Dysfunctional Prefrontal Regional Specialization and Compensation in Schizophrenia
Abstract
Objective: It has been suggested that in healthy persons higher-order cognitive processing engaged by incremental working memory load hierarchically employs more dorsal than ventral prefrontal resources in healthy individuals. Given that working memory performance is impaired in schizophrenia, especially at higher executive loads, the authors investigated how this prefrontal functional organization might be altered in disease, independent of performance deficits. Method: Using N-back working memory functional magnetic resonance imaging (fMRI) data, the authors studied 15 patients with schizophrenia and 26 healthy comparison subjects. Subgroups based on median performance accuracy at 2-back were analyzed; high performers included eight schizophrenia patients and 14 comparison subjects, and low performers included seven patients and 12 comparison subjects. Results: High-performing but not low-performing comparison subjects responded to incremental working memory executive load with disproportionately greater dorsal but not ventral prefrontal cortex activation, which also predicted performance accuracy. In the high- and low-performing patient groups, incremental working memory load caused a disproportionate increase in ventral but not dorsal prefrontal cortex activation relative to the respective comparison group, which also correlated with accuracy. Functional connectivity between the ventral prefrontal cortex and posterior parietal cortex was relatively greater in patients, whereas comparison subjects had greater functional connectivity between the dorsal prefrontal cortex and posterior parietal cortex. Conclusions: The hierarchical organization of the prefrontal cortex may be compromised in schizophrenia, resulting in loss of functional specialization and integration at the dorsal prefrontal cortex and in compensatory activation from the ventral prefrontal cortex, which may ultimately affect working memory and executive cognition.
Working memory enables us to temporarily hold and manipulate information, and it underlies higher-order thinking, language, and goal-directed behavior (1) . Impairment of working memory is an important component of the cognitive dysfunction seen in schizophrenia (2 – 4) . Functional magnetic resonance imaging (fMRI) experiments (5 , 6) , complemented by primate single-neuron recordings and neurocomputational models (7) , suggest that in healthy persons, higher-order working memory executive processes (e.g., manipulation of information versus simple maintenance) preferentially engage dorsal (Brodmann’s areas 9 and 46) relative to ventral (Brodmann’s areas 44, 45, and 47) regions of the prefrontal cortex. Related work also suggests that the prefrontal cortex is hierarchically organized, with higher-order processes in the dorsal prefrontal cortex exerting control over more ventral regions that are responsible for simpler processes (8 , 9) , such as rehearsal of information within working memory (10 , 11) .
Few investigations have explicitly examined the functional hierarchy of distinct dorsal and ventral regions in the prefrontal cortex in patients with schizophrenia, although schizophrenia patients have been found to be selectively more impaired on behavioral working memory tasks that have higher executive demands (12 , 13) , where dysfunction in the dorsal prefrontal cortex may be a key factor. Although a number of functional imaging experiments support the presence of dorsal prefrontal cortex dysfunction in schizophrenia (14 – 19) , studies have variously reported increases and decreases in activation of the prefrontal cortex, depending on the specific task paradigm used, and illness-related differences in behavioral performance and capacity constraints of task load (15 , 20β2) . Moreover, it has been suggested that ventral regions contribute significantly to observed hyperactivity in the prefrontal cortex in some patients and comparison subjects matched on working memory performance (23 , 24) .
In this study we sought to further examine how dorsal-ventral prefrontal functional organization might be altered in schizophrenia, independent of performance deficits. In order to examine higher-order working memory executive processing and dorsal prefrontal cortex function, we focused on the differential activation between 1-back and 2-back tasks, reflecting incremental working memory load (number of target items stored) and information updating (the executive process of continuous selection and updating of target items) (25) . We studied patients and healthy comparison subjects with similar performance indices and analyzed subgroups defined in terms of memory performance: high-performing comparison subjects, high-performing patients, low-performing comparison subjects, and low-performing patients. Although both ventral and dorsal regions are commonly coactivated in the distributed working memory network, we expected the high-performing comparison subjects to adapt to increased working memory executive load predominantly via activation of the dorsal prefrontal cortex. In patients, greater activation in the ventral prefrontal cortex might occur in addition to or in lieu of activation elsewhere in the prefrontal cortex and could reflect compensatory processes if behavioral performance is maintained. In other words, if patients with schizophrenia have a primary defect in dorsal prefrontal cortex processing, they may resort to cognitive strategies that preferentially engage ventral circuitry as a means of maintaining performance. While this might be a less efficient strategy, at least when manipulation of information becomes more critical, it would be consistent with patients’ also having diminished working memory capacity (15) .
To examine illness effects on the dorsal and ventral prefrontal cortex, we also analyzed functional connectivity between prefrontal cortex regions and the posterior parietal cortex. The prefrontal cortex-posterior parietal cortex coupling during working memory tasks has long been implicated in human and primate studies of functional and structural anatomy (8 , 26 , 27) . We expected to find that schizophrenia patients would have relatively lower functional connectivity between the dorsal prefrontal cortex and posterior parietal cortex, reflecting loss of its specialized role in the working memory network, as would be consistent with recent positron emission tomography (PET) findings (24) . Conversely, patients would have greater functional connectivity between the ventral prefrontal cortex and posterior parietal cortex compared with healthy comparison subjects, if indeed the ventral activation was compensatory.
Method
We initially studied 24 patients with schizophrenia and 26 healthy comparison subjects, all right-handed as assessed by the Edinburgh Inventory (28) . Patients were recruited from the Clinical Brain Disorders Branch Sibling Study (NIH Protocol 95-M-6150). Comparison subjects were recruited from the National Institutes of Health Clinical Research Volunteer Program. The study was approved by the institutional review board of the Intramural Program of the National Institute of Mental Health. All study subjects gave written consent before participation. Ten subjects in this study were included in a previous report (17) .
All participants were assessed with a Structured Clinical Interview for DSM-IV, a neurological examination, a battery of neuropsychological tests, an EEG, and a screening MRI examination. Exclusion criteria included an inability to give informed consent, IQ below 70, a history of substance abuse within the past 6 months, a history of significant neurological illness, and any focal abnormalities on EEG or MRI. All schizophrenia patients were on a stable regimen of antipsychotic medication.
Acquisition of N-back fMRI data was done largely as previously described (17) . Briefly, the N-back task consisted of continual presentation of visual stimuli in which every number was both a probe and a target (i.e., 100% target). The numbers 1–4 appeared randomly every 1.8 sec for 500 msec at set locations at the points of a diamond-shaped box. Subjects were to recall the stimulus seen N previously by means of a fiber-optic response box with buttons arrayed in the same configuration as the stimuli presented on the screen. The task was presented as two counterbalanced runs in which 30-sec epochs of 0-back alternated with either 1-back or 2-back.
Whole brain blood-oxygen-level-dependent fMRI data were collected on a 3-T GE scanner (General Electric Systems, Milwaukee) with a GE-EPI pulse sequence acquisition of 24 contiguous slices (echo time=30 msec, repetition time=2 seconds, flip angle=90°, field of view=24 cm, matrix=64×64, voxel dimensions=3.75×3.75×6 mm). All fMRI data were processed and spatially normalized to a common stereotaxic space (Montreal Neurologic Institute template), analyzed with SPM99 software (Wellcome Department of Imaging Neuroscience, London, http://www.fil.ion.ucl.ac.uk/spm), and individually examined for motion artifacts as described previously (15) .
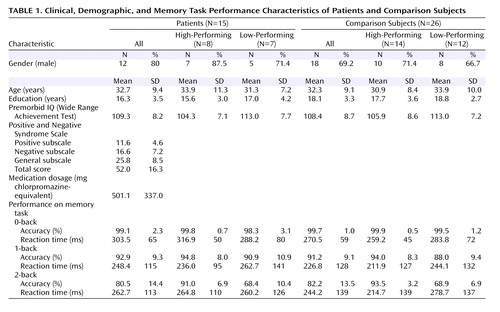
Because activation of the prefrontal cortex has been shown to be sensitive to behavioral performance differences (15 , 20) , we matched patients and comparison subjects for 1-back and 2-back performance. We excluded seven patients whose performance was more than two standard deviations below the mean of the comparison subjects and two patients because of movement artifacts during scanning. The patient and comparison groups were each divided into two subgroups based on median performance accuracy at 2-back, thus forming a cohort of eight high-performing patients and 14 high-performing comparison subjects, and another cohort of seven low-performing patients and 12 low-performing comparison subjects ( Table 1 ).
There were no significant group differences in age, gender, years of education, or premorbid IQ between the four groups. For N-back reaction time between groups, there were no significant effects of group, working memory load, or group-by-load interaction. As expected, high-performing patients had better accuracy than low-performing patients at 2-back (t=5.0, df=13, p<0.001) but not at 1-back (t=0.79, df=13, n.s.). Similarly, high-performing comparison subjects had better accuracy than low-performing comparison subjects at 2-back (t=11.9, df=24, p<0.001) but not at 1-back (t=1.73, df=24, n.s.).
Within the high-performing cohort, participants maintained high performance accuracy between 1-back and 2-back, with no within-group differences. There were also no accuracy differences between high-performing comparison subjects and high-performing patients at 1-back or 2-back. Within the lower-performing cohort, accuracy was lower at 2-back than at 1-back in each group (low-performing patients: t=4.93, df=6, p=0.003; low-performing comparison subjects: t=7.77, df=11, p<0.001). There were no significant differences in accuracy between low-performing comparison subjects and low-performing patients at 1-back or 2-back.
Single-subject contrast images were entered into a second level of analysis with subject as a random factor. In a preliminary step, we explored the main effect of each working memory task condition (i.e., 1-back or 2-back versus 0-back) for each of the four subgroups and across groups in each performance cohort. We also explored the effects of working memory executive load (2-back versus 1-back) in the combined sample, in all patients versus all comparison subjects, and in pairwise contrasts across the four subgroups. Coordinates were transformed to standard space (29) and reported as z scores with a significance threshold of p<0.005 (uncorrected), except for the working memory executive load contrast with the combined sample, which was threshold corrected for false-discovery rate (30) at p<0.05.
In a confirmatory analysis, functional regions of interest in the dorsal and ventral prefrontal cortex were subsequently identified from the working memory executive load contrast involving the combined sample. Note that this contrast was orthogonal and unbiased to the group-by-load interaction effects of interest. Mean fMRI signal intensities were extracted from the individual subject contrast maps at 2-back and 1-back by use of a 10-mm diameter sphere centered on one peak in the dorsal prefrontal cortex and another in the ventral prefrontal cortex. Repeated-measures ANOVA was then used to study these two regions of interest to confirm the main group-by-load interactions identified in the exploratory voxelwise contrasts.
In the functional connectivity analyses, the same dorsal and ventral prefrontal cortex regions of interest were used as seed volumes. From each of these, the median time series was computed for each individual at 2-back and cross-correlated with the time series of all other brain voxels with Pearson’s correlation coefficients. Group differences in functional connectivity were then computed by combining the correlation maps with group as a random factor (31) . In accordance with our a priori hypothesis and on the basis of known distributed functional and structural anatomy of working memory (8 , 26 , 27) , our analyses were restricted to the posterior parietal cortex (Brodmann’s areas 7 and 40) by masking through use of WFU PickAtlas software (32) . The results combining all patient versus all comparison groups are reported and thresholded at p<0.05 with small-volume correction.
Results
Exploratory Voxelwise Contrasts
In each group of patients and comparison subjects, a similar frontoparietal working memory network was activated at 1-back versus 0-back and 2-back versus 0-back. At each load level in the high-performing cohort, patients had greater prefrontal cortex activation than comparison subjects, whereas in the low-performing cohort, patients had regions of lower and greater prefrontal cortex activation compared with comparison subjects, similar to findings reported previously (17) . A figure containing surface renderings of 2-back activations across performance-matched cohorts is included in a data supplement that accompanies the online version of this article.
The effects of working memory executive load and putatively higher-order working memory complexity were subsequently explored using the contrast 2-back versus 1-back. While the combined groups engaged dorsal and ventral prefrontal cortex to process working memory executive load, patients had relatively greater activation in the left ventral prefrontal cortex. A table listing the regions activated in the combined sample and in each patient and comparison group as well as their between-group contrasts is included in the data supplement that accompanies the online version of this article.
Regions activated in the subgroups and their contrasts are shown in Figure 1 ; additional details are presented in a table in the data supplement that accompanies the online version of this article. In high-performing comparison subjects, incremental working memory executive load elicited activation in the right dorsal prefrontal cortex. In low-performing comparison subjects, no significant differences in dorsal prefrontal cortex activation were elicited at the chosen threshold. In high-performing patients, an increase in working memory executive load elicited prefrontal activation in the left ventral prefrontal cortex. Similar regions were activated in low-performing patients.

a High-performing comparison subjects responded to incremental working memory executive load with dorsal prefrontal cortex activation, whereas patient groups (high-performing and low-performing patients) had ventral prefrontal cortex activation. A threshold of p<0.005 uncorrected and random-effects analysis were applied.
The effects of performance category on working memory executive load were explored in comparison subjects with the contrast of high-performing comparison subjects versus low-performing comparison subjects, and vice versa. The high-performing comparison subjects had a greater increase in activation at the right dorsal prefrontal cortex (peak: Brodmann’s area 46; x=44, y=23, z=27; z score=2.81) relative to low-performing comparison subjects. The ventral prefrontal cortex and reverse contrasts did not elicit significant activations at the chosen threshold.
The effects of performance category on the processing of working memory executive load were explored in schizophrenia patients with the contrast of high-performing patients versus low-performing patients and vice versa. There was no significant increase in activation in the first contrast at the chosen threshold. In the reverse contrast, a region in the left ventral prefrontal cortex had greater activation in low-performing patients compared with high-performing patients (peak: Brodmann’s area 44; x=–55, y=15, z=10; z score=2.75).
The effects of illness on incremental working memory executive load in high-performing subjects were explored with the contrast of high-performing patients versus high-performing comparison subjects and vice versa. A region in the left ventral prefrontal cortex (peak: Brodmann’s area 47; x=–45, y=37, z=–7; z score=2.62) was found to have greater activation in high-performing patients. The reverse contrast did not yield significant activations at the chosen threshold.
The effects of illness on working memory executive load in low-performing subjects were explored with the contrast of low-performing patients versus low-performing comparison subjects and vice versa. A similar region in the left ventral prefrontal cortex (peak: Brodmann’s area 47; x=–30, y=14, z=–2; z score=2.62) was found to have greater activation in low-performing patients. The reverse contrast did not yield activations at the chosen threshold. Note that while the effects reported have so far been leniently thresholded, this result independently replicated that obtained with the high-performing cohort.
Functional Regions-of-Interest Analyses
In a confirmatory analysis to reexamine the group effects from the series of exploratory voxelwise contrasts, parameter estimates for each subject were extracted from ventral and dorsal prefrontal cortex regions identified in an orthogonal contrast of 2-back versus 1-back combining all subjects (see Figure 2 ; see also the first table in the data supplement that accompanies the online version of this article). The parameter estimates for each N-back task condition in the right dorsal prefrontal cortex (Brodmann’s area 46; x=44, y=34, z=26) showed an effect of load in all subjects (F=33.3, df=1, 37, p<0.001), and, in comparison subjects, effects of load (F=31.4, df=1, 24, p<0.001) and load-by-performance-group interaction (F=6.0, df=1, 24, p=0.022). Here, consistent with the voxelwise contrasts, high-performing comparison subjects had a disproportionately greater increase in activation between 1-back and 2-back relative to low-performing comparison subjects (see Figure 2 , lower left panel). Parameter estimates from the left ventral prefrontal cortex (Brodmann’s area 47; x=–37, y=25, z=–6) yielded load effects in all subjects (F=55.2, df=1, 37, p<0.001), driven by a load-by-group interaction in which patients had disproportionately increased activation (F=7.51, df=1, 37, p=0.009) (see Figure 2 , lower right panel). This result was also consistent with the voxelwise contrasts. In addition, there was a group-by-region interaction (F=7.61, df=1, 39, p=0.009) in which comparison subjects had greater regional activation at the dorsal relative to the ventral prefrontal cortex, whereas patients lacked this regional differentiation and had greater ventral prefrontal cortex activation than comparison subjects. Finally, in high-performing comparison subjects, 2-back activation at the dorsal prefrontal cortex region of interest correlated with performance accuracy (r=0.48, p=0.041), but not at the ventral prefrontal cortex region of interest (r=–0.23, n.s.). In patients in both performance cohorts, ventral prefrontal cortex activation correlated with accuracy (r=0.52, p=0.024), but not dorsal prefrontal cortex activation (r=–0.19, n.s.).

a Images are in neurological convention, and thresholded at p<0.05 corrected for false discovery rate in the whole brain, with random-effects analysis. Parameter estimates were then extracted and charted from the right dorsal and the left ventral prefrontal cortex. Consistent with the exploratory voxelwise contrasts, the functional regions-of-interest analysis showed a load-dependent disproportionate increase in dorsal prefrontal cortex activation in high-performing relative to low-performing comparison subjects (lower left) and ventral prefrontal cortex activation in patients relative to comparison subjects (lower right).
b t=7.30, df=13, p<0.001.
c t=2.75, df=6, p=0.033.
d t=2.83, df=7, p=0.025.
e t=6.09, df=6, p=0.001.
Functional Connectivity Analyses
In the functional connectivity analyses, seed regions were derived from regions of interest in the dorsal and ventral prefrontal cortex. In comparison subjects relative to patients, the dorsal prefrontal cortex region of interest had greater functional connectivity with the bilateral inferior parietal lobules (Brodmann’s area 40; x=34, y=–64, z=54; z score=4.87; and x=–49, y=–49, z=48; z score=4.39). Conversely, in patients relative to comparison subjects, the ventral prefrontal cortex region of interest had greater connectivity with the left superior parietal lobule (Brodmann’s area 7; x=–11, y=–82, z=42; z score=3.90). For each individual subject, the mean correlation coefficients for the connectivity between the respective right dorsal prefrontal cortex and posterior parietal cortex and the left ventral prefrontal cortex and posterior parietal cortex were subsequently extracted from 10-mm diameter spheres centered on the peaks at the respective right (x= –34, y=–64, z=54) and left posterior parietal cortex (x=–11, y=–82, z=42). The functional connectivity levels of these regions were inversely correlated with each other, with lower connectivity between the dorsal prefrontal cortex and posterior parietal cortex predicting higher connectivity between the ventral prefrontal cortex and posterior parietal cortex, and vice versa (r=–0.41, p=0.008) ( Figure 3 ). Correlations within each group, however, did not reach statistical significance.
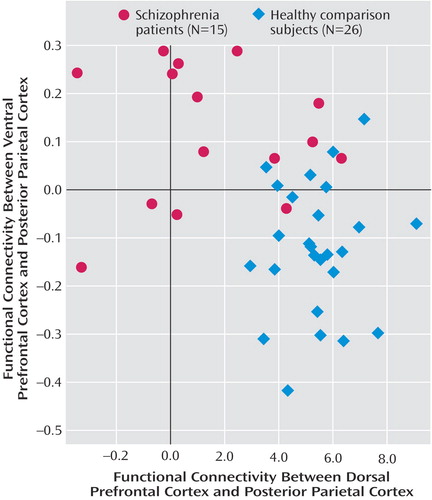
a The functional connectivity of the left ventral prefrontal cortex and right dorsal prefrontal cortex regions of interest with the respective posterior parietal cortex regions had an inverse relationship (p<0.01). Lower connectivity between the dorsal prefrontal cortex and posterior parietal cortex predicted higher compensatory connectivity between the ventral prefrontal cortex and posterior parietal cortex.
Discussion
Dorsal Prefrontal Cortex and Higher-Order Information Processing in Healthy Individuals
The greater response in the dorsal but not the ventral prefrontal cortex in high-performing relative to low-performing comparison subjects (see Figure 2 ) suggests that this region was selectively involved in successfully processing additional working memory executive load between 1-back and 2-back. This interpretation is supported by the greater activation in the dorsal prefrontal cortex observed in comparison subjects (relative to the ventral prefrontal cortex in patients in a group-by-region interaction) and its correlation with performance accuracy. It is also consistent with conceptualizations about the role of the dorsal prefrontal cortex in higher-order information processing (6 – 9) . The less prominent change in activation in the dorsal prefrontal cortex in low-performing comparison subjects suggests that activation may have reached maximum levels, which is consistent with the putative capacity constraints on activation in this region (22) .
Ventral Prefrontal Cortex and Higher-Order Information Processing in Schizophrenia
Both patient groups had disproportionately greater activation in the ventral but not the dorsal prefrontal cortex in response to working memory executive load between 1-back and 2-back relative to performance-matched comparison subjects. This result supports conceptualizations of physiological inefficiency in the diseased prefrontal cortex, where a greater degree of activation is required to perform at the same or a lower level (15) . Moreover, the correlations between prefrontal cortex activation and performance accuracy occurred at the dorsal prefrontal cortex in comparison subjects but at the ventral prefrontal cortex in patients. Therefore, while the dorsal prefrontal cortex might successfully drive working memory performance in comparison subjects, patients required additional activation from the ventral prefrontal cortex, a region normally utilized in simpler working memory rehearsal operations (6 , 7 , 10 , 11) .
These results suggest that physiological inefficiency in the diseased prefrontal cortex could involve a relative loss of specialized function in higher-order processing regions in the dorsal prefrontal cortex and compensatory activation from the ventral prefrontal cortex. This interpretation is further supported by the double-dissociation in functional connectivity with the posterior parietal cortex, which was relatively lower between the dorsal prefrontal cortex and posterior parietal cortex but higher between the ventral prefrontal cortex and posterior parietal cortex in patients relative to comparison subjects. The loss of functional connectivity at well-defined functional and structural connections between the dorsal prefrontal cortex and posterior parietal cortex (8) is consistent with recent electrophysiological (33) and PET (24) findings implicating dysfunction at the dorsal prefrontal cortex in schizophrenia. The increased functional connectivity between the ventral prefrontal cortex and posterior parietal cortex, together with the increased activation of the ventral prefrontal cortex and the correlation with performance, raises the possibility that this region plays a compensatory role, supporting connectivity within the working memory network to an extent that similar behavioral performance is maintained between patients and comparison subjects. The overall inverse relationship between functional connectivity between the ventral and dorsal prefrontal cortex and the posterior parietal cortex in the combined sample also suggests a possible continuum whereby increasing connectivity between the ventral prefrontal cortex and posterior parietal cortex compensates for decreasing connectivity between the dorsal prefrontal cortex and posterior parietal cortex, with patients and comparison subjects residing at opposing ends of this spectrum ( Figure 3 ). Whether this relationship also exists within groups remains to be fully evaluated.
Another finding, although only a preliminary one because of the leniently thresholded voxelwise contrasts, may extend the concept that the ventral prefrontal cortex could be compensatory but only to a certain extent, as might be the case if it were not specialized for the task. It was observed that low-performing patients had disproportionately greater activation with incremental working memory load in another region of the left ventral prefrontal cortex relative to high-performing patients. Notably, at 1-back, there were no performance differences between high-performing patients and low-performing patients, and no activation differences at this location; however, at 2-back, only low-performing patients had increased activation there. Thus, this increased activation failed to maintain performance.
The nature of dysfunctional regional specialization and compensation in the diseased prefrontal cortex deserves evaluation in future studies. We cannot rule out, for example, that schizophrenia patients prefer a more concrete cognitive strategy with a greater dependence on verbal rehearsal (10 , 11) , although preliminary analyses of strategy reported by a subset of subjects (i.e., use of subvocal rehearsal, spatial positioning, or both) do not suggest that patients utilized more overt verbal rehearsal than comparison subjects (data available on request). We also need to further evaluate the hypothesis that activation of the ventral prefrontal cortex not only is a compensatory mechanism but also is related to the cause of their poorer performance. Thus, if verbal rehearsal is a prepotent cognitive strategy because it is simpler and more concrete, then better performers may be more adept at inhibiting this approach and thus are able to maximize dorsal prefrontal cortex engagement and the more efficient and effective neural processing of the task that follows.
Ultimately, determinations as to whether the dorsal, ventral, or both regions of the prefrontal cortex are primarily dysfunctional in schizophrenia will have to be made in conjunction with other studies. Based on findings of deficient functional connectivity in this study and in other functional neuroimaging experiments of working memory in schizophrenia using a variety of tasks (14 – 17 , 23 , 24) as well as other anatomical findings independent of fMRI (34 – 37) , we favor the explanation that the dorsal prefrontal cortex is primarily dysfunctional in schizophrenia. Our findings on the ventral prefrontal cortex extend previous observations that suggest that activations in ventral regions are intact (14 , 18 , 24) or increased (17 , 38) in patients with schizophrenia, possibly in compensation for dysfunctional dorsal prefrontal cortex activation (23) . Furthermore, a recent postmortem study found abnormalities in the dorsal but not the ventral prefrontal cortex in schizophrenia patients (39) . Thus, at another level, the increased activation of the ventral prefrontal cortex could reflect recruitment of parallel networks with computational properties required to supplement executive processes affected in schizophrenia, perhaps by loss of inhibitory elements in the dorsal prefrontal cortex (40) or by an inability to maintain adequate signal-to-noise differentiation within the overall dysfunctional prefrontal cortex-posterior parietal cortex network (41) .
Thus far we have focused mainly on the prefrontal cortex, although our findings of functional connectivity with the posterior parietal cortex also suggest that patients and comparison subjects differ in anatomically and functionally distinct regions within the parietal cortex. This proposition will be evaluated in a future report. Finally, the roles of antipsychotic medication and genotype in mediating compensatory activations of the prefrontal cortex need to be explored, along with the question of how these findings could reflect neuroplastic adaptations in the prefrontal cortex germane to the developmental pathophysiology of schizophrenia.
Study Limitations
Because interpretation of prefrontal cortex activation has been shown to be confounded by behavioral performance differences (15 , 20) , our study design included careful performance matching of patients and comparison subjects. This feature constrains the generalizability of our findings to less impaired patients, and presumably to those in whom compensatory processes are more active. In examining subtle activation differences between 1-back and 2-back in comparison subjects versus the relatively small subgroups of patients, the exploratory contrasts might not have revealed all regions differentially implicated in processing incremental working memory executive load. This problem is mitigated by contrasts involving all patients versus comparison subjects, which showed similar findings in the ventral prefrontal cortex. The exploratory contrasts were also complemented by convergent results from analyses involving regions of interest, functional connectivity, and brain-behavioral performance correlations.
Conclusion
This study extends conceptualizations of physiological inefficiency in the neural processing of higher-order working memory executive function in schizophrenia. While high-performing comparison subjects optimally utilized the dorsal prefrontal cortex, schizophrenia patients had greater ventral prefrontal cortex involvement. This compensatory ventral response may reflect loss of hierarchical functional specialization in the diseased prefrontal cortex, which may eventually fail to maintain cognitive performance.
1. Baddeley AD: Working memory: looking back and looking forward. Nat Rev Neurosci 2003; 4:829–839Google Scholar
2. Silver H, Feldman P, Bilker W, Gur RC: Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry 2003; 160:1809–1816Google Scholar
3. Goldman-Rakic PS: Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 1994; 6:348–357Google Scholar
4. Goldberg TE, Weinberger DR, Berman KF, Pliskin NH, Podd MH: Further evidence for dementia of the prefrontal type in schizophrenia? a controlled study of teaching the Wisconsin Card Sorting Test. Arch Gene Psychiatry 1987; 44:1008–1014Google Scholar
5. Smith EE, Jonides J: Storage and executive processes in the frontal lobes. Science 1999; 283:1657–1661Google Scholar
6. D’Esposito M, Postle BR, Ballard D, Lease J: Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn 1999; 41:66–86Google Scholar
7. Deco G, Rolls ET, Horwitz B: “What” and “where” in visual working memory: a computational neurodynamical perspective for integrating fMRI and single-neuron data. J Cogn Neurosci 2004; 16:683–701Google Scholar
8. Fuster JM: The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe, 3rd ed. Philadelphia, Lippincott Williams Wilkins, 1997Google Scholar
9. Koechlin E, Ody C, Kouneiher FT: The architecture of cognitive control in the human prefrontal cortex. Science 2003; 302:1181–1185Google Scholar
10. Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S: Dissociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychol Science 1996; 7:25–31Google Scholar
11. Paulesu E, Frith CD, Frackowiak RS: The neural correlates of the verbal component of working memory. Nature 1993; 362:342–345Google Scholar
12. Goldberg TE, Patterson KJ, Taqqu Y, Wilder K: Capacity limitations in short-term memory in schizophrenia: tests of competing hypotheses. Psychol Med 1998; 28:665–673Google Scholar
13. Kim JH, Glahn DC, Nuechterlein KH, Cannon TD: Maintenance and manipulation of information in schizophrenia: further evidence for impairment in the central executive component of working memory. Schizophr Res 2004; 68:173–187Google Scholar
14. Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, Cohen JD: Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry 2001; 58:280–288Google Scholar
15. Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR: Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078–1092Google Scholar
16. Manoach DS, Press DZ, Thangara JV, Searl MM, Goff DC, Halpern E, Saper CB, Warach S: Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task as measured by fMRI. Biol Psychiatry 1999; 45:1128–1137Google Scholar
17. Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR: Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry 2003; 160:2209–2215Google Scholar
18. Perlstein WM, Carter CS, Noll DC, Cohen JD: Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry 2001; 158:1105–1113Google Scholar
19. Weinberger DR, Berman K, Zec R: Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional cerebral blood flow evidence. Arch Gen Psychiatry 1986; 43:114–124Google Scholar
20. Ramsey NF, Koning HA, Welles P, Cahn W, Van Der Linden JA, Kahn RS: Excessive recruitment of neural systems subserving logical reasoning in schizophrenia. Brain 2002; 125(part 8):1793–1807Google Scholar
21. Manoach DS: Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res 2003; 60:285–298Google Scholar
22. Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR: Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex 1999; 9:20–26Google Scholar
23. Tan H-Y, Choo W-C, Fones CSL, Chee MWL: fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. Am J Psychiatry 2005; 162:1849–1858Google Scholar
24. Kim J-J, Kwon JS, Park HJ, Youn T, Kang DH, Kim MS, Lee DS, Lee MC: Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: a [ 15 O]H 2 O PET study. Am J Psychiatry 2003; 160:919–923 Google Scholar
25. Goldberg T, Egan M, Gscheidle T, Coppola R, Weickert T, Kolachana B, Goldman D, Weinberger D: Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 2003; 60:889–896Google Scholar
26. Goldman-Rakic PS: Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci 1988; 11:137–156Google Scholar
27. Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR: The role of parietal cortex in verbal working memory. J Neurosci 1998; 18:5026–5034Google Scholar
28. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Google Scholar
29. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
30. Genovese CR, Lazar NA, Nichols T: Thresholding of statistical maps in functional imaging using the false discovery rate. Neuroimage 2002; 15:870–878Google Scholar
31. Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF: Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 2005; 62:379–386Google Scholar
32. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH: An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239Google Scholar
33. Peled A, Geva AB, Kremen WS, Blankfeld HM, Esfandiarfard R, Nordahl TE: Functional connectivity and working memory in schizophrenia: an EEG study. Int J Neurosci 2001; 106:47–61Google Scholar
34. Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CTW, Frank JA, Tedeschi G, Weinberger DR: Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry 1996; 153:1554–1563Google Scholar
35. Garey LJ, Ong WY, Patel TS, Kanna IM, Davis A, Mortimer AM, Barnes TRE, Hirsh SR: Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry 1998; 65:446–453Google Scholar
36. Glantz LA, Lewis DA: Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000; 57:65–73Google Scholar
37. Selemon LD, Goldman-Rakic PS: The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17–25Google Scholar
38. Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI: Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp 2005; 25:60–69Google Scholar
39. Selemon LD, Mrzljak J, Kleinman JE, Herman MM, Goldman-Rakic PS: Regional specificity in the neuropathologic substrates of schizophrenia: a morphometric analysis of Broca’s area 44 and area 9. Arch Gen Psychiatry 2003; 60:69–77Google Scholar
40. Lewis DA, Hashimoto T, Volk DW: Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6:312–324Google Scholar
41. Winterer G, Weinberger DR: Genes, dopamine, and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci 2004; 27:683–690Google Scholar