Neurobiological Measures of Schizotypal Personality Disorder: Defining an Inhibitory Endophenotype?
Abstract
OBJECTIVE: Subjects with schizotypal personality disorder demonstrate deficits in inhibition when assessed on prepulse inhibition, P50 suppression, and antisaccade paradigms. This study determined if distinct subgroups of subjects with schizotypal personality disorder could be identified on the basis of performance on these measures and whether endophenotypes could be defined for future genetic study by using measures of inhibitory function. METHOD: Prepulse inhibition, P50 suppression, and antisaccade paradigms were assessed in 21 subjects with schizotypal personality disorder. RESULTS: Seven subjects with schizotypal personality disorder had deficits on each paradigm; seven had no deficits on any paradigm. P50 and antisaccade deficits were present in five of the same subjects and significantly correlated. CONCLUSIONS: These results suggest that P50 and antisaccade performance reflects a common endophenotype and that prepulse inhibition identifies a separate endophenotype reflecting different neurobiological substrate(s) in subjects with schizotypal personality disorder. This pattern may generalize to schizophrenia spectrum disorder patients.
Schizotypal personality disorder defines a heterogeneous group of individuals with a mix of social/interpersonal, perceptual, and disorganized symptoms that were empirically derived on the basis of symptoms exhibited by nonpsychotic relatives of schizophrenia patients. Subjects with schizotypal personality disorder may have some genetic vulnerability for schizophrenia, but they do not have many of the confounding variables (chronic illness, medication effects, and multiple hospitalizations) seen in schizophrenia patients. It is possible, however, that phenocopies of schizotypal personality disorder exist because of a range of genetic and environmental factors that are unrelated to the schizophrenia spectrum. The study of endophenotypic markers in subjects with schizotypal personality disorder has become increasingly important because it provides a means of assessing endophenotypic traits of the schizophrenia spectrum that may be more central to schizophrenia than are symptoms and DSM-IV diagnoses (1). Although the diagnostic criteria for schizotypal personality disorder probably reflect relatively remote factors that may predispose to schizophrenia, the neurobiological deficits may be more closely associated with specific neural substrate abnormalities (1).
It has long been hypothesized that subjects with schizophrenia spectrum disorders have deficits in inhibitory functioning that lead to difficulty in filtering (or inhibiting) trivial internal and external stimuli, perhaps accounting for the observed attention and cognitive abnormalities (2). Schizophrenia patients, their relatives, and subjects with schizotypal personality disorder have deficits in a variety of psychophysiological paradigms designed to study central inhibition, including prepulse inhibition of the startle response (3), suppression of the P50 event-related potential (4, 5), and the antisaccade task (6, 7). While the prepulse inhibition, P50 suppression, and antisaccade paradigms are all thought to relate to inhibitory phenomena, different, but perhaps overlapping, neural substrates regulate the three functions (8–10). Exploring the correlative relationship of task performance in patients with schizophrenia spectrum disorders is a first step in defining an inhibitory endophenotype.
The aims of the present study were to determine 1) if the deficits in inhibition observed on measures of prepulse inhibition, P50 suppression, and antisaccade paradigms are present in all subjects with schizotypal personality disorder or if distinct schizotypal personality disorder subgroups can be identified on the basis of their performance on these measures and 2) whether an endophenotype for use in future genetic studies can begin to be defined by using measures of inhibitory functions within a population of subjects with schizotypal personality disorder.
Method
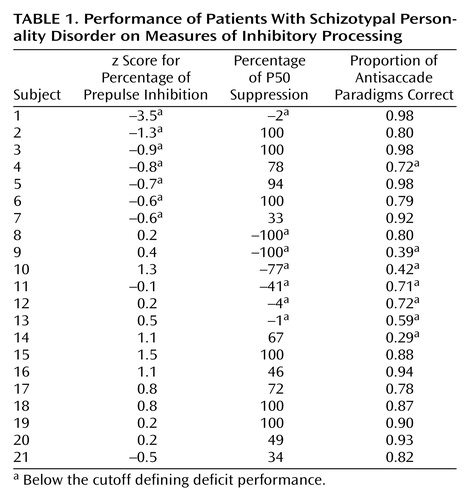
Subjects included 10 men and 11 women (N=21) with schizotypal personality disorder who were tested on the prepulse inhibition, P50 suppression, and antisaccade paradigms. Details of the experimental methods for each of the paradigms have been reported elsewhere (3, 5, 7). Four of the 21 subjects were receiving psychotropic medication, and 11 reported a family history of psychiatric illness. Before entry into the study, all subjects provided written informed consent after receiving an explanation of the study. Subjects received one of two startle paradigms (3). The percentage of prepulse inhibition (30-msec interstimulus interval) was converted to z scores. The mean prepulse inhibition of comparison subjects (z=0.2) minus 1 standard deviation (z=0.8) from a previous report (3) was chosen in determining a deficit cutoff z score of –0.6. We previously reported that subjects with schizotypal personality disorder had deficits in P50 suppression relative to those in normal comparison subjects (5). A cutoff score of 19% P50 suppression was determined on the basis of the mean (64%) for the normal comparison subjects minus one standard deviation (45%) in this study. Cutoff scores were determined for the antisaccade paradigm on the basis of the mean proportion correct (0.84) for the normal comparison subjects minus one standard deviation (0.12) (cutoff score=0.72)(7).
Results
Seven (33%) of the subjects with schizotypal personality disorder scored below the prepulse inhibition cutoff, seven scored below the P50 suppression cutoff, and seven scored below the antisaccade cutoff, while seven (33%) had no deficits on any paradigm (Table 1). Of the seven subjects who had P50 suppression deficits, five (71%) also had antisaccade deficits. Additionally, 81% (N=17) of the subjects with schizotypal personality disorder had concordant scores (both abnormal or both normal) on P50 suppression and antisaccade paradigm performance. The observed frequencies of performance on the P50 suppression and antisaccade paradigms were analyzed to assess the independence of the measures. The null hypothesis was rejected (χ2=6.9, df=1, p<0.01; p<0.02, Fisher’s exact test), suggesting that performance on the P50 suppression and antisaccade paradigms is not independent. Other tests of association (prepulse inhibition and P50 suppression, prepulse inhibition and antisaccade task) were nonsignificant. Demographic and clinical ratings were also similarly assessed, and there were no significant relationships found between gender, family history of psychotic illness, clinical rating, or psychotropic medication use and any of the physiological paradigms.
The distributions of the P50 suppression and prepulse inhibition data were skewed, and the P50 suppression and antisaccade data were not normally distributed. One outlier was identified in the prepulse inhibition data (z=–3.5) and was excluded from the analyses. Spearman’s rank-order correlations revealed a significant correlation between P50 suppression and antisaccade performance (r=0.49, p<0.05, N=20). Correlations between prepulse inhibition and the other measures were nonsignificant.
Discussion
Individuals diagnosed with schizotypal personality disorder have a high rate of inhibitory deficits (3, 5, 7), consistent with the pattern seen in schizophrenia patients and their relatives. These preliminary results, with liberal cutoff points, suggest that the inhibitory deficits measured by the P50 suppression and antisaccade paradigms are moderately associated and present in a significant subgroup of subjects with schizotypal personality disorder, while the prepulse inhibition paradigm appears to identify a different subgroup of subjects with schizotypal personality disorder. Neurobiological measures, versus clinical characteristics, may provide simpler, empirically derived endophenotypes with discernible patterns of inheritance due to major genetic effects in schizophrenia spectrum illness (11). These results should be replicated in a larger group of subjects with schizotypal personality disorder as well as in normal and schizophrenia subjects. Comparisons between the P50 suppression and prepulse inhibition paradigms in normal human and animal subjects (12–14) are consistent with the idea that these two “gating” measures do not correlate with each other. In contrast, a significant relationship between P50 suppression and antisaccade performance has been reported in schizophrenia patients and their relatives, and a composite P50 suppression/antisaccade paradigm endophenotype has been linked to a marker on chromosome 22q in these families (15).
The convergence, as well as divergence, in performance data on these measures of inhibitory functioning suggests that there may be overlap and independence in the modulatory neural circuitry and genetic architecture of the three tasks. For example, there are commonalities in the neural substrates of P50 suppression and antisaccade performance (e.g., basal ganglia and frontal circuits) and temporal characteristics that may prove important in future neurophysiological and genetic studies.
 |
Presented in part at the 39th annual meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, Dec. 10–14, 2000. Received April 4, 2001; revision received Oct. 2, 2001; accepted Oct. 10, 2001. From the Department of Psychiatry, University of California, San Diego. Address reprint requests to Dr. Cadenhead, Department of Psychiatry, 0810, University of California, San Diego, 9500 Gilman Dr., La Jolla, CA 92093-0810; [email protected] (e-mail). Supported in part by NIMH grants MH-01124, MH-18399, MH-51129, and MH-42228; Young Investigator Awards (to Drs. Cadenhead and McDowell) from the National Alliance for Research on Schizophrenia and Depression; and a grant from the Department of Veterans Affairs VISN 22 Mental Illness Research Education and Clinical Center. The authors thank Kathleen Shafer, Colleen Brenner, and Joyce Sprock for their support of this research.
1. Braff DL, Freedman R: The Importance of Endophenotypes in Studies of the Genetics of Schizophrenia. Baltimore, Lippincott Williams & Wilkins (in press)Google Scholar
2. Venables P: Input dysfunction in schizophrenia, in Progress in Experimental Personality Research. Edited by Maher B. New York, Academic Press, 1964, pp 1-41Google Scholar
3. Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL: Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry 2000; 157:1660-1668; correction, 157:1904Link, Google Scholar
4. Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD: Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry 1983; 18:537-551Medline, Google Scholar
5. Cadenhead KS, Light GA, Geyer MA, Braff DL: Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. Am J Psychiatry 2000; 157:55-59Link, Google Scholar
6. Ross RG, Harris JG, Olincy A, Radant A, Adler LE, Freedman R: Familial transmission of two independent saccadic abnormalities in schizophrenia. Schizophr Res 1998; 30:59-70Crossref, Medline, Google Scholar
7. Brenner CA, McDowell JE, Cadenhead KS, Clementz BA: Saccadic inhibition among schizotypal personality disorder and schizophrenia subjects. Psychophysiology 2001; 38:399-403Crossref, Medline, Google Scholar
8. Pierrot-Deseilligny C: Saccade and smooth-pursuit impairment after cerebral hemispheric lesions. Eur Neurol 1994; 34:121-134Crossref, Medline, Google Scholar
9. Swerdlow N, Caine S, Braff D, Geyer M: The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. J Psychopharmacol 1992; 6:176-190Crossref, Medline, Google Scholar
10. Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R: Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 1998; 24:189-202Crossref, Medline, Google Scholar
11. Coon H, Plaetke R, Holik J, Hoff M, Myles-Worsley M, Waldo M, Freedman R, Byerley W: Use of a neurophysiological trait in linkage analysis of schizophrenia. Biol Psychiatry 1993; 34:277-289Crossref, Medline, Google Scholar
12. Oranje B, van Berckel BN, Kemner C, van Ree JM, Kahn RS, Verbaten MN: P50 suppression and prepulse inhibition of the startle reflex in humans: a correlational study. Biol Psychiatry 1999; 45:883-890Crossref, Medline, Google Scholar
13. Schwarzkopf SB, Lamberti JS, Smith DA: Concurrent assessment of acoustic startle and P50 evoked potential measures of sensory inhibition. Biol Psychiatry 1993; 33:815-828Crossref, Medline, Google Scholar
14. Ellenbroek BA, van Luijtelaar G, Frenken M, Cools AR: Sensory gating in rats: lack of correlation between auditory evoked potential gating and prepulse inhibition. Schizophr Bull 1999; 25:777-788Crossref, Medline, Google Scholar
15. Myles-Worsley M, Coon H, McDowell J, Brenner C, Hoff M, Lind B, Bennett P, Freedman R, Clementz B, Byerley W: Linkage of a composite inhibitory phenotype to a chromosome 22q locus in eight Utah families. Am J Med Genet 1999; 88:544-550Crossref, Medline, Google Scholar



