Complexity of Prefrontal Cortical Dysfunction in Schizophrenia: More Than Up or Down
Abstract
OBJECTIVE: Numerous neuroimaging studies have examined the function of the dorsolateral prefrontal cortex in schizophrenia; although abnormalities usually are identified, it is unclear why some studies find too little activation and others too much. The authors’ goal was to explore this phenomenon. METHOD: They used the N-back working memory task and functional magnetic resonance imaging at 3 T to examine a group of 14 patients with schizophrenia and a matched comparison group of 14 healthy subjects. RESULTS: Patients’ performance was significantly worse on the two-back working memory task than that of healthy subjects. However, there were areas within the dorsolateral prefrontal cortex of the patients that were more active and areas that were less active than those of the healthy subjects. When the groups were subdivided on the basis of performance on the working memory task into healthy subjects and patients with high or low performance, locales of greater prefrontal activation and locales of less activation were found in the high-performing patients but only locales of underactivation were found in the low-performing patients. CONCLUSIONS: These findings suggest that patients with schizophrenia whose performance on the N-back working memory task is similar to that of healthy comparison subjects use greater prefrontal resources but achieve lower accuracy (i.e., inefficiency) and that other patients with schizophrenia fail to sustain the prefrontal network that processes the information, achieving even lower accuracy as a result. These findings add to other evidence that abnormalities of prefrontal cortical function in schizophrenia are not reducible to simply too much or too little activity but, rather, reflect a compromised neural strategy for handling information mediated by the dorsolateral prefrontal cortex.
Abnormal dorsolateral prefrontal cortex activation during neuroimaging studies remains a linchpin in the argument for the central role that dorsolateral prefrontal cortex neuropathology plays in schizophrenia. The most replicated finding has been one of relatively less dorsolateral prefrontal cortex function (so-called hypofrontality), but several functional magnetic resonance imaging (fMRI) studies of patients with schizophrenia have either failed to find hypofrontality (1, 2) or found greater dorsolateral prefrontal cortex activation (hyperfrontality) (3–6). Extrinsic factors such as differences in clinical populations or medication effects may contribute to these divergent findings, but variability of the response in the dorsolateral prefrontal cortex of patients with schizophrenia may arise from an intrinsic abnormality in information processing that manifests as greater or less dorsolateral prefrontal cortex activation depending on how patients handle the experimental conditions.
Using a parametric version of the N-back working memory task, we demonstrated that healthy subjects operate on an inverted-U-shaped curve as their working memory load increases and thus become relatively hypofrontal when working memory capacity is exceeded (7). Furthermore, Rypma and D’Esposito (8) demonstrated greater activation but similar performance in healthy subjects with longer reaction times compared with faster-responding subjects (reduced efficiency of information processing).
Although the relationship between working memory performance and dorsolateral prefrontal cortex activation has been demonstrated for healthy subjects (7), this relationship remains unclear in schizophrenia. Two possibilities might be considered. First, patients and healthy subjects might operate on the same proximal limb of the load-response curve, but patients might reach their capacity sooner (consistent with their limited working memory capacity [3, 4]), fall off the curve sooner, and thus appear to be hypofrontal compared with healthy subjects beyond this point. In other words, if fMRI activation is represented on the y axis and working memory load on the x axis, then at any given level of performance the activation of healthy subjects will be equal to or greater than that of patients.
The second possibility is that patients and comparison subjects operate on separable and distinct load-response curves. In this case, one would not be able to predict activation differences between patients and comparison subjects for a given level of performance. Both possibilities are illustrated in Figure 1. It is important to note that no single experiment has as yet demonstrated the existence of such curves. Nonetheless, we have favored the latter interpretation, given the demonstration of greater activation when performance is near normal in patients (4, 5). In other words, greater activation at a point where the patient’s working memory capacity has been exceeded violates the expectations of an overlapping inverted U-shaped curve. Separate curves, with patients with schizophrenia shifted to the left, appear to show that patients are hyperfrontal at relatively higher performance and hypofrontal at lower performance. Because no study has resolved this conundrum, the discussion of dorsolateral prefrontal cortex function in schizophrenia remains largely one of greater versus less activity than comparison subjects.
In fact, either scenario may be an oversimplification, or elements of both concepts may apply. Certainly, hypofrontality and hyperfrontality studies greatly outnumber negative studies, which suggests that illness results in a fundamental alteration or alterations in neural strategy adopted within the schizophrenic dorsolateral prefrontal cortex.
As part of a continuing program of examining the dynamics of dorsolateral prefrontal cortex function during executive cognition in patients with schizophrenia, we used fMRI at high field (3 T) to compare dorsolateral prefrontal cortex activation in patients with schizophrenia and healthy comparison subjects performing the N-back working memory task. We hypothesized that these data would replicate our previous finding of hyperfrontality in schizophrenic patients (4). In addition, since our group of patients with schizophrenia included both high-performing and low-performing patients, we further hypothesized that the prefrontal response of each subgroup might be differentiated on the basis of performance.
In this comparison we found the first evidence to our knowledge of concomitant areas of greater and lesser dorsolateral prefrontal cortex activation, supporting the notion that dysfunction of the dorsolateral prefrontal cortex in schizophrenia is complex and likely a reflection of the organization of neural activity, i.e., the neural strategy for information processing, rather than simply reflecting too much or too little prefrontal activity.
Method
We studied 14 patients with schizophrenia and 14 matched healthy comparison subjects. Patients were recruited from the Clinical Brain Disorders Branch Sibling Study (Protocol 95-M-0150). Patients were recruited from local and national sources advertised in print, by word of mouth, and on the Internet. Patients with DSM-IV diagnoses of schizophrenia and schizoaffective disorder were selected. Healthy volunteers were recruited from the National Institutes of Health Clinical Research Volunteer Program and were screened in an identical fashion to patients (see below). This study was approved by the Institutional Review Board of the Intramural Program of the National Institute of Mental Health. All subjects gave written, informed consent before participation.
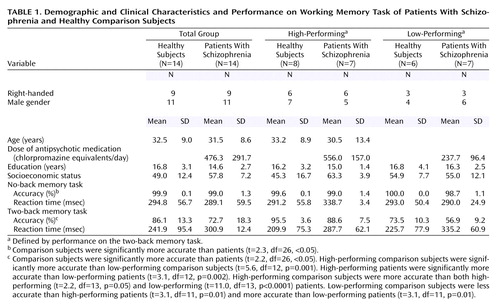
All subjects were given a Structured Clinical Interview for DSM-IV to determine the presence of any psychiatric illnesses, a neurological examination, a battery of neuropsychological tests, an EEG, and a screening MRI examination (9). Exclusion criteria included inability to give informed consent, IQ below 70, a history of substance abuse within the past 6 months, a history of significant neurological illness, and any focal abnormalities found by EEG or MRI. Healthy comparison subjects were matched to patients for age, gender, years of education, handedness, and socioeconomic status as closely as possible. All patients were receiving a stable regimen of antipsychotic medication (including either typical or atypical antipsychotics) (Table 1).
All subjects underwent a training session immediately before fMRI data acquisition to practice the N-back task. Before the fMRI scanning session, all subjects were trained to the point at which their performance (accuracy) for the task remained constant. In contrast to other versions of this task, our version of the N-back task consisted of continual presentation of visual stimuli in which every number was both a probe and a target (i.e., 100% target). The numbers 1–4 appeared randomly every 1.8 seconds for 500 msec at set locations at the points of a diamond-shaped box. Instructions displayed above the diamond informed subjects to recall the stimulus seen “N” previously. The stimuli were generated by a standard desktop computer running in-house software (R. Coppola) and projected onto an opaque screen at the subjects’ feet. Individuals who wore glasses were fitted with nonferromagnetic glasses matching their current prescription.
Responses were relayed to the computer by means of a fiber-optic response box with buttons arrayed in the same configuration as the stimuli presented on the screen and relayed back to the computer for tabulation of performance accuracy. The task was presented as one continual run in which 30-second epochs of either no back or two back (the latter entailing a delay between stimuli of 3.6 seconds). We recorded both accuracy (percent correct responses) and reaction time (RT).
Whole brain blood-oxygen-level-dependent fMRI data were collected on a 3-T General Electric scanner (General Electric Systems, Milwaukee) with a GE-EPI RT pulse sequence acquisition of 24 contiguous slices (echo time=30 msec, repetition time=2 seconds, flip angle=90°, field of view=24 cm, matrix=64×64, voxel dimensions=3.75×3.75×6 mm). All fMRI data were processed and spatially normalized to a common stereotaxic space (Montreal Neurologic Institute template) and analyzed with SPM 99 software (Wellcome Department of Cognitive Neurology, London [http://www.fil.ion.ucl.ac.uk/spm/]) as described previously (4, 10). Individual data were examined for excessive interscan and intrascan motion artifacts as described previously (11). Single-subject contrast maps were created by contrasting two-back and no-back tasks with a one-sample t test. All individual maps showed activation of the typical working memory network as described previously for this task (4, 7). Two one-sample t test maps for each group were combined to create a mask that limited further comparisons to those regions activated by both groups.
Single-subject contrast images were entered into a second level of analysis comparing patients with comparison subjects using analysis of variance (ANOVA). Coordinates were transformed to the standard space of Talairach and Tournoux (12) and are reported as z scores with a significance threshold of p<0.05 (uncorrected) and a minimum cluster size of 8.
Because of the performance differences noted below, the group comparison between patients and comparison subjects was rerun as an analysis of covariance (ANCOVA) with performance as a nuisance variable. Finally, both the patient group and the comparison group were subdivided to create equal subgroups that differed significantly in working memory performance. The definitions of “hypofrontality” and “hyperfrontality” refer to results taken from the statistical parametric mapping analysis (e.g., “hyperfrontality” was used when patients showed greater dorsolateral prefrontal cortex activation than comparison subjects).
There were no demographic differences between subgroups (Table 1). The eight high-performing comparison subjects were significantly more accurate than the six low-performing comparison subjects. The seven high-performing patients were significantly more accurate than the seven low-performing patients. High-performing comparison subjects were more accurate than both high-performing and low-performing patients. Low-performing comparison subjects were less accurate than high-performing schizophrenic patients and more accurate than low-performing patients (Table 1).
Group comparisons with ANOVA were made between high-performing comparison subjects and high- and low-performing patients and then between low-performing comparison subjects and high- and low-performing patients. Since our primary hypotheses involved prefrontal activation, only these results are reported here.
Results
Overall Group Differences
Because subjects were matched on a case-by-case basis, no significant demographic differences were found (Table 1). Speed of response did not differ between groups, but patients with schizophrenia performed less accurately than comparison subjects at both no-back and two-back tasks. At no-back, although performance was different, both groups performed highly accurately and near ceiling. At two-back, the patients’ performance was significantly worse, although still well above chance (25% accuracy for the N-back task) (Table 1). There were no significant differences in the number of responses (i.e., number of omitted responses) found for any comparison.
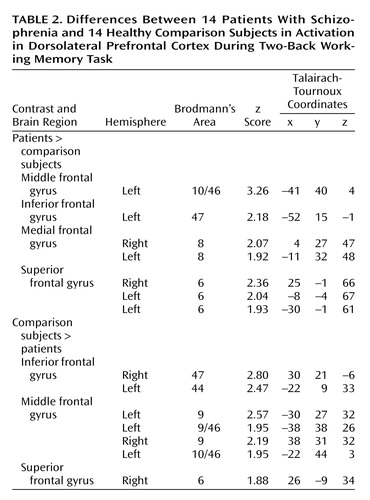
In agreement with our previous findings with this memory task (4), the most significant overall group difference in activation of the dorsolateral prefrontal cortex seen on fMRI was an area of greater activation (hyperfrontal) in patients in spite of overall poorer accuracy (Table 2, Figure 2). However, unlike our previous comparison of patients and healthy subjects during the two-back task, we also identified in this study group a region within the dorsolateral prefrontal cortex in which patients showed less activation. For example, patients were hypofrontal in the left middle frontal gyrus (Brodmann’s area 10/46). Elsewhere in the prefrontal cortex, other working memory areas, including the superior frontal gyrus (Brodmann’s area 6) and inferior frontal gyrus (Brodmann’s area 47), showed both greater and less activation by patients. Patients showed an exclusively greater response in bilateral medial frontal gyrus (Brodmann’s area 8). In contrast, areas in which comparison subjects showed an exclusively greater response included bilateral (Brodmann’s area 9) and left (Brodmann’s area 44) inferior frontal gyrus. These dorsolateral prefrontal cortex differences, together with widespread differences throughout the working memory network, suggest that although both groups used a similar working memory network, different neural strategies were engaged during information processing. ANCOVA with performance as the covariate did not alter these relationships, suggesting that these results were a simple linear effect of performance differences.
Performance Subgroup Analyses
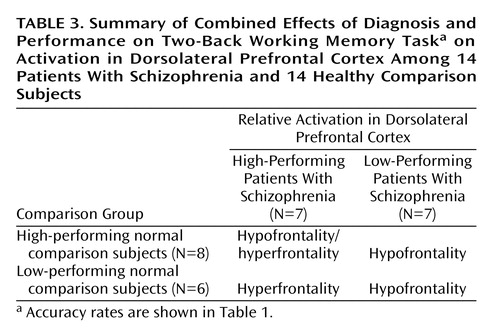
To further explore the impact of performance differences on these data, both groups were subdivided on the basis of their performance differences. The comparison of high-performing healthy subjects and high-performing patients reiterated the findings from the whole study—areas of both hypofrontality and hyperfrontality were found. High-performing comparison subjects showed greater dorsolateral prefrontal cortex activation bilaterally than high-performing patients (right Brodmann’s area 9 [Talairach-Tournoux x, y, and z coordinates=34, 35, 37], z score=2.91, p=0.002; right Brodmann’s area 9/46 [30, 38, 26], z score=1.84, p=0.03; left Brodmann’s area 9 [–30, 31, 32], z score=2.28, p=0.01) and low-performing patients (right Brodmann’s area 46 [34, 35, 9], z score=3.42, p<0.001; left Brodmann’s area 9/46 [–34, 41, 26], z score=3.44, p<0.001; left Brodmann’s area 46 [–34, 38, 15], z score=3.20, p=0.001) (Figure 3).
Low-performing comparison subjects showed no areas of greater dorsolateral prefrontal cortex activation than high-performing patients but did show greater dorsolateral prefrontal cortex activation than low-performing patients (left Brodmann’s area 46 [–34, 38, 15], z score=2.51, p=0.006; left Brodmann’s area 9/46 [–41, 38, 26], z score=2.36, p=0.009) (Figure 3). In contrast, high-performing patients showed greater left dorsolateral prefrontal cortex activation than high-performing comparison subjects (Brodmann’s area 46 [–41, 40, 4], z score=3.98, p<0.001) and low-performing comparison subjects (left Brodmann’s area 46 [–41, 40, 4], z score=2.95, p=0.002) (Figure 3). The results of these analyses are summarized in Table 3.
In summary, low-performing patients were uniformly hypofrontal. However, hyperfrontality arose as patient performance approached or surpassed that of comparison subjects, although areas of hypofunction were found in relatively higher-performing patients as well.
Discussion
In a single group of patients performing a working memory task, we found areas of both greater and less dorsolateral prefrontal cortex activation than in healthy comparison subjects performing the same task. These data support the contention that patients operate on a distinct inverted-U-shaped curve compared with healthy subjects (Figure 1). At low performance, patients with schizophrenia show primarily hypofrontality, as suggested by the comparison of low-performing patients with high- and low-performing healthy subjects. Patients whose performance was closer to that of the normal comparison subjects, however, primarily demonstrated hyperfrontality. Both findings suggest that, likely as a result of dorsolateral prefrontal cortex pathology, patients possess an abnormal neural strategy and handle working memory information differently than healthy comparison subjects do. These data suggest that neither hypofrontality nor hyperfrontality per se is a sufficient explanation for working memory deficits in schizophrenia.
The finding of less dorsolateral prefrontal cortex function in poor-performing patients resembles previous findings in positron emission tomography (PET) and fMRI (see reference 13 for review). The finding of greater activation of the left dorsolateral prefrontal cortex in patients performing well is also similar to data from previous fMRI reports in patients with schizophrenia (3, 4). Notable additional similarities include greater activation of the hippocampus in poor-performing patients than healthy subjects. Regions other than the dorsolateral prefrontal cortex also show both greater and less activation differences between groups—including the anterior cingulate, inferior parietal lobule, basal ganglia, and superior frontal gyrus.
Although pathology in these other nodes of the working memory circuit may play a role in these regional findings, our supposition is that they are secondary to dorsolateral prefrontal cortex function. This contention is supported by studies finding that only dorsolateral prefrontal cortex levels of N-acetylaspartate (measured by proton magnetic spectroscopic imaging) predicted activation in the dorsolateral prefrontal cortex and these other locales (4, 14).
The finding of right-sided hypofrontality is similar to the finding of the majority of previous PET and fMRI studies (e.g., references 15, 16). However, the finding of simultaneous hypofrontality and hyperfrontality within subregions of the working memory network is unique. This study may have also benefited from the use of a strong field magnet (3 T). Physiological signal-to-noise ratios increase with field strength, and it may be that this contributed to our ability to identify areas of greater and less dorsolateral prefrontal cortex function. Another potential explanation is that the performance difference between comparison subjects and patients is the smallest yet reported. We compared the effect sizes (Cohen’s d) for fMRI studies that published means and standard deviations for performance data (e.g., d=3.26 in Manoach et al. [3]; d=4.92 in Callicott et al. [11] ; d=1.76 in Manoach et al. [5]; d=0.92 in Callicott et al. [4]; d=1.2 in Perlstein et al. [15]; and d=1.5 in Barch et al. [16]) and found the effect size in our current data set to be smaller (d=0.84). Furthermore, the difference in performance was smaller than in our previous study, which found hyperfrontality (4), again suggesting that as patient performance approaches that of comparison subjects more areas that appear overactive might be identified.
The contention that patients and comparison subjects operate on distinct load-response curves has been posited as a way to unify the diverse findings of both greater and less dorsolateral prefrontal cortex activation in past fMRI and PET studies (13). We think it important to note that there was no single dorsolateral prefrontal cortex response in our subjects but, rather, distinct group differences in several dorsolateral prefrontal cortex nodes. Given our limited understanding of the role of functional specialization within the dorsolateral prefrontal cortex, we are unable to assign particular importance to multiple areas of group difference beyond the notion that the data implicate widespread information processing dysfunction within the dorsolateral prefrontal cortex. Depending on the particular characteristics of the groups studied and the demands of the task, one could imagine that these nonoverlapping curves could be captured in a region in which patients showed greater activation per unit performance than comparison subjects and the contrary finding of greater activation for comparison subjects. These data are consistent with this contention.
Hypofrontality characterized the patients’ dorsolateral prefrontal cortex response when they performed significantly worse than healthy subjects (e.g., high-performing comparison subjects versus high-performing patients and low-performing comparison subjects versus low-performing patients). However, hyperfrontality characterized aspects of the patients’ dorsolateral prefrontal cortex response when their performance fell within the normal range (e.g., high-performing patients versus high-performing comparison subjects and high-performing patients versus low-performing comparison subjects). However, these data are an incomplete justification of the construct of two load-response curves because they do not directly demonstrate that a given dorsolateral prefrontal cortex region moves from hyperfrontality to hypofrontality as performance falls. Since it is not likely that dorsolateral prefrontal cortex activation increases indefinitely, one might assume that hyperfrontal dorsolateral prefrontal cortex regions will eventually become hypofrontal beyond capacity (as has been demonstrated for healthy subjects pushed past capacity [7]). Alternatively, it might be that hypofrontal regions are always hypofrontal, given a general failure of patients to recruit or use the proper working memory network.
Finally, the data do not suggest a single load-response curve in the dorsolateral prefrontal cortex but, rather, a family of curves depending on the node examined. Again, factors that give rise to multiple curves within the dorsolateral prefrontal cortex are not understood at this time. The data are most compelling at demonstrating that patients differ most from healthy subjects in the way in which they organize neural activity and maintain proper levels of activation in the working memory network. To perform near normal, patients engage the working memory system in an inefficient manner. When performing poorly, they do not appropriately engage and sustain the working memory system. These data suggest that neither findings of patient hypofrontality nor findings of patient hyperfrontality are erroneous; rather, they both reflect aspects of dorsolateral prefrontal cortex dysfunction in schizophrenia.
Several alternate explanations for these findings must be considered. First, one could argue that the identification of areas of greater activation by both groups in separate locales resulted from areas of nonoverlapping activation in each group that, when compared, produced apparent group differences. Thus, instead of identifying pathology-specific activation differences, we have simply identified two groups of subjects activating a working memory network at separate locales. However, since group comparisons were limited to areas activated by both groups, this explanation is unlikely. In addition, the number of subjects studied is small. Although the statistical parametric mapping analysis should allow generalization to the general population, these results require replication. In particular, alternate prefrontal tasks should be used; such work is currently underway.
In summary, we have demonstrated that patients with relatively intact working memory function showed areas of both greater and less dorsolateral prefrontal cortex activation simultaneously. These data lend credence to the idea that although patients operate grossly within a working memory network similar to that used by healthy subjects, there is a distinction in the neural subfields recruited during these tasks. Hypofrontality seems to be associated with gross failure in working memory performance, and hyperfrontality seems to arise in the setting of better performance. Patients with schizophrenia do not appropriately process and maintain information in the working memory system, a deficit that might extend to other cognitive functions.
 |
 |
 |
Received Feb. 26, 2003; revision received May 6, 2003; accepted May 8, 2003. From the Clinical Brain Disorders Branch, NIMH/NIH. Address all correspondence to Dr. Callicott, Clinical Brain Disorders Branch, NIMH/NIH, Bldg. 10, Rm. 4D-20, MSC 1389, Bethesda, MD 20892-1389; [email protected] (e-mail).
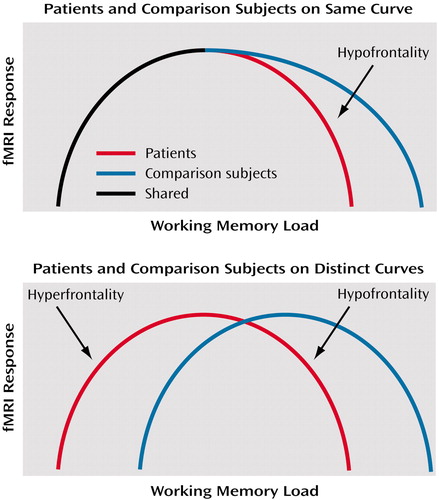
Figure 1. Inverted-U-Shaped Curve Representing Theoretical Response of the Dorsolateral Prefrontal Cortex to Increasing Working Memory Load in Patients With Schizophrenia and Healthy Comparison Subjectsa
aIncreasing working memory load is represented on the x axis with the theoretical fMRI response on the y axis. In the first graph, patients and comparison subjects operate on the same working memory curve until patients become relatively hypofrontal as they reach their limited working memory capacity sooner. This model is insufficient to explain hyperfrontality. In the second graph, patients and comparison subjects operate on distinct curves. Patients reach the peak of the inverted U sooner and thus appear hyperfrontal at lower working memory load. However, their working memory capacity is eventually breached and hypofrontality results. The peak height of the curves may or may not be similar, but, as drawn, this model would predict that patients and comparison subjects reach similar magnitudes of prefrontal activation but at different levels of working memory load, which has been reported (4).
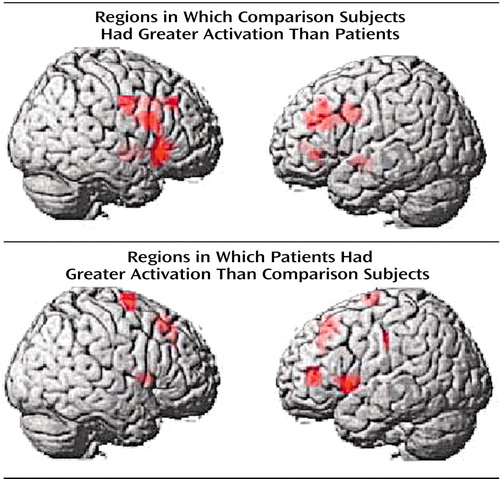
Figure 2. Hypofrontality and Hyperfrontality During Two-Back Working Memory Task in 14 Patients With Schizophrenia Compared With 14 Healthy Comparison Subjectsa
aFor all comparisons, p<0.05, minimum cluster size=8, according to SPM 99, uncorrected.
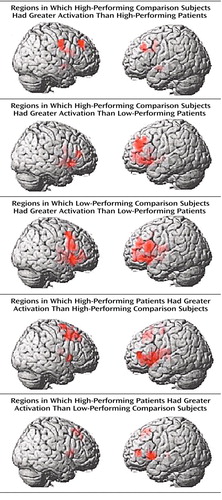
Figure 3. Impact of Differences in Performance of Two-Back Working Memory Task on Activation in Dorsolateral Prefrontal Cortex of 14 Patients With Schizophrenia Compared With 14 Healthy Comparison Subjectsa
aFor all comparisons, p<0.05, minimum cluster size=8, according to SPM 99, uncorrected.
1. Curtis VA, Bullmore ET, Morris RG, Brammer MJ, Williams SC, Simmons A, Sharma T, Murray RM, McGuire PK: Attenuated frontal activation in schizophrenia may be task dependent. Schizophr Res 1999; 37:35–44Crossref, Medline, Google Scholar
2. Volz H, Gaser C, Hager F, Rzanny R, Ponisch J, Mentzel H, Kaiser WA, Sauer H: Decreased frontal activation in schizophrenics during stimulation with the Continuous Performance Test—a functional magnetic resonance imaging study. Eur Psychiatry 1999; 14:17–24Crossref, Medline, Google Scholar
3. Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S: Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry 1999; 45:1128–1137Crossref, Medline, Google Scholar
4. Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR: Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078–1092Crossref, Medline, Google Scholar
5. Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL: Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 2000; 48:99–109Crossref, Medline, Google Scholar
6. Ramsey NF, Koning HA, Welles P, Cahn W, Van Der Linden JA, Kahn RS: Excessive recruitment of neural systems subserving logical reasoning in schizophrenia. Brain 2002; 125(part 8):1793–1807Google Scholar
7. Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR: Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex 1999; 9:20–26Crossref, Medline, Google Scholar
8. Rypma B, D’Esposito M: The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci USA 1999; 96:6558–6563Crossref, Medline, Google Scholar
9. Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR: Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry 2000; 157:1309–1316Link, Google Scholar
10. Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, Berman KF, Goldberg TE, Weinberger DR: Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage 2000; 12:268–275Crossref, Medline, Google Scholar
11. Callicott JH, Ramsey NF, Tallent K, Bertolino A, Knable MB, Coppola R, Goldberg T, van Gelderen P, Mattay VS, Frank JA, Moonen CT, Weinberger DR: Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology 1998; 18:186–196Crossref, Medline, Google Scholar
12. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. Stuttgart, Germany, Georg Thieme, 1988Google Scholar
13. Callicott JH, Weinberger DR: Functional brain imaging: future prospects for clinical practice, in Psychiatry in the New Millenium. Edited by Weisman S, Sabshin M, Eist H. Washington, DC, American Psychiatric Press, 1999, pp 119–135Google Scholar
14. Bertolino A, Esposito G, Callicott JH, Mattay VS, Van Horn JD, Frank JA, Berman KF, Weinberger DR: Specific relationship between prefrontal neuronal N–acetylaspartate and activation of the working memory cortical network in schizophrenia. Am J Psychiatry 2000; 157:26–33Link, Google Scholar
15. Perlstein WM, Carter CS, Noll DC, Cohen JD: Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry 2001; 158:1105–1113Link, Google Scholar
16. Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A III, Noll DC, Cohen JD: Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry 2001; 58:280–288Crossref, Medline, Google Scholar



