colleagues (19), primate studies show that the ventrolateral prefrontal cortex receives its largest input from posterior and temporal lobe regions (40), supporting its role in maintenance and activation of episodic information in working memory. However, the notion that the region’s role is limited to semantic working memory was recently challenged by Otten and colleagues (15), who found the expected left ventrolateral prefrontal cortex activation for deep versus shallow encoding. However, they also found that ventrolateral prefrontal cortex activation predicted successful word recognition regardless of encoding condition. These findings suggest a more general role for the ventrolateral prefrontal cortex. In the current study we did not investigate retrieval success because too few events would have resulted if we followed recommendations (15) to limit analysis to words that were recognized with a high level of confidence. However, in the context of the analyses that were performed, the results seem to support the conclusion that the left ventrolateral prefrontal cortex facilitates depth-of-processing in both groups by activating and maintaining semantic representations in working memory.
Left hemisphere overactivation during encoding suggests a residual disturbance in distributed mnemonic or semantic processing in schizophrenia. Group comparisons revealed overactivation in the patients in the left hemisphere in the thalamus, hippocampus, and lingual gyrus during encoding and in the frontal pole during retrieval. Left temporal hyperactivity has been documented in previous studies of schizophrenia (41), and evidence of more diffuse patient activation is consistent with previous findings (10). In an earlier study, we speculated that parahippocampal overactivation could be explained by a model of disrupted or reversed functional connectivity of prefrontal and temporal-limbic structures (42). It is notable that prefrontal activation was reduced, consistent with the reversal hypothesis. Similar reversals were seen in levels-of-processing studies, with lowered prefrontal and increased superior temporal activation during encoding (19) and increased prefrontal and reduced hippocampal activity during cued recall (17, 18).
Unlike previous studies, the current study found evidence of hippocampal overactivation during encoding despite unimpaired left prefrontal response. This dissociation of prefrontal and temporal-limbic function does not fit the classic reversal model and suggests that hippocampal hyperactivity may be independent of top-down prefrontal control. It is possible that overactivation reflects reduced inhibition of lexical networks in schizophrenia. Inclusion of left-handers does not appear to be responsible, as overactivation in the patients was also found when data for the two left-handed patients were removed. A number of studies have demonstrated increased semantic associative priming in schizophrenia, especially when symptoms of formal thought disorder are present (43). During semantic processing, patients may have experienced increased spread of activation (44), resulting in functional overactivation. In an exploratory analysis we directly tested the role of thought disorder by using a two-sample t test in SPM2 to contrast patients with (N=6) and without thought disorder (N=8) as measured by the SAPS (analysis available on request). We did not find any subgroup differences in the BOLD response to deep correct encoding minus shallow correct encoding in the hippocampus or other areas of patient overactivation even at a liberal significance threshold of p<0.01 (uncorrected). However, the range of thought disorder was restricted in the current group of subjects, and a more robust test of the role of thought disorder in hippocampal overactivation during semantic processing will require testing of patients with a wider range of thought disorder.
During recognition testing, semantic processing appeared sufficient to support robust levels-of-processing effects on performance. However, activity in the right prefrontal cortex was not as robust in the patients, suggesting possible differences in retrieval strategies. Although both groups activated the right visual association areas, only the comparison subjects produced above-threshold effects in a right prefrontal region traditionally associated with episodic retrieval (45). This group difference appeared to be a matter of degree, as right prefrontal activity was seen in the patients at a lower threshold and no difference between groups was found in a direct contrast. Nevertheless, the less robust right prefrontal activation in the patients may be due to the patients’ relying more on a sense of familiarity than on episodic knowledge during retrieval (46). The increased spread of activation in the patients during semantic encoding might result in a less secure memory trace, leading to greater reliance on familiarity effects.
Previously we found a more conservative response bias in patients after deep encoding and concluded that this response may reflect problems in source monitoring (11). Although we did not replicate this finding in the current study, the patients’ response bias for deep words was in a more conservative direction. Lack of group differences may have been due to reduced power because of the smaller number of subjects. Analysis of fMRI data acquired with source memory probes will permit direct examination of memory trace and retrieval strategies.
Several concerns raised by the current study should be considered. Unlike previous studies (17, 18), we did not find evidence of hippocampal activation in the comparison subjects. Our ability to detect hippocampal overactivation in the patients argues against a problem in fMRI sensitivity. A likely explanation is that the recognition task did not require subjects to make a conscious recollection of the memory event, which likely reduced hippocampal demands (47). A future study employing recognition and recall probes may help resolve this issue. Our results were also inconsistent with those of a study that found attenuated left ventrolateral prefrontal cortex response in patients with schizophrenia (19). It is unclear whether this discrepancy is due to the patients’ heterogeneity or to differences in experimental design and analysis procedures. One design difference is that the previous study required more frequent alternation between encoding conditions. Greater response alternation demands may have increased frontal lobe activation in the patients during shallow encoding and accounted for the lack of differences between conditions.
We studied patients who were taking medication, and the patients were more closely matched to comparison subjects than in many of our previous studies. These factors limit the generalizability of the results to more acutely ill and thought-disordered patients. However, the approach also helped control potential confounding variables and ensured that the patients were able to complete the study. It is unlikely that medication explains the current fMRI results, as previous studies have not found medication effects on prefrontal or mesial temporal function (10, 17, 18) (see reference 48 for exception). However, in an exploratory analysis, a subgroup of patients receiving typical neuroleptics (N=3) did appear to have more severe negative symptoms and worse performance during semantic encoding and recognition, compared to patients receiving atypical neuroleptics (N=11). This finding is consistent with the notion that atypical neuroleptics may have beneficial cognitive effects (37) and argues for larger-scale studies that can test the effect of typical versus atypical medications on fMRI activation. Finally, the finding that recognition memory and left prefrontal function can be restored in clinically stable patients holds promise for remediation efforts. A worthy goal is to develop interventions to facilitate patients’ use of organizational strategies during initial encoding and to test if these interventions improve memory and functional outcome.
 |
 |
 |
Presented at the Conference on Human Brain Mapping, Budapest, Hungary, June 13–17, 2004. Received July 22, 2004; revision received Sept. 27, 2004; accepted Oct. 1, 2004. From the Departments of Psychiatry and Radiology, University of Pennsylvania; and the Health Psychology Program, University of the Sciences in Philadelphia. Address correspondence and reprint requests to Dr. Ragland, Department of Psychiatry, 10th Floor Gates Bldg./HUP, University of Pennsylvania, Philadelphia, PA 19104; [email protected] (e-mail). Supported by NIH grants MH-62103, NS-045839, and M01 RR-0040.The authors thank Wendy Snyder for subject accrual and Colleen Martin for data management.
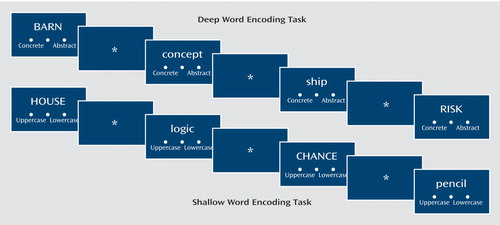
Figure 1. Word Presentation During the Shallow and Deep Word Encoding Tasksa
aEncoding stimuli included a representation of the three-button response pad to remind participants when to make a left button press (concrete or uppercase) or a right button press (abstract or lowercase) when the word is presented. During recognition (not illustrated) a left button press was made for a previously seen “old” word, and a right button press was made for a “new” word that was not part of the encoding list. In both tasks words were presented for 3 seconds followed by a visual fixation cross-hair that was presented for 0–9 seconds during encoding and 0–12 seconds during recognition (jittered interstimulus interval).
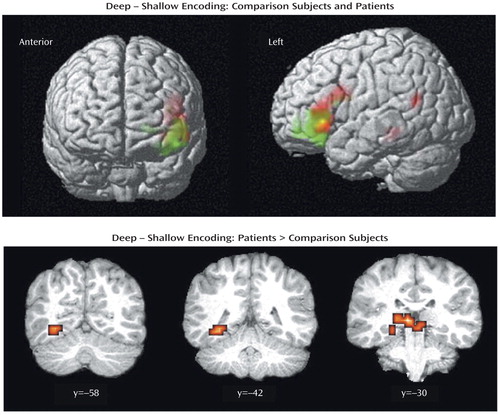
Figure 2. Brain Images Showing Similarities and Differences Between Comparison Subjects (N=14) and Patients With Schizophrenia (N=14) in Blood-Oxygen-Level-Dependent fMRI Signal Change During Correct Deep Minus Shallow Word Encodinga
aIn the upper panel statistical parametric maps are surface-rendered on smoothed brain images to illustrate similarities in left inferior frontal activation in the patients (red color) and the comparison subjects (green color). Overlapping activation is illustrated in yellow. In the lower panel statistical parametric maps are rendered on coronal slices to illustrate greater activation in the patients, versus the comparison subjects, in left lingual gyrus (y=–58), hippocampus (y=–42), and thalamus (y=–30). Between-group contrasts showed no areas of significantly greater activation in the comparison subjects versus the patients. Colored areas indicate a difference in signal change that exceeds a threshold corresponding to a corrected p value of 0.05.
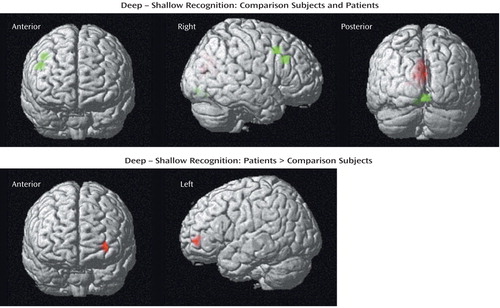
Figure 3. Brain Images Showing Similarities and Differences Between Comparison Subjects (N=14) and Patients With Schizophrenia (N=14) in Blood-Oxygen-Level-Dependent fMRI Signal Change During Correct Deep Minus Shallow Word Recognitiona
aIn the upper panel statistical parametric maps are surface-rendered on smoothed brain images to illustrate activation of the occipital cortex in the patients (red color) and the comparison subjects (green color) and activation of the right middle frontal gyrus in the comparison subjects (green color). In the lower panel statistical parametric maps are surface-rendered on smoothed brain images to illustrate greater activation in the left frontal pole (red color) in the patients versus the comparison subjects. Between-group contrasts showed no areas of significantly greater activation in the comparison subjects, versus the patients. Colored areas indicate a difference in signal change that exceeds a threshold corresponding to a corrected p value of 0.05.
1. Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
2. Blanchard JJ, Neale JM: The neuropsychological signature of schizophrenia: generalized or differential deficit? Am J Psychiatry 1994; 151:40–48Link, Google Scholar
3. Gold JM, Randolph C, Carpenter CJ, Goldberg TE, Weinberger DR: Forms of memory failure in schizophrenia. J Abnorm Psychol 1992; 101:487–494Crossref, Medline, Google Scholar
4. Heinrichs RW, Awad AG: Neurocognitive subtypes of chronic schizophrenia. Schizophr Res 1993; 9:49–58Crossref, Medline, Google Scholar
5. Paulsen JS, Heaton RK, Sadek JR, Perry W, Delis DC, Braff D, Kuck J, Zisook S, Jeste DV: The nature of learning and memory impairments in schizophrenia. J Int Neuropsychol Soc 1995; 1:88–99Crossref, Medline, Google Scholar
6. Heaton R, Paulsen JS, McAdams LA, Kuck J, Zisook S, Braff D, Harris J, Jeste DV: Neuropsychological deficits in schizophrenics: relationship to age, chronicity, and dementia. Arch Gen Psychiatry 1994; 51:469–476Crossref, Medline, Google Scholar
7. McClain L: Encoding and retrieval in schizophrenics’ free recall. J Nerv Ment Dis 1983; 171:471–479Crossref, Medline, Google Scholar
8. Aleman A, Hijman R, de Haan EHF, Kahn RS: Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry 1999; 156:1358–1366Abstract, Google Scholar
9. Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE: Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry 2001; 158:1114–1125Link, Google Scholar
10. Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE: Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry 2004; 161:1004–1015Link, Google Scholar
11. Ragland JD, Moelter ST, McGrath C, Hill SK, Gur RE, Bilker WB, Siegel SJ, Gur RC: Levels-of-processing effect on word recognition in schizophrenia. Biol Psychiatry 2003; 54:1154–1161Crossref, Medline, Google Scholar
12. Craik FIM, Lockart RS: Levels of processing: a framework for memory research. J Verbal Learning Verbal Behavior 1972; 11:671–684Crossref, Google Scholar
13. Buckner RL, Koutstaal W, Schacter DL, Dale AM, Rotte M, Rosen BR: Functional-anatomic study of episodic retrieval, II: selective averaging of event-related fMRI trials to test the retrieval success hypothesis. Neuroimage 1998; 7:163–175Crossref, Medline, Google Scholar
14. Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD: Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci 1995; 15:5870–5878Crossref, Medline, Google Scholar
15. Otten LJ, Henson RN, Rugg MD: Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain 2001; 124:399–412Crossref, Medline, Google Scholar
16. Weiss AP, Heckers S: Neuroimaging of declarative memory in schizophrenia. Scand J Psychol 2001; 42:239–250Crossref, Medline, Google Scholar
17. Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM: Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1998; 1:318–323Crossref, Medline, Google Scholar
18. Weiss AP, Schacter DL, Goff DC, Rauch SL, Alpert NM, Fischman AJ, Heckers S: Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry 2003; 53:48–55Crossref, Medline, Google Scholar
19. Kubicki M, McCarley RW, Nestor PG, Huh T, Kikinis R, Shenton ME, Wible CG: An fMRI study of semantic processing in men with schizophrenia. Neuroimage 2003; 20:1923–1933Crossref, Medline, Google Scholar
20. Nelson HE: The National Adult Reading Test (NART). Windsor, UK, Nelson, 1982Google Scholar
21. Shtasel DL, Gur RE, Mozley PD, Richards J, Taleff MM, Heimberg C, Gallacher F, Gur RC: Volunteers for biomedical research: recruitment and screening of normal controls. Arch Gen Psychiatry 1991; 48:1022–1025Crossref, Medline, Google Scholar
22. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (SCID-I/NP), version 2.0. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
23. First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L: Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II): User’s Guide. Washington, DC, American Psychiatric Press, 1997Google Scholar
24. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
25. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
26. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
27. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
28. Chute DL, Westall RF: Power Laboratory. Devon, Pa, MacLaboratory, 1997Google Scholar
29. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
30. Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS: Time course of EPI of human brain function during task activation. Magn Reson Med 1992; 25:390–397Crossref, Medline, Google Scholar
31. Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
32. Snodgrass JG, Corwin J: Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 1988; 117:34–50Crossref, Medline, Google Scholar
33. SAS/STAT User’s Guide, version 8. Cary, NC, SAS Institute, 1999Google Scholar
34. Andersson JL: How to estimate global activity independent of changes in local activity. Neuroimage 1997; 6:237–244Crossref, Medline, Google Scholar
35. Pelizzari CA, Chen GT, Spelbring DR, Weichselbaum RR, Chen CT: Accurate three-dimensional registration of CT, PET and/or MR images of the brain. J Comput Assist Tomogr 1989; 13:20–26Crossref, Medline, Google Scholar
36. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33:636–647Crossref, Medline, Google Scholar
37. Sharma T, Hughes C, Soni W, Kumari V: Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl) 2003; 169:398–403Crossref, Medline, Google Scholar
38. Gur RC, Gur RE: Hypofrontality in schizophrenia: RIP. Lancet 1995; 345:1383–1384Crossref, Medline, Google Scholar
39. Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD: Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 1999; 10:15–35Crossref, Medline, Google Scholar
40. Petrides M, Pandya DN: Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 2002; 16:291–310Crossref, Medline, Google Scholar
41. Gur RE: Left hemisphere dysfunction and left hemisphere overactivation in schizophrenia. J Abnorm Psychol 1978; 87:226–238Crossref, Medline, Google Scholar
42. Friston KJ, Frith CD: Schizophrenia: a disconnection syndrome? Clin Neurosci 1995; 3:89–97Medline, Google Scholar
43. Moritz S, Mersmann K, Kloss M, Jacobsen D, Wilke U, Andresen B, Naber D, Pawlik K: “Hyper-priming” in thought-disordered schizophrenic patients. Psychol Med 2001; 31:221–229Crossref, Medline, Google Scholar
44. Spitzer M: A cognitive neuroscience view of schizophrenic thought disorder. Schizophr Bull 1997; 23:29–50Crossref, Medline, Google Scholar
45. Desgranges B, Baron JC, Eustache F: The functional neuroanatomy of episodic memory: the role of the frontal lobes, the hippocampal formation, and other areas. Neuroimage 1998; 8:198–213Crossref, Medline, Google Scholar
46. Danion JM, Rizzo L, Bruant A: Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Arch Gen Psychiatry 1999; 56:639–644Crossref, Medline, Google Scholar
47. Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA: Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci 2000; 3:1149–1152Crossref, Medline, Google Scholar
48. Medoff DR, Holcomb HH, Lahti AC, Tamminga CA: Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus 2001; 11:543–550Crossref, Medline, Google Scholar



