A Concordance Study of Three Electrophysiological Measures in Schizophrenia
Abstract
OBJECTIVE: The authors evaluated concordance rates among three electrophysiological measures in patients with schizophrenia, nonschizophrenic first-degree relatives of schizophrenia patients, and healthy comparison subjects. The purpose of the study was to provide data for defining a common endophenotype for genetic studies of schizophrenia and for improving the criteria for diagnosis. METHOD: P50 event-related potential inhibition, antisaccade, and smooth pursuit eye tracking paradigms were measured. Data for all three paradigms were available for 81 patients with schizophrenia, 25 parents of patients with schizophrenia, and 60 healthy comparison subjects. RESULTS: The schizophrenia patients and the patients’ parents showed a high rate of inhibitory deficits measured by the P50 inhibition and antisaccade paradigms. Both groups had a high prevalence of eye tracking dysfunction. Smooth pursuit gain and the error rate in the antisaccade paradigm were significantly correlated in the schizophrenia patients and the parents, whereas P50 inhibition showed no correlation with smooth pursuit gain or antisaccade paradigm measurements. CONCLUSIONS: Despite superficial similarities, two paradigms designed to measure central inhibition processes (antisaccade and P50 inhibition) do not appear to reflect the same neurobiological substrates. In contrast, the convergence in performance data for the antisaccade and eye tracking paradigms suggests that the neural circuitry underlying these tasks may overlap. P50 inhibition and antisaccade errors were the optimal paradigms for discrimination between comparison subjects, patients with schizophrenia, and the parents of patients with schizophrenia.
Schizophrenia is a complex disorder in which several genes contribute to susceptibility. The identification of heritable neurobiological markers or endophenotypes associated with schizophrenia may contribute to the genetic dissection of this disease. Furthermore, these endophenotypes are probably more closely related to brain dysfunction than are the clinical phenotypes and may allow improved understanding of the neurobiology of the disease. Endophenotypes would also be useful for establishing a biological underpinning for diagnosis and classification of schizophrenia. Three electrophysiological endophenotypes are routinely studied in schizophrenia: eye tracking dysfunction, deficits in P50 event-related potential inhibition in a two-auditory-click conditioning test paradigm, and saccadic inhibition deficits.
Several studies have shown that eye tracking dysfunction is present in a high percentage of subjects with schizophrenia and their nonschizophrenic relatives (1, 2). In a functional magnetic resonance imaging study, Tregellas et al. (3) found greater activity in the posterior hippocampus and subtle activity deficits in the frontal eye field and cingulate gyrus during smooth pursuit eye movements in subjects with schizophrenia, relative to healthy comparison subjects. These data suggest that abnormalities in smooth pursuit eye movements may also involve failure of inhibition in the hippocampus. Both frontal eye fields and supplementary eye fields make important contributions to predictive aspects of smooth pursuit (for review, see reference 4). A significant linkage of eye tracking dysfunction to markers on chromosome 6p21 has been reported (5). There is also evidence that eye tracking dysfunction is associated with a dopamine D3 receptor gene polymorphism (6).
Previous studies showed that patients with schizophrenia and their nonschizophrenic relatives have deficits in P50 inhibition, relative to healthy subjects (7–9). Findings in animal and human studies suggest a role for septohippocampal cholinergic activity in sensory gating (for review, see reference 10). A linkage between P50 inhibition deficits and a genetic marker at the locus of the α7 subunit of the nicotinic receptor in families of patients with schizophrenia has been reported (11). Moreover, several studies have shown that promoter variants (in the α7 gene) or variants located to exon 6 (in the α7-like gene) are associated with P50 inhibition deficits (12–14).
Patients with schizophrenia demonstrate impaired ability to suppress a reflexive saccade to a visual peripheral target when they are instructed to look as quickly as possible at the opposite location of the cue. This antisaccade task measures saccadic inhibition. Patients with schizophrenia generate a higher proportion of errors and have higher antisaccade latencies, relative to comparison subjects (15) (for review, see reference 16). In addition, patients’ nonschizophrenic first-degree relatives generate a higher proportion of antisaccade errors (17, 18). The frontal cortex contains three areas that contribute to programming of saccades (the frontal eye field, the dorsomedial supplementary motor area, and the dorsolateral prefrontal cortex). The eye fields of the frontal lobes project directly or indirectly to the superior colliculus, to the brainstem reticular formation, and to the cerebellum. Volitional saccades depend on the frontal eye fields. Parietal cortical areas contribute to shifting visual attention and also to initiating saccades (for review, see reference 4). Disinhibition on antisaccade tasks may reflect impairment in the dorsolateral prefrontal cortex and its associated circuitry (19, 20).
The antisaccade and P50 inhibition paradigms, designed to measure central inhibition or sensory gating, show a number of neurobiological similarities, and both have been found to be disturbed in patients with schizophrenia. For example, in a study by Cadenhead et al. (21), P50 inhibition and antisaccade deficits were significantly correlated in 71% of the subjects with schizotypal personality disorders. A composite P50 suppression/antisaccade paradigm endophenotype has been linked to a marker on chromosome 22q in some families of patients with schizophrenia (22).
We simultaneously measured these three paradigms in patients with schizophrenia, healthy comparison subjects, and nonschizophrenic parents of schizophrenia patients to examine the correlations between these markers, with the aim of defining a common endophenotype for genetic studies and improving the diagnostic criteria for schizophrenia.
Method
Subjects
Patients (N=144) who met the DSM-IV criteria for schizophrenia were recruited from the psychiatric hospital with which the authors are affiliated. The patients’ diagnoses were confirmed by two trained psychiatrists using the French version of the Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (SADS-LA) (23). The patients were required to be clinically stable for at least 15 days, with no change in neuroleptic dose at the time of the study. All patients were receiving traditional neuroleptic treatment, except for six patients who were receiving risperidone and three who were receiving olanzapine. None of the patients were treated with clozapine, benzodiazepines, or lithium at the time of the study. Eighty-two patients completed the Wisconsin Card Sorting Test; the number of criteria (ways to classify the cards, e.g., color or number of figures on the card) found and the percentage of perseverative errors were measured in those patients (24). All patients were interviewed with the Positive and Negative Syndrome Scale (25).
The unrelated healthy comparison subjects, who had no personal or family history of neurological or psychiatric disease and were free of any psychotropic treatment, were recruited from the hospital staff.
The parents of the patients with schizophrenia were recruited at the admission of the patients. Both parents were recruited if they did not have schizophrenia, were younger than age 65 years, and agreed to participate in the study. All parents were interviewed with the SADS-LA. In eight cases, one parent had schizotypal personality disorder, according to the French translation of the Schedule for Schizotypal Personalities (26). The parents with schizotypal personality disorder were included in the study.
Subjects with neurological disease, mental retardation, or alcohol or substance abuse at the time of the study were excluded from the study. Visual acuity of all subjects was normal or corrected to normal. After complete description of the study to the subjects, written informed consent was obtained. The study was approved by the hospital’s ethical committee. Subjects were asked to abstain from cigarette smoking for 1 hour before the electrophysiological studies.
Electrophysiological measures
Oculomotor measures
Horizontal eye movements were recorded with an infrared photoelectric limbus eye tracking device (IRIS eye tracker) (Skalar Medical, Delft, the Netherlands). For calibration and for the antisaccade paradigm, the target system consisted of an array of light-emitting diodes placed horizontally (–30° to 30° of visual angle) on a flat screen placed 110 cm in front of the subject. For the pursuit paradigm, the target was a projected light from a mirror mounted on a galvanometer (see also reference 27).
Eye and target movements were sampled online at 200 Hz by using a 12-bit analog-to-digital converter. Subjects were tested in two paradigms (smooth pursuit and antisaccades) assigned in a random order.
Smooth pursuit paradigm
Subjects were asked to track a projected laser spot for 1 minute. The projected laser spot moved in a horizontal sinusoidal waveform at 0.4 Hz with an amplitude of plus or minus 15°. After removing blinks and saccades, slow eye movement velocity was recalculated by using the 2-point central difference algorithm with a 50-msec step size. Amplitude of eye velocity modulation was calculated by least-square fitting a sinusoid on slow phase velocity. The pursuit gain was computed as the ratio of the amplitude of eye velocity to the amplitude of target velocity.
Antisaccade paradigm
Subjects were instructed to fixate on a central light-emitting diode. After 2–4 seconds, the fixation light-emitting diode was turned off, and the subjects were asked to look as quickly and as accurately as possible at the opposite location from the peripheral target light-emitting diode, which appeared 15° to the left or right as soon as the central light-emitting diode was extinguished. The peripheral light-emitting diode was extinguished after 0.5 seconds.
Sixty trials were administered; the first 10 trials were considered practice trials and were not counted. Reflexive saccades in the wrong direction, i.e., toward the peripheral target, were considered to be errors. The latency of the antisaccades and the number of errors (a key performance measure) were considered.
P50 inhibition paradigm recording procedures
Auditory stimuli were delivered in a conditioning-testing paradigm consisting of click pairs (S1, conditioning click; S2, testing click; 500-msec interclick interval; 10-second interpair interval) (see also reference 28). The first five stimulus pairs were not included in the analysis. After the first five stimulus pairs were presented, three sets of 30 stimulus pairs were delivered with a 1-minute rest between each set.
Electroencephalographic activity was monitored and averaged on a Nihon Kohden (Tokyo) computer. EEG measurements were recorded from an electrode fixed to the vertex and referenced to linked ears. Electrical activity was amplified with a band pass filter of 1–200 Hz and digitized at 1000 Hz for averaging by a digital computer.
All trials contaminated by ocular movements and movement artifacts were automatically rejected by the computer (criterion=40 μV). A single average was compiled from the three sets. The conditioning P50 amplitude was identified as the most positive peak between 40 and 80 msec after the first stimulus (amplitude >0.5 μV). The test P50 amplitude was identified at the same latency plus or minus 10 msec. Amplitude was measured as the difference between the peak of the P50 wave and the immediately preceding negative peak, in both the conditioning and test responses. Test/conditioning ratios were calculated by dividing the test P50 amplitude by the conditioning P50 amplitude.
Statistical Analysis
All data were expressed as means and standard deviations. All p values were two-tailed. Chi-square tests were used for analysis of categorical variables. Other data were analyzed with analysis of variance (ANOVA) or a nonparametric test (Mann-Whitney U test). Post hoc analyses were done with Bonferroni or Newman-Keuls tests.
An ANOVA by general linear model was used to compare smooth pursuit gain, antisaccade latency, percentage of perseverative errors, and test/conditioning ratios between the comparison subjects, the patients with schizophrenia, and the parents of the schizophrenia patients. To examine first-rank interactions, the ANOVA took into account four factors: age (age ≤30 years, age >30 and <50 years, age ≥50 years), gender (male, female), clinical status (comparison subjects, patients with schizophrenia, parents), and smoking status (smoker, nonsmoker).
Spearman’s rank-order correlation was used to examine the relationship between the electrophysiological paradigms and between the paradigms and the demographic variables. Receiver operating curve analysis was performed 1) to calculate a cutoff score for each paradigm on the basis of the mean value of the comparison group, which led to a specificity of 80% for the corresponding paradigm, and 2) to discriminate between comparison subjects, patients with schizophrenia, and parents by using the three paradigms.
Results
Demographic and Clinical Data
One hundred thirteen healthy comparison subjects, 144 patients with schizophrenia, and 35 subjects who were nonschizophrenic parents of patients with schizophrenia were included in this study (Table 1). Among the 144 patients with schizophrenia, the following DSM-IV subtypes were observed: paranoid (N=61), undifferentiated (N=40), disorganized (N=23), and residual (N=7). Thirteen patients fulfilled the criteria for schizoaffective disorder. The mean age at onset of the disease was 22.4 years (SD=5.4). The mean neuroleptic dose was 456 mg/day (SD=322) (chlorpromazine equivalents). The mean tropatepine dose was 4.1 mg/day (SD=5) (57 of the 144 patients were receiving tropatepine). The mean Wisconsin Card Sorting Test scores were as follows: 4.05 (SD=1.5) for the number of criteria and 29.9 (SD=21.6) for the percentage of perseverative errors. The mean Positive and Negative Syndrome Scale scores were 69 (SD=17) for the total score, 14.5 (SD=5.9) for the positive subscale score, 20.5 (SD=6.4) for the negative subscale score, and 33.9 (SD=8.4) for the general psychopathology score.
As for the gender ratio, both the comparison group and the parent group differed significantly from the schizophrenia patient group. The three groups differed significantly in age. The comparison group and the parent group differed significantly from the patient group in smoking status.
Smooth Pursuit Paradigm
Eye movement smooth pursuit was measured in 73 comparison subjects, 111 schizophrenia patients, and 27 parents (Table 2). The mean pursuit gain was significantly lower in the patients, relative to the comparison subjects, and was slightly, but not significantly, lower in the patients’ parents, relative to the comparison subjects. The pursuit gain measures showed no age effect (F=0.47, df=2, 164, p=0.62), no gender effect (F=1.82, df=1, 164, p=0.18), and no effect of smoking status (F=0.45, df=1, 164, p=0.50).
Within each group, there was no significant correlation between gain and any of the demographic and clinical variables (age, number of cigarettes per day, Positive and Negative Syndrome Scale scores, neuroleptic treatment dose, Wisconsin Card Sorting Test scores), except for duration of the disease (rs=–0.22, N=111, p<0.03).
Antisaccade Paradigm
The antisaccade paradigm was measured in 73 comparison subjects, 104 schizophrenia patients, and 31 parents (Table 2). The latency of antisaccades was significantly higher in the schizophrenia patients, relative to the comparison group and the parent group. The latency of antisaccades was slightly but not significantly higher in the parents, relative to the comparison subjects. The antisaccade measures showed no age effect (F=2.83, df=2, 158, p=0.06), no gender effect (F=0.38, df=1, 158, p=0.54), and no effect of smoking status (F=1.01, df=1, 158, p=0.31).
Within the schizophrenia group, there were significant correlations between the latency of antisaccades and age (rs=0.27, N=104, p=0.005), Positive and Negative Syndrome Scale negative subscale scores (rs=0.23, N=94, p=0.02), duration of the disease (rs=0.23, N=104, p=0.02), and Wisconsin Card Sorting Test scores (number of criteria: rs=–0.28, N=66, p=0.02). No correlation was found between antisaccade latency and the number of cigarettes per day or the neuroleptic treatment dose.
The number of errors was higher in the schizophrenia group, relative to the comparison group and the parent group. In the parent group, the number of errors was slightly but not significantly higher, relative to the comparison group. The number of errors showed no age effect (F=2.39, df=2, 158, p=0.10), no gender effect (F=2.19, df=1, 158, p=0.14), and no effect of smoking status (F=0.42, df=1, 158, p=0.51).
Within the schizophrenia patient group and the parent group, there were significant correlations between the number of errors and age (rs=0.42, N=104, p=0.001 and rs=0.39, N=31, p=0.02, respectively). Within the schizophrenia patient group, there were significant correlations between the number of errors and the duration of the disease (rs=0.35, N=104, p<0.01) and between the number of errors and the Wisconsin Card Sorting Test scores (number of criteria: rs=–0.31, N=62, p<0.01; percentage of perseverative errors: rs=0.39, N=62, p<0.01). No correlation was observed between the number of errors and the number of cigarettes per day or the neuroleptic treatment dose.
Test/Conditioning Ratio
The test/conditioning ratio was measured in 100 comparison subjects, 124 schizophrenia patients, and 33 parents (Table 2). The mean test/conditioning ratio was significantly higher in the schizophrenia patients and the parents, relative to the comparison subjects. There were no differences between the schizophrenia patients and the parents in the mean test/conditioning ratio. The test/conditioning ratio showed no age effect (F=0.47, df=2, 223, p=0.62), no gender effect (F=0.26, df=1, 223, p=0.61), and no effect of smoking status (F=0.04, df=1, 223, p=0.84).
There were no significant correlations in any group between test/conditioning ratios and clinical or demographic variables (including the Positive and Negative Syndrome Scale scores, neuroleptic treatment dose, and Wisconsin Card Sorting Test scores), except for a significant correlation between the test/conditioning ratio and age (rs=–0.45, N=33, p<0.01) in the parent group and a significant correlation between the test/conditioning ratio and duration of the disease in the schizophrenia patient group (rs=0.19, N=124, p=0.03).
For each paradigm, no significant difference was found within the parent group between subjects with and without schizotypal personality disorder.
For each paradigm, no significant difference was found within the schizophrenia group between the patients who received typical neuroleptics and those who received atypical neuroleptics (including olanzapine [N=3], risperidone [N=6], and amisulpride [N=16]) (p>0.10, Mann-Whitney U test), except for a significant difference in the test/conditioning ratio (p<0.01, Mann-Whitney U test). However, the results of the statistical analyses remained unchanged when the schizophrenia patients who received atypical neuroleptics were excluded from the analyses. No significant differences related to DSM-IV schizophrenia categories (schizoaffective disorder and paranoid, undifferentiated, residual, and disorganized subtypes) were found.
Concordance of the Three Paradigms
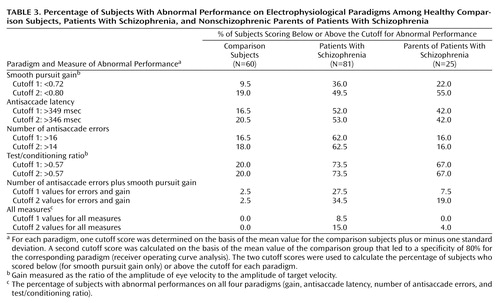
Data for the three paradigms—P50 suppression, smooth pursuit, and antisaccade—were available for 60 comparison subjects, 81 schizophrenia patients, and 25 parents. For each paradigm, we determined two deficit cutoff scores (Table 3). For this purpose, two strategies were used: 1) a first cutoff score was determined on the basis of the mean value for the comparison subjects plus or minus one standard deviation, and 2) a second cutoff score was calculated on the basis of the mean value for the comparison subjects that led to a specificity of 80% for the corresponding paradigm (receiver operating curve analysis). The percentage of subjects who scored below (for smooth pursuit gain only) or above each cutoff score was calculated (Table 3).
Performances on the P50 suppression, antisaccade, and smooth pursuit gain paradigms (categorized as normal or abnormal by using the cutoff values) were analyzed with Fisher’s exact test to assess the independence of the measures. First, by using the 80th percentile cutoff scores, the hypothesis of independence of the smooth pursuit gain and antisaccade performances (null hypothesis) was rejected in the parent group, suggesting that the number of errors in the antisaccade paradigm and smooth pursuit gain were not independent (p<0.02) (in the schizophrenia group, p<0.10). Other tests of association were not considered significant (association of gain and antisaccade latency, gain and test/conditioning ratio, antisaccade latency and test/conditioning ratio, number of antisaccade errors and test/conditioning ratio), except for the tests of association between antisaccade latency and number of errors in the comparison group and in the schizophrenia group (p<0.006 and p<0.02, respectively). Second, by using the comparison group’s mean value plus or minus one standard deviation, the null hypothesis of the independence of the smooth pursuit gain and antisaccade performances was rejected in the schizophrenia group, suggesting that the number of antisaccade errors and smooth pursuit gain were not independent (p<0.05). Other tests of association between the electrophysiological parameters were not significant, except for the test of association between antisaccade latency and number of errors in the comparison subjects (p<0.003). The association between antisaccade latency and number of errors approached significance in the schizophrenia group (p=0.07).
Furthermore, the relationships between the electrophysiological parameters were analyzed by using Spearman’s rank order correlations. In the schizophrenia group, a significant correlation between antisaccade latency and number of errors (rs=0.42, N=81, p<0.001) and a significant correlation between smooth pursuit gain and number of antisaccade errors (rs=–0.27, N=81, p<0.006) were found; the correlation between gain and antisaccade latency in that group approached significance (rs=–0.16, N=81, p<0.11). In the comparison group, there was a significant correlation between antisaccade latency and number of errors (rs=0.22, N=60, p<0.05). In the parent group, there was a significant correlation between smooth pursuit gain and antisaccade latency (rs=–0.40, N=25, p<0.04) and between gain and number of antisaccade errors (rs=–0.54, N=25, p<0.004).
The results remained unchanged when the schizophrenia patients who were receiving atypical neuroleptics were excluded from the analyses.
Diagnosis
The three paradigms were used to discriminate between the comparison subjects, parents, and patients with schizophrenia. The area under the receiver operating curve was measured. The optimal paradigms for discriminating between the comparison subjects and the schizophrenia patients were the test/conditioning ratio and the number of errors in the antisaccade paradigm (area under the curve=0.86, SD=0.02, and area under the curve=0.83, SD=0.03, respectively). The optimal paradigm for discriminating between the parents and the schizophrenia patients was the number of errors in the antisaccade paradigm (area under the curve=0.80, SD=0.04).
Odds ratios were calculated for each paradigm to approach the relative risk for schizophrenia. The cutoff scores used to define abnormal values were determined by using receiver operating curve analysis (see the previous section, titled Concordance of the Three Paradigms). The odds ratios were 4.2 (95% confidence interval [CI]=2.0–9.1) for smooth pursuit gain, 4.2 (95% CI=2.1–9.2) for antisaccade latency, 8.8 (95% CI=4.2–20) for number of antisaccade errors, and 10.9 (95% CI=5.7–22.2) for the test/conditioning ratio. When the schizophrenia patients who received atypical neuroleptics were excluded from the analyses, the odds ratios slightly, but not significantly, improved.
Discussion
Patients with schizophrenia showed a high rate of inhibitory deficits measured by the P50 inhibition and antisaccade paradigms. The nonschizophrenic parents of the schizophrenia patients also had a higher rate of inhibitory deficits, relative to the healthy comparison subjects. These results are consistent with the pattern usually reported (for review, see reference 29). The fact that the mean test/conditioning ratios in the patient group and the parent group did not significantly differ may be partly explained by the mean age of the parent group. However, Rasco et al. (30) found no difference when the mean test/conditioning ratios of comparison subjects age >55 years were compared with those of younger subjects. Moreover, when age was taken into account in our statistical analyses, no interaction with age was observed. The highest mean number of antisaccade errors was observed in the schizophrenia patients, and the lowest in the comparison subjects, as previously reported (18, 31). The latency of antisaccades was not significantly different between the parents and comparison subjects, as previously observed (32). Latency differences on voluntary tasks are not as robust in a population at risk for schizophrenia as they are in the patients themselves and may in fact be related to the disease process or its treatment rather than to disease liability (33).
We focused on gain as an indicator of the oculomotor pursuit system. Schizophrenia patients had a high prevalence of eye tracking dysfunction. Abnormal eye tracking was also observed in approximately 50% of the parent group; this finding was consistent with the findings of previous studies (2, 34) (for review, see reference 35).
The role of neuroleptic treatment may be considered. Treatment with typical neuroleptics has not been reported to cause significant smooth pursuit eye movement abnormalities in schizophrenia patients (36). With the newer atypical antipsychotics, one cross-sectional study found that patients treated with risperidone and olanzapine had better pursuit gain than those treated with typical neuroleptics, but this finding only approached significance (37). As for the antisaccade paradigm, the evidence available to date suggests that typical neuroleptic treatment does not affect the antisaccade error rate. Antagonism of serotonin 5-HT2 receptors and, indirectly, action on prefrontal dopaminergic transmission may reduce the antisaccade error rate (38). Treatment with atypical antipsychotics may normalize antisaccade latency in previously untreated or drug-naive patients with schizophrenia; however, risperidone may prolong latency (for review, see references 39, 40). Typical neuroleptic treatment does not seem to affect P50 sensory gating deficits in subjects with schizophrenia (41). In a randomized longitudinal 12-week study, Arango et al. (42) found no significant effect of olanzapine and haloperidol on a P50 sensory gating index in patients with treatment-resistant schizophrenia. However, clozapine, olanzapine, and risperidone may be associated with improvements in sensory gating in cross-sectional studies (43–45).
We observed no significant differences between patients who received typical neuroleptics and those who received atypical neuroleptics in any of the paradigms we used, except for the test/conditioning ratio. In addition, all statistically significant results remained significant when the patients who were receiving atypical neuroleptics were excluded from the analyses.
Our results suggest that, despite superficial similarities between the three paradigms we used, there is little evidence to suggest that these paradigms, particularly those measuring sensory gating, evaluate similar brain processes. Each paradigm may be important for investigating the neurobiological disturbances observed in patients with schizophrenia. Similarly, comparisons between measures of P50 suppression and measures of prepulse inhibition of the startle reflex in humans have demonstrated low evidence for correlation between these gating measures, suggesting that they are probably mediated by different neuronal mechanisms (46, 47). In contrast, Cadenhead et al. (21) reported a high rate of concordance between P50 suppression, prepulse inhibition, and antisaccade paradigm performances in a small group of subjects with schizotypal personality disorder (33% of the subjects in that study had no deficits on any paradigm). Myles-Worsley et al. (22) studied a composite P50/antisaccade inhibitory phenotype (subjects with abnormal findings on the P50 suppression or antisaccade paradigms or on both) and reported a linkage to a marker on chromosome 22 (25% of the subjects with schizophrenia and 38% of their relatives in that study had no deficits on either paradigm). In our study, no concordance was observed between P50 inhibition and antisaccade paradigm performances.
However, we found significant correlations between smooth pursuit gain and the antisaccade error rate in the schizophrenia patients and the nonschizophrenic parents. These results suggest that eye tracking dysfunction and saccades generated during the antisaccade paradigm may stem from the same putative prefrontal cortical dysfunction. Berman et al. (48) reported that in healthy humans, common cortical networks (especially the frontal eye fields) subserved smooth pursuit and saccadic eye movements. Human studies and nonhuman primate studies have confirmed activation in the dorsolateral prefrontal cortex, frontal eye fields, and anterior cingulate cortex during antisaccades. Both frontal eye fields and the supplementary eye field make important contributions to predictive aspects of smooth pursuit (for review, see reference 4). In the frontal eye fields, γ-aminobutyric acid inhibitory circuits may play a central role in eye-movement generation (49). Frontal eye field dysfunction may be particularly important in the low gain component of pursuit dysfunction in schizophrenia (50). Positron emission tomography studies showed that relatives of schizophrenia patients with eye tracking dysfunction, compared to relatives with normal tracking and to healthy subjects, failed to activate the frontal eye fields during eye tracking tasks (51). Furthermore, McDowell et al. (20) observed that patients with schizophrenia did not demonstrate the increased prefrontal cortical activity during antisaccade performance that was apparent in comparison subjects. Similarly, Matsue et al. (52) and Sereno and Holzman (53) reported a relationship between smooth pursuit performance and antisaccade error rate in a group of patients with schizophrenia, whereas Hutton (54) did not. Furthermore, O’Driscoll et al. (33) found that pursuit quality scores and error rate in the antisaccade task were negatively correlated in subjects with high “schizotypal” ratings, relative to comparison subjects.
Finally, of the three paradigms used as diagnostic tools to discriminate between comparison subjects, patients with schizophrenia, and nonschizophrenic parents of patients, P50 inhibition and antisaccade errors were considered the optimal paradigms. In a study by Siegel et al. (8), only 8% of schizophrenia patients and 28% of patients’ first-degree relatives showed neither eye tracking nor P50 inhibition deficits when the two paradigms were studied concomitantly. In our study, 5% of the schizophrenia patients and 12% of the parents showed neither deficit when antisaccade and P50 paradigms were studied concomitantly, compared with 4% and 4%–12% (depending which of the two cutoff scores was used) of the patients and parents, respectively, when the eye tracking, antisaccade, and P50 paradigms were measured together. Conversely, in the study by Siegel et al. (8), the subjects with schizophrenia were 2.5 times more likely than their relatives to have both deficits (61.5% versus 23%) when eye tracking dysfunction and P50 inhibition were measured, compared with the 3.5- to eightfold greater likelihood (depending on the cutoff scores used) when the eye tracking dysfunction, P50 inhibition, and antisaccade paradigms were used in our study. These three endophenotypes (particularly the P50 inhibition and antisaccade paradigms) may be useful in establishing a biological underpinning for diagnosis and classification in schizophrenia.
 |
 |
 |
Received Dec. 6, 2003; revision received May 18, 2004; accepted May 21, 2004. From the Department of Psychiatry, Rouen University Hospital Charles Nicolle; and Le Rouvray Hospital Center, INSERM Unit 614, University of Medicine, Rouen, France. Address Correspondence and reprint requests to Prof. Thibaut, Department of Psychiatry, Rouen University Hospital Charles Nicolle, 1 rue de Germont 76031 Rouen Cedex, France; [email protected] (e-mail). Supported by grants from Pfizer and the French Health Ministry. The authors thank the study patients and their families for their participation, colleagues from the University Hospital of Caen (particularly Prof. P. Denise) and from the Le Rouvray Hospital Center (particularly Prof. Petit, Dr. Allio, Dr. Haouzir, and Dr. Morcamp) for their contributions, Richard Medeiros for assistance with the manuscript, and the anonymous reviewers for their comments.
1. Holzman PS, Proctor LR, Hughes DW: Eye-tracking patterns in schizophrenia. Science 1973; 181:179–181Crossref, Medline, Google Scholar
2. Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW: Eye-tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry 1974; 31:143–151Crossref, Medline, Google Scholar
3. Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R: Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry 2004; 161:315–321Link, Google Scholar
4. Leigh RJ, Zee DS: The Neurology of Eye Movements, 3rd ed. New York, Oxford University Press, 1999Google Scholar
5. Arolt V, Lener R, Nolte A, Muller-Myhsok B, Purmann S, Shurmann M, Leutelt J, Pinnow M, Schwinger E: Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. Am J Med Genet 1996; 67:564–579Crossref, Medline, Google Scholar
6. Rybakowski JK, Borkowska A, Czerski PM, Hauer J: Dopamine D3 receptor (DRD3) gene polymorphism is associated with the intensity of eye movement disturbances in schizophrenic patients and healthy subjects. Mol Psychiatry 2001; 6:718–724Crossref, Medline, Google Scholar
7. Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R: Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 1982; 17:639–654Medline, Google Scholar
8. Siegel C, Waldo M, Mizner G, Adler LA, Freedman R: Deficit in sensory gating in schizophrenic patients and their relatives. Arch Gen Psychiatry 1984; 41:607–612Crossref, Medline, Google Scholar
9. Clementz BA, Geyer MA, Braff DL: Poor P50 suppression among schizophrenia patients and their first-degree biological relatives. Am J Psychiatry 1998; 155:1691–1694Link, Google Scholar
10. Adler LE, Freedman R, Ross RG, Olincy A, Waldo M: Elementary phenotypes in the neurobiological and genetic study of schizophrenia. Biol Psychiatry 1999; 46:8–18Crossref, Medline, Google Scholar
11. Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hoff M, Rosenthal J, Waldo MC, Reimberr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LA, Kruglyak L, Leonard S, Byerler W: Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 1997; 94:587–592Crossref, Medline, Google Scholar
12. Raux G, Bonnet-Brilhault F, Louchart S, Houy E, Gantier R, Levillain D, Allio G, Haouzir S, Martinez M, Frébourg T, Thibaut F, Campion D: The -2 bp deletion in exon 6 of “alpha 7-like” nicotinic receptor subunit gene is a risk factor for the P50 sensory gating deficit. Mol Psychiatry 2002; 7:1006–1011Crossref, Medline, Google Scholar
13. Leonard S, Gault J, Hopkins J, Hogel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota O, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R: Association of promoter variants in the α7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry 2002; 59:1085–1096Crossref, Medline, Google Scholar
14. Houy E, Raux G, Thibaut F, Belmont A, Demily C, Allio G, Haouzir S, Fouldrin G, Petit M, Frébourg T, Campion D: The promoter -194 C polymorphism of the nicotinic alpha 7 receptor gene has a protective effect against the P50 sensory gating deficit. Mol Psychiatry 2004; 9:320–322Crossref, Medline, Google Scholar
15. Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, Kato M: Disturbances of voluntary control of saccadic eye movements in schizophrenia patients. Biol Psychiatry 1988; 23:670–677Crossref, Medline, Google Scholar
16. Lee KH, Williams LM: Eye movement dysfunction as a biological marker of risk for schizophrenia. Aust NZ J Psychiatry 2000; 34:S91-S100Google Scholar
17. Crawford TJ, Sharma T, Puri BK, Murray RM, Berridge DM, Lewis SW: Saccadic eye movements in families multiply affected with schizophrenia: the Maudsley Family Study. Am J Psychiatry 1998; 155:1703–1710Link, Google Scholar
18. McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA: Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology 1999; 36:138–141Crossref, Medline, Google Scholar
19. Pierrot-Deseilligny C: Saccade and smooth-pursuit impairment after cerebral hemispheric lesions. Eur Neurol 1994; 34:121–134Crossref, Medline, Google Scholar
20. McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, Braff DL: Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biol Psychiatry 2002; 51:216–223Crossref, Medline, Google Scholar
21. Cadenhead KS, Light GA, Geyer MA, McDowell JE, Braff DL: Neurobiological measures of schizotypal personality disorder: defining an inhibitory endophenotype? Am J Psychiatry 2002; 159:869–871Link, Google Scholar
22. Myles-Worsley M, Coon H, McDowell J, Brenner C, Hoff M, Lind B, Bennett P, Freedman R, Clementz B, Byerley W: Linkage of a composite inhibitory phenotype to a chromosome 22q locus in eight Utah families. Am J Med Genet 1999; 88:544–550Crossref, Medline, Google Scholar
23. Fyer AJ, Mannuzza SM, Klein DF, Endicott J: Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (SADS-LA). New York, New York State Psychiatric Institute, 1985Google Scholar
24. Nelson HE: A modified card sorting test sensitive to frontal lobe defects. Cortex 1976; 12:313–324Crossref, Medline, Google Scholar
25. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
26. Baron M, Asnis L, Gruen R: The Schedule for Schizotypal Personalities (SSP): a diagnostic interview for schizotypal features. Psychiatry Res 1981; 4:213–228Crossref, Medline, Google Scholar
27. Nkam I, Thibaut F, Denise P, Van Der Elst A, Segard L, Brazo P, Ménard JF, Théry S, Halbeck I, Delamilleure P, Vasse T, Etard O, Dollfus S, Campion D, Levillain D, Petit M: Saccadic and smooth-pursuit eye movements in deficit and non-deficit schizophrenia. Schizophr Res 2001; 48:145–153Crossref, Medline, Google Scholar
28. Louchart-de la Chapelle S, Levillain D, Ménard JF, Van Der Elst A, Allio G, Haouzir S, Dollfus S, Campion D, Petit M, Thibaut F: P50 inhibitory gating deficit is correlated with the negative symptomatology of schizophrenia. Psychiatry Res (in press)Google Scholar
29. Waldo MC, Adler LE, Leonard S, Olincy A, Ross RG, Harris JG, Freedman R: Familial transmission of risk factors in the first-degree relatives of schizophrenic people. Biol Psychiatry 2000; 47:231–239Crossref, Medline, Google Scholar
30. Rasco L, Skinner RD, Garcia-Rill E: Effect of age on sensory gating of the sleep state-dependent P1/P50 midlatency auditory evoked potential. Sleep Res Online 2000; 3:97–105Medline, Google Scholar
31. Ross RG, Harris JG, Olincy A, Radant A, Adler LE, Freedman R: Familial transmission of two independent saccadic abnormalities in schizophrenia. Schizophr Bull 1998; 30:59–70Crossref, Google Scholar
32. Clementz B, McDowell JE, Zisool S: Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol 1994; 109:277–287Crossref, Google Scholar
33. O’Driscoll GA, Lenzenger MF, Holzman PS: Antisaccades and smooth pursuit eye tracking and schizotypy. Arch Gen Psychiatry 1998; 55:837–843Crossref, Medline, Google Scholar
34. Karoumi B, Saoud M, d’Amato T, Rosenfeld F, Denise P, Gutknecht C, Gaveau V, Beaulieu FE, Daléry J, Rochet T: Poor performance in smooth pursuit and antisaccadic eye-movement tasks in healthy siblings of patients with schizophrenia. Psychiatry Res 2001; 101:209–219Crossref, Medline, Google Scholar
35. Lencer R, Malchow CP, Trillenberg-Krecher K, Schwinger E, Arolt V: Eye-tracking dysfunction in families with sporadic and familial schizophrenia. Biol Psychiatry 2000; 47:391–401Crossref, Medline, Google Scholar
36. Campion D, Thibaut F, Denise P, Courtin P, Pottier M, Levillain D: SPEM impairment in drug-naive schizophrenic patients: evidence for a trait marker. Biol Psychiatry 1992; 32:891–902Crossref, Medline, Google Scholar
37. Hutton SB, Crawford TJ, Gibbins H, Cuthbert I, Barnes TR, Kennard C, Joyce EM: Short and long term effects of antipsychotic medication on smooth pursuit eye tracking in schizophrenia. Psychopharmacology (Berl) 2001; 157:284–291Crossref, Medline, Google Scholar
38. Burke JG, Reveley MA: Improved antisaccade performance with risperidone in schizophrenia. J Neurol Neurosurg Psychiatry 2002; 72:449–454Medline, Google Scholar
39. Ettinger U, Kumari V: Pharmacological studies of smooth pursuit and antisaccade eye movements in schizophrenia: current status and directions for future research. Curr Neuropharmacol 2003; 1:1–16Crossref, Google Scholar
40. Brownstein J, Krastoshevsky O, McCollum C, Kundamal S, Matthysse S, Holzman PS, Mendell NR, Levy DL: Antisaccade performance is abnormal in schizophrenia patients but not in their biological relatives. Schizophr Res 2003; 63:13–25Crossref, Medline, Google Scholar
41. Adler LE, Waldo MC, Tatcher A, Cawthra E, Baker N, Freedman R: Lack of relationship of auditory gating defects to negative symptoms in schizophrenia. Schizophr Res 1990; 3:131–138Crossref, Medline, Google Scholar
42. Arango C, Summerfelt A, Buchanan RW: Olanzapine effects on auditory sensory gating in schizophrenia. Am J Psychiatry 2003; 160:2066–2068Link, Google Scholar
43. Nagamato HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R: Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biol Psychiatry 1996; 40:181–188Crossref, Medline, Google Scholar
44. Yee CM, Nuechterlein KH, Morris SE, White PM: P50 suppression in recent-onset schizophrenia: clinical correlates and risperidone effects. J Abnorm Psychol 1998; 107:691–698Crossref, Medline, Google Scholar
45. Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL: Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am J Psychiatry 2000; 157:767–771Link, Google Scholar
46. Schwarzkopf SB, Lamberti JS, Smith DA: Concurrent assessment of acoustic startle and auditory P50 evoked potential measures of sensory inhibition. Biol Psychiatry 1993; 33:815–828Crossref, Medline, Google Scholar
47. Oranje B, Van Berckel NM, Kemner C, Van Ree JM, Kahn RS, Verbaten MN: P50 suppression and prepulse inhibition of the startle reflex in humans: a correlation study. Biol Psychiatry 1999; 45:883–890Crossref, Medline, Google Scholar
48. Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA: Cortical networks subserving pursuit and saccadic eye movements in humans: an fMRI study. Hum Brain Mapp 1999; 8:209–225Crossref, Medline, Google Scholar
49. Schiller PH, Tehovnik EJ: Cortical inhibitory circuits in eye-movement generation. Eur J Neurosci 2003; 18:3127–3133Crossref, Medline, Google Scholar
50. Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, Carl JR: Eye tracking abnormalities in schizophrenia: evidence for dysfunction in the frontal eye fields. Biol Psychiatry 1998; 44:698–708Crossref, Medline, Google Scholar
51. O’Driscoll GA, Benkelfat C, Florencio PS, Wolff AL, Joober R, Lal S, Evans AC: Neural correlates of eye tracking deficits in first-degree relatives of schizophrenic patients: a positron emission tomography study. Arch Gen Psychiatry 1999; 56:1127–1134Crossref, Medline, Google Scholar
52. Matsue Y, Saito H, Osakabe K, Awata S, Ueno T, Matsuoka H, Chiba H, Fuse Y, Sato M: Smooth pursuit eye movements and voluntary control of saccades in the antisaccade task in schizophrenic patients. Jpn J Psychiatry Neurol 1994; 48:13–22Medline, Google Scholar
53. Sereno AB, Holzman PS: Antisaccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry 1995; 37:394–401Crossref, Medline, Google Scholar
54. Hutton SB, Crawford TJ, Puri BK, Duncan LJ, Chapman M, Kennard C, Barnes TRE, Joyce EM: Smooth pursuit and saccadic abnormalities in first-episode schizophrenia. Psychol Med 1998; 28:685–692Crossref, Medline, Google Scholar



