Saccadic Eye Movements in Families Multiply Affected With Schizophrenia: The Maudsley Family Study
Abstract
Objective:Family studies have shown that abnormalities of smooth pursuit eye movement are increased in the adult relatives of schizophrenic probands as well as in the probands themselves. More recently, an inability of schizophrenic subjects to inhibit reflexive saccades reliably has been shown. This study aimed to test the hypothesis that the latter dysfunction is part of the extended schizophrenia phenotype.Method:With the use of infrared oculography, measurements of reflexive saccades and antisaccades were undertaken in 29 probands with schizophrenia, 50 of their nonpsychotic first-degree relatives, and 38 unrelated healthy volunteers. Results:Probands, relatives, and healthy subjects showed no overall differences in the generation of reflexive saccades. However, in the antisaccade task, probands showed more saccadic distractibility when they were required to inhibit reflexive saccades. Analysis of corrective saccades showed that this was not due to failed comprehension or motivation. Relatives of the probands with high saccadic distractibility showed a higher distractibility rate than relatives of the probands with normal distractibility. Across all subjects, females showed a higher rate of distractibility errors than males. Conclusions:The ability to suppress reflexive saccades is an objective neurocognitive measure that is impaired in schizophrenic patients and in a proportion of their biological relatives. This antisaccade abnormality may be a vulnerability marker in a subset of schizophrenic patients and their families. Am J Psychiatry 1998; 155: 1703-1710
The study of eye movements as a possible trait marker for schizophrenia was established in the 1970s. Holzman’s group and other researchers (1–4) reported that abnormal patterns of smooth pursuit eye movement were more common in schizophrenic subjects than in healthy volunteers. Furthermore, these abnormalities were evident in a greater proportion of the first-degree relatives of schizophrenic patients than relatives of healthy control subjects or probands with bipolar disorder. The observation that smooth pursuit abnormalities did not necessarily segregate with schizophrenia in these families led to a proposed model of inheritance in which an autosomal dominant gene of variable expressivity was presumed to predispose to schizophrenia, abnormal pursuit eye movements, or both (5).
More recently, the study of saccadic, as opposed to pursuit, eye movements has attracted attention. The study of saccadic eye movements as a measure of information processing has advantages over more traditional neuropsychological approaches. It offers precise yet unobtrusive measures of performance, a variety of experimental manipulations is possible, and the neural systems subserving saccadic eye movements are well-charted from primate studies (6–10). The saccadic abnormality most frequently reported in schizophrenia emerges during the so-called antisaccade task (11–14). This task demands the ability both to inhibit a reflexive saccadic eye movement in response to the sudden appearance of a visual target and then to generate a voluntary saccadic eye movement in the opposite direction. Thus, subjects are instructed not to look at a target presented peripherally in their visual field but to move their eyes “in the opposite direction to the target.” Failures on this task can be of commission, by failing to inhibit the initial reflexive saccade, or omission, by failing adequately to generate an opposite saccade. Crawford et al. (15) found that schizophrenic patients performed within the normal range in a reflexive saccade task but displayed more errors in the antisaccade task than did healthy control subjects, thus indicating a specific difficulty with the inhibition of reflexive saccades. This error rate was correlated with perseveration scores on the Wisconsin Card Sorting Test and was independent of antipsychotic medication (16; see also 17–20).
Lesions in the dorsolateral prefrontal cortex cause similar deficits (21). The dorsolateral prefrontal cortex contains spatially organized memory fields that are active during retention in working memory (22) and involve the dopamine D1 receptor (23). A positron emission tomography study in healthy volunteers (24) has confirmed the active role of the dorsolateral prefrontal cortex, the basal ganglia, and the cingulate cortex in memory-guided saccades, a task that involves the intentional suppression of reflexive saccades.
In an attempt to characterize the functional abnormality underlying abnormal saccadic distractibility, Crawford et al. (25) compared schizophrenic patients with and without such distractibility who were closely matched on a wide range of demographic and clinical variables. With the use of high-resolution single photon emission tomography measuring regional cerebral blood flow (CBF) with [99mTc]HMPAO, each of the two schizophrenic groups was activated with the antisaccade task. In comparison with the patients who had normal error rates, the patients who had high distractibility rates showed significantly reduced blood flow in the striatum, anterior cingulate, and superior temporal areas. Thus, the subset of schizophrenic patients who had abnormal levels of antisaccadic distractibility was also characterized by reduced regional CBF at discrete neural centers.
There are few published studies of saccadic distractibility in families of schizophrenic probands. Clementz et al. (26) found that antisaccade errors proved to be a more discriminating measure than smooth pursuit abnormalities for distinguishing between schizophrenic patients and control subjects and reported increased antisaccade abnormalities in the patients compared with relatives. They also found a small increase in the relatives in comparison with the control subjects, but the variance was large in relation to the size of the effect, which may have been attributable to the inclusion of some relatives with a diagnosis of schizophrenia. Katsanis et al. (27) demonstrated a significant increase in antisaccade errors of patients with schizophrenia and their biological relatives compared with control subjects. However, the specificity of the eye movement abnormality was not clear, since data from a noninhibitory condition (i.e., a reflexive saccade task) were not reported. In a published abstract, Hellewell and Deakin (28) reported increased saccadic abnormality in relatives of schizophrenic subjects compared with control subjects, together with a bimodal distribution of error rates in their large sample of relatives. These reports require further investigation and replication.
The present study was designed to address the following questions. 1) Is the abnormality of saccadic distractibility detectable in the nonpsychotic first-degree relatives of probands with strongly familial schizophrenia compared with unrelated healthy subjects? 2) If so, is the eye movement abnormality confined to relatives of probands who express high saccadic distractibility rates?
METHOD
Twenty-nine schizophrenic patients (15 male and 14 female; mean age=37.0 years, SD=10.4, range=24–67) and 50 of their nonschizophrenic first-degree biological relatives (19 male and 31 female; mean age=45.6 years, SD=15.1, range=18–68) from 18 families multiply affected with schizophrenia participated in this study. We ascertained families multiply affected with schizophrenia as part of the Maudsley Family Study, described previously (29). Families were classified as multiply affected if two or more members (from among first- and second-degree relatives) suffered from schizophrenia. All schizophrenic probands met the DSM-III-R criteria for schizophrenia. These families were recruited from clinics and voluntary care organizations throughout the United Kingdom. Five of the relatives had axis I recurrent unipolar major depression, and one was bulimic. Three relatives had an axis II diagnosis of schizotypal personality disorder. Thirty-eight unrelated healthy volunteers (19 male and 19 female; mean age=34.9 years, SD=12.1, range=20–77) with no personal or family history of psychotic illness were recruited from the local community through newspaper advertisements. This group of healthy subjects was matched to our group of schizophrenic subjects on age (within 3 years), gender, handedness, and parental social class.
Patients, relatives, and unrelated healthy comparison subjects were assessed on a variety of clinical scales. All subjects were individually assessed by an interviewer who was blind to diagnosis and family status. Research Diagnostic Criteria diagnoses (30) as well as DSM-III-R diagnoses were made by means of a modified version of the Schedule for Affective Disorders and Schizophrenia—Lifetime Version (31). Additional information regarding psychopathology was obtained from family members and hospital records. An extensive family history was obtained by personal interview with the use of the Family History Research Diagnostic Criteria (32). A modified version of the Schedule for Schizotypal Personalities (33) was also administered to assess schizotypal traits. Exclusion criteria for the study were a history of head trauma resulting in loss of consciousness, a disorder affecting the central nervous system, and substance abuse or heavy alcohol abuse in the past 12 months. In addition, potential healthy comparison subjects were excluded if there was a personal or family history of mental illness. After complete description of the study to the subjects, written informed consent was obtained.
Eye Movement Recording and Measurements
Eye movement measurements were conducted in the eye movement laboratory at the Charing Cross and Westminster Medical School (London) by one of us (T.J.C.), who was blind to clinical and neuropsychological data. Full details of the saccade paradigms have been reported in previous publications (15, 16, 25) and are therefore only briefly described here.
The target display consisted of four red light-emitting diode (LED) targets (diameter=0.25°) placed ±7.5° and ±15.0° from the central fixation LED. The experiment was conducted in the dark. Eye movements were recorded with the use of an infrared limbus reflection device (Skalar IRIS). The stimulus display and data sampling were controlled by a PDP 11/73 computer.
The two types of saccade and the details of the paradigms used to elicit them were as follows.
1. Reflexive saccade. The trial began with a red central fixation LED that the subject was asked to fixate. After 800 msec the central LED was switched off, and simultaneously a peripheral target LED was illuminated for 1000 msec and a 200-msec buzzer signal was initiated. The subject was asked to direct his or her gaze quickly and accurately at the newly illuminated target LED and then to return to central fixation, ready for the next trial.
2. Antisaccade. A central LED was illuminated at the start of the trial. After 800 msec the central LED was extinguished, and simultaneously a peripheral LED was illuminated and the buzzer sounded, precisely as in the reflexive saccade trials. However, this time the subject was instructed to direct the gaze toward a position in space equidistant from, but in the opposite direction to, the peripheral stimulus, i.e., the mirror image location. Thus, to be scored as a correct response, a target to the left of center required a saccade to the subject’s right, and vice versa. Each paradigm consisted of 24 trials, and the direction and eccentricity of the targets were varied pseudorandomly to prevent predictive eye movements. The reflexive saccade trials were conducted first to allow all participants to adapt to the requirements of the eye recording device (e.g., head stability) in a nondemanding task.
Eye position was determined by means of a Kaiser window low-pass filter; the signal was then differentiated with the use of a 2-point central difference algorithm to yield eye velocity. Saccade detection was based on a velocity criterion of 30°/sec in addition to an acceleration across three consecutive samples. The latency and spatial accuracy (or gain, i.e., saccade amplitude divided by target amplitude) of the initial saccade on each trial was analyzed. Final eye position was measured by taking the mean fixation location during the maximal period of fixation stability after all secondary and corrective saccades were complete.
In the antisaccade task, the number of trials in which a distractibility error was made was counted for each subject. A saccadic distractibility error was recorded when the initial saccadic movement was made in the direction of the target instead of its mirror image projection in the opposite hemifield. After an antisaccade error was made, subjects frequently and spontaneously followed this with a “corrective” saccade in the appropriate (i.e., antitarget) direction. These saccadic distractibility corrections provided a useful demonstration that subjects had understood the instructions to generate a saccade in the opposite direction to the target and yet were still unable to suppress the initial reflexive saccade.
Statistical Analyses
A proband’s response is likely to be more correlated with that from a member of his or her family (regardless of whether that member is another proband or a relative) than with the response given by an unrelated comparison subject or a member of another proband’s family. Therefore, a statistical model should not only relate a response to the explanatory variables, namely, age, gender, and group (proband, relative, comparison subject), but also take into account this potential “dependence” between members of the same family. One such model is the multilevel model (34), which may be fitted by using the statistical software package Mln (35). In order to locate the source of the eye movement effects, the Wald test (34, 35) was used to conduct pairwise comparisons.
While studies have found that schizophrenic patients as a group show a significant elevation in mean saccadic distractibility errors, there is a large variability in performance(15, 17, 22, 25, 36–38). A previous study (25) used a statistical threshold to discriminate abnormal saccadic distractibility rates in a group of schizophrenic patients. Since this method discriminated between two subgroups of the schizophrenic patients on the basis of both saccadic distractibility error rate and regional cerebral blood flow, we applied the same criteria in this study. The probands were first classified on the basis of their saccadic distractibility error scores. In the antisaccade paradigm, the mean saccadic distractibility error rate from a group of 67 healthy subjects in our laboratory was 21% (SD=15%). This information was used as a guide in setting appropriate criteria for distinguishing quantitatively between probands who were normal and poor performers on this task and for contrasting their corresponding first-degree biological relatives. The threshold for the classification of high saccadic distractibility errors, with the use of mean proband distractibility rate per family, was one standard deviation above the mean score of the group of healthy comparison subjects. The cutoff for the normal error rate classification was one standard deviation below the mean of the group of schizophrenic patients. When applied to this study, these criteria yielded 19 relatives of probands with normal saccadic distractibility (from four families) and 25 relatives of probands with high saccadic distractibility (from 11 families). Six relatives of probands with intermediate scores (from three families) could not be assigned to either group.
RESULTS
Antisaccade Eye Movement Task
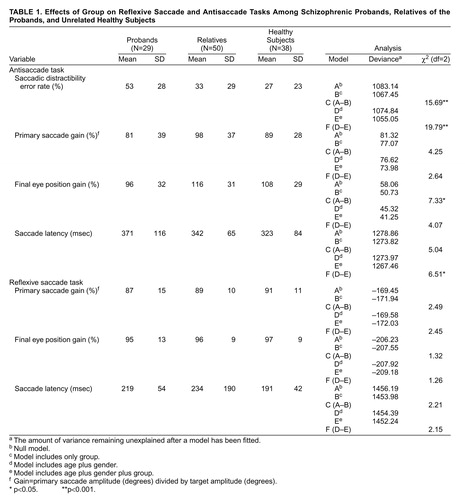
The saccadic distractibility score represents the frequency of the disinhibited reflexive saccades directed toward the target in the antisaccade task. The mean error rates for each of the three groups of subjects are shown in table 1. Table 1 also shows the effect of the explanatory variables—group and a combined variable (age and gender)—on the reflexive saccade and antisaccade tasks by showing the deviance (the amount of variance remaining unexplained after a model has been fitted) of the null model and the deviances when each explanatory variable was included in the model separately. Four models were fitted to each eye movement variable: 1) model A, the null model, which included the intercept/constant term, or “grand mean,” but none of the explanatory variables; 2) model B, which included only the explanatory variable group as a factor at three discrete levels (proband, relative, healthy comparison subject); 3) model D, which included age as a continuous covariate and gender as a factor at two discrete levels; and 4) model E, which included age, gender, and group simultaneously. The deviances for models A, B, D, and E are shown in table 1. To test whether there was a significant group effect irrespective of age and gender, for each saccadic parameter we compared the difference in deviance between models A and B; these differences are also given in table 1. To test for a significant difference between the three groups after adjustment for age and gender, we examined the change in deviance when moving from model D to model E. The level of significance for differences was determined with reference to the chi-square distribution with two degrees of freedom.
Table 1 shows that for saccadic distractibility, the deviance for the model that included only group was 1067.45. When subtracted from the deviance for the null model, this figure produced a significant chi-square value of 15.69. Pairwise comparisons (with the Wald test in Mln) showed that the schizophrenic probands displayed a higher saccadic distractibility error rate on the antisaccade task (mean=53%) than both the healthy comparison subjects (mean=27%, estimate=25.6, standard error=6.396, df=1, p<0.001) and the first-degree relatives (mean=33%, estimate=22.28, standard error=5.553, df=1, p<0.001). (Statistical significance was determined by dividing the parameter estimate by the standard error for the degrees of freedom.) There was no significant difference in saccadic distractibility between the relatives and the healthy comparison subjects. However, all groups showed a high proportion of corrective antisaccades following the errors (probands, 86%; relatives, 91%; healthy subjects, 94%). There was a significant main effect of gender (χ2=5.49, df=1, p<0.05): female subjects had consistently higher saccadic distractibility error rates across all groups (table 2). The effect of age across all groups was not reliable (χ2=3.55, df=1, n.s.). (These data yielded similar results with conventional analysis of variance [ANOVA] and nonparametric analyses.)
The findings in patients and unrelated healthy volunteers resemble those of previous studies in our laboratory (15, 25). Overall, the relatives, when considered as a homogeneous group, did not show a significantly elevated rate of saccadic distractibility errors compared with the healthy subjects. However, further analyses revealed that the relatives of the schizophrenic probands with normal saccadic distractibility error rates (N=19) had a mean saccadic distractibility error rate of 17%, which did not differ significantly from the mean of the healthy subjects (27%), whereas the relatives of the probands with high saccadic distractibility error rates (N=25) had a mean saccadic distractibility error rate of 39%. A one-way between-groups ANOVA to compare these independent groups (the relatively high ratio of families to relatives rendered these data unsuitable for Mln modeling) showed that the error rate of the relatives of the probands with high errors was significantly greater than that of the relatives of the probands with normal errors (F=7.24, df=1, 43, p<0.02) and that of the healthy comparison subjects (F=4.91, df=1, 56, p<0.05). There were no significant differences on any other saccadic parameter between the relatives of the probands with high errors and the relatives of the probands with normal errors.
The demographic profile of the group of relatives differed from that of both the probands and the healthy subjects on mean age and male/female ratio. We therefore conducted further analyses to examine the possible effect of these factors on saccadic distractibility errors. There was a highly significant group effect on antisaccadic distractibility even after we took into account the variance attributable to age and gender (χ2=19.79) (table 1).
A number of considerations undermine the view that a specific age-related parameter could account for these results. 1) Age correlated only poorly with saccadic distractibility errors across all groups, accounting for only 2.9% of the overall variance (r=0.17, N=117, R2=0.03). The correlation was also poor in the group of relatives (r=–0.05, N=50, n.s.) and in the group of healthy subjects (r=0.24, N=38, n.s.), although there was a positive correlation for the probands (r=0.45, N=29, p<0.05). 2) The schizophrenic probands and the healthy subjects, who showed highly significant differences on saccadic distractibility errors, were closely matched on mean age (see above). 3) The relatives of the probands with high saccadic distractibility errors (mean age=47.9 years, SD=15.6) and probands with normal saccadic distractibility errors (mean age=45.5 years, SD=14.2) were also matched on mean age.
Primary saccade gain is defined as primary saccade amplitude divided by target amplitude. There was no main effect of group on primary antisaccade gain (χ2=4.25) (table 1). The mean final eye position of antisaccades was mildly hypermetric for the relatives compared with both the probands, who had mildly hypometric eye positions, and the healthy comparison subjects (table 1). The group factor accounted for a significant proportion of the final eye position variance (χ2=7.33); however, the effect was no longer evident once age and gender were introduced in the model (χ2=4.07). No group effect on antisaccade latency was found (χ2=5.04), although a weak group effect was obtained in the covariance analysis that included age and gender (χ2=6.51) (table 1).
Reflexive Saccade Task
In the reflexive saccade task there were no significant group effects for saccade gain, final eye position, or mean saccade latency (table 1).
DISCUSSION AND CONCLUSIONS
The primary findings of this study can be summarized as follows. 1) The ability to inhibit reflexive saccades was selectively impaired, in comparison with the generation of reflexive saccades, in probands with schizophrenia from families multiply affected with the disorder. 2) Across all groups, female subjects tended to have a higher rate of saccadic distractibility than male subjects. 3) The presence of saccadic distractibility in unaffected first-degree biological relatives was related to its presence in probands (see also reference 37).
These findings replicate the previously reported antisaccade abnormality in schizophrenia—on this occasion, in familial schizophrenia. The results imply that the presence of the antisaccade abnormality in these families is most likely in first-degree biological relatives of probands with familial schizophrenia who also express the antisaccade abnormality. The neuropathophysiological impairment that underlies the high ratio of antisaccade errors in schizophrenic patients may also determine the performance of first-degree relatives of schizophrenic patients with abnormality of antisaccades. Thus, it may constitute a marker for the transmission of vulnerability in a subset of schizophrenic patients and their families. This hypothesis is consistent with two recent studies showing that abnormality of the proband (37) and a family history of schizophrenia (38) are important factors in predicting the antisaccade abnormality in first-degree relatives (these studies were published while this article was in revision).
There is a consensus regarding the abnormalities of oculomotor control in schizophrenia. Despite varying study methods, the abnormality of smooth pursuit eye tracking that was first reported by Diefendorf and Dodge (39) has been widely replicated since the report of Holzman et al. (40), although the role of antipsychotic medication in these findings is still debated (41,42).
In addition to showing low smooth pursuit eye tracking gain, patients with schizophrenia generate more unwarranted saccades during smooth pursuit eye tracking than normal subjects and patients with other psychiatric disorders (43–49). This observation forms the central component of one hypothesis that has been suggested (15, 50, 51), which is that the primary oculomotor deficit in schizophrenia might be one of saccadic distractibility due to impairment of the neural circuitry responsible for saccadic inhibition, given that accurate smooth pursuit eye tracking requires the active inhibition of reflexive saccades. This hypothesis is undermined by the observation that “catch-up” saccades proved to be the variable that best discriminated between the smooth pursuit of schizophrenic subjects and that of subjects with bipolar affective disorder (52), not intrusive saccades as predicted by the saccadic inhibition hypothesis. However, there is evidence that smooth pursuit impairment and saccadic disinhibition may be related: Sereno and Holzman (13) found that impaired pursuit tracking cosegregated with antisaccade abnormality. Express saccades have been used as a measure of saccadic disinhibition (53,54) and have been found to correlate with impairment of smooth pursuit. Moreover, two recent family studies (55, 56) reported that the failure to inhibit “task inappropriate” saccades was the measure that best discriminated the abnormality of smooth pursuit eye tracking in schizophrenic offspring from the normal eye tracking of control subjects. Further clarification must await research comparing the different forms of saccadic intrusions during pursuit with the inhibition of “inappropriate” saccades in both schizophrenic probands and their biological relatives. The robust gender effects on saccadic distractibility in the current study have methodological implications for future studies; clearly, studies should be careful to match subject groups by gender. In contrast, age appears to have little impact on this saccadic distractibility.
Smooth pursuit and saccadic eye movements appear to be under some degree of genetic control (57). Smooth pursuit deficits have now been highly replicated in the nonpsychotic relatives of schizophrenic probands. Recently, this finding was confirmed by Keefe et al. (4), who showed that qualitatively rated smooth pursuit deficits best discriminated relatives of schizophrenic subjects from unrelated control subjects. In that study there was no segregation of smooth pursuit deficits with other purported trait markers of attentional deficits or schizotypal symptoms. The finding that qualitatively rated smooth pursuit deficits, and not quantitatively rated measures, were discriminatory raises a problem of replicability in this area. Studying deficits in saccadic inhibition has the advantage of examining a manipulable paradigm with objective measures; in addition, the antisaccade task is more closely related to the well-established executive and attentional deficits in schizophrenia (15,17).
This study and other recent studies suggest that saccadic distractibility should also be further explored as a useful biological trait marker for schizophrenia. Abnormal saccadic distractibility in schizophrenia is reported across diverse cultural groups (58) and has not been widely reported in bipolar affective disorder or neurotic disorder (15, 16). However, Katsanis et al. (27) recently reported an increased saccadic distractibility impairment in schizophrenic subjects and first-degree biological relatives compared with healthy control subjects but also a significant level of abnormality in their small sample of bipolar patients. It is important to note that the insensitivity of antisaccades to neuroleptic medication contrasts markedly with the evidence from studies of Parkinson’s disease (16, 59, 60) and neuropharmacology studies (23) of dopamine sensitivity for memory-guided and predictive saccades.
Given the recent findings showing the sensitivity of smooth pursuit eye tracking in detecting subtle deficits in children at high risk (55) and in childhood-onset schizophrenia (56,61), further research should 1) determine whether this is also true of saccadic distractibility errors and 2) ascertain the degree of convergence in the pathophysiology of these two oculomotor subsystems. The use of saccadic distractibility in latent trait designs may be useful in the search for genetic susceptibility loci.
Received June 23, 1997; revision received June 1, 1998; accepted June 10, 1998. From the Mental Health and Neural Systems Research Unit, Department of Psychology, Lancaster University; the Department of Psychological Medicine, Institute of Psychiatry, London; the Robert Steiner MRI Unit, Royal Postgraduate Medical School, Hammersmith Hospital, London; and the School of Psychiatry and Behavioural Science, University of Manchester, Manchester. Address reprint requests to Dr. Crawford, Mental Health and Neural Systems Research Unit, Department of Psychology, Lancaster University, Lancaster LA1 4YF, United Kingdom; [email protected] (e-mail). Supported in part by a grant from the Wellcome Trust to Dr. Crawford.The authors thank Drs. S. Lech and S. Sigmundsson for assistance with clinical assessments and Prof. C. Kennard for laboratory facilities.
 |
 |
1. Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW: Eye-tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry 1974; 31:143–151Crossref, Medline, Google Scholar
2. Blackwood DHR, St Clair DM, Juir WJ, Duffy JC: Auditory P300 and eye tracking dysfunction in schizophrenia pedigrees. Arch Gen Psychiatry 1991; 48:899–909Crossref, Medline, Google Scholar
3. Levy DL, Holzman PS, Matthysse S, Mendell NR: Eye tracking and schizophrenia: a selective review. Schizophr Bull 1994; 20:47–62Crossref, Medline, Google Scholar
4. Keefe SE, Silverman JM, Mohs RC, Siever LJ, Harvey PD, Friedman L, Roitman SEL, DuPre RL, Smith CJ, Schmeidler J, Davis KL: Eye tracking attention and schizotypal symptoms in nonpsychotic relatives of patients with schizophrenia. Arch Gen Psychiatry 1997; 54:169–176Crossref, Medline, Google Scholar
5. Holzman PS, Kringlen E, Matthysse S, Flanagan S, Lipton RB, Cramer G, Levin S, Lange K, Levy DL: A single dominant gene can account for eye tracking dysfunctions and schizophrenia in offspring of discordant twins. Arch Gen Psychiatry 1988; 45:641–647Crossref, Medline, Google Scholar
6. Bruce CJ, Goldberg ME: Primate frontal eye fields, I: single neurones discharging before saccades. J Neurophysiol 1985; 53:603–635Crossref, Medline, Google Scholar
7. Hikosaka O, Wurtz RH: The basal ganglia, in The Neurobiology of Saccadic Eye Movements. Edited by Wurtz RH, Goldberg ME. Amsterdam, Elsevier, 1991, pp 257–282Google Scholar
8. Schiller PH, True SD, Conway JL: Deficits in eye movements following frontal eye field and superior colliculus ablations. J Neurophysiol 1980; 44:1175–1189Crossref, Medline, Google Scholar
9. Schlag J, Schlag-Rey M: Evidence for a supplementary eye field. J Neurophysiol 1987; 57:179–200Crossref, Medline, Google Scholar
10. Segraves MA, Goldberg ME: Functional properties of corticotectal neurons in the monkey’s frontal eye field. J Neurophysiol 1987; 58:1387–1419Crossref, Medline, Google Scholar
11. Radant AD, Claypoole K, Wingerson DK, Cowley DS, Roy-Byrne PP: Relationships between neuropsychological and oculomotor measures in schizophrenia patients and normal controls. Biol Psychiatry 1997; 42:797–805Crossref, Medline, Google Scholar
12. Fukushima J, Fukishima K, Miyasaka K, Yamashita I: Further analysis of the control of voluntary saccadic eye movements in schizophrenic patients. Biol Psychiatry 1990; 28:943–958Crossref, Medline, Google Scholar
13. Sereno AB, Holzman PS: Antisaccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry 1995; 37:394–401Crossref, Medline, Google Scholar
14. Matsue Y, Saito H, Osakabe K, Awata S, Ueno T, Matsuoka H, Chiba H, Fus Y, Sato M: Smooth pursuit eye movements and voluntary control of saccades in the antisaccade task in schizophrenic patients. Jpn J Psychiatry Neurol 1994; 48:13–22Medline, Google Scholar
15. Crawford TJ, Haegar B, Kennard C, Reveley MA, Henderson L: Saccadic abnormalities in psychotic patients, I: neuroleptic-free psychotic patients. Psychol Med 1995; 25:461–471Crossref, Medline, Google Scholar
16. Crawford TJ, Haegar B, Kennard C, Reveley MA, Henderson L: Saccadic abnormalities in psychotic patients, II: the role of neuroleptic treatment. Psychol Med 1995; 25:473–483Crossref, Medline, Google Scholar
17. Rosse RB, Schwartz BL, Kim SY, Deutsch SI: Correlation between antisaccade and Wisconsin Card Sorting Test performance in schizophrenia. Am J Psychiatry 1993; 150:333–335Link, Google Scholar
18. Tien AY, Ross DE, Pearlson G, Strauss ME: Eye-movements and psychopathology in schizophrenia and bipolar disorder. J Nerv Ment Dis 1996; 184:331–338Crossref, Medline, Google Scholar
19. Radant AD, Claypoole K, Wingerson DK, Cowley DS, Roy-Byrne PP: Relationships between neuropsychological and oculomotor measures in schizophrenia patients and normal controls. Biol Psychiatry 1997; 42:797–805Crossref, Medline, Google Scholar
20. Karoumi B, Ventre-Dominey J, Vighetto A, Dalery J, D’Amato T: Saccadic eye movements in schizophrenic patients. Psychiatry Res 1998; 77:9–19Crossref, Medline, Google Scholar
21. Guitton D, Buchtal HA, Douglas RM: Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal directed saccades. Exp Brain Res 1985; 58:455–474Crossref, Medline, Google Scholar
22. Park S, Holzman PS: Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 1992; 49:975–982Crossref, Medline, Google Scholar
23. Williams GV, Goldman-Rakic PS: Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 1995; 376:572–575Crossref, Medline, Google Scholar
24. O’Sullivan EP, Jenkins IH, Henderson L, Kennard C, Brooks DJ: The functional anatomy of remembered saccades: a PET study. Neuroreport 1995; 6:2141–2144Crossref, Medline, Google Scholar
25. Crawford TJ, Puri BK, Nijran KS, Jones B, Kennard C, Lewis SW: Abnormal saccadic distractibility in patients with schizophrenia: a 99mTc-HMPAO SPET study. Psychol Med 1996; 26:265–277Crossref, Medline, Google Scholar
26. Clementz BA, McDowell JE, Zisook S: Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol 1994; 103:277–287Crossref, Medline, Google Scholar
27. Katsanis S, Kortenkamp S, Iacono WG, Grove WM: Antisaccade performance in patients with schizophrenia and affective disorder. J Abnorm Psychol 1997; 106:468–472Crossref, Medline, Google Scholar
28. Hellewell JSE, Deakin JFW: Impaired frontal lobe function in schizophrenia is familial. Schizophr Res 1994; 11:149–150Crossref, Google Scholar
29. Frangou S, Sharma T, Alarcon G, Sigmudsson T, Takei N, Binnie C, Murray RM: The Maudsley Family Study, 2: endogenous event-related potentials in familial schizophrenia. Schizophr Res 1997; 23:45–53Crossref, Medline, Google Scholar
30. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
31. Endicott J, Spitzer RL: A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 1978; 35:837–844Crossref, Medline, Google Scholar
32. Endicott J, Andreasen NC, Spitzer RL: Family History Research Diagnostic Criteria. New York, New York State Psychiatric Institute, Biometrics Research, 1975Google Scholar
33. Baron M, Asnis L, Gruen R: The Schedule for Schizotypal Personalities (SSP): a diagnostic interview for schizotypal features. Psychiatry Res 1981; 4:213–228Crossref, Medline, Google Scholar
34. Goldstein H: Multilevel Statistical Models. New York, Halsted Press, 1995Google Scholar
35. Woodhouse G: A Guide to Mln for New Users. London, Institute of Education, 1995Google Scholar
36. Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, Kato M: Voluntary control of saccadic eye movements in patients with schizophrenia and affective disorders. J Psychiatr Res 1990; 24:29–24Crossref, Google Scholar
37. McDowell JE, Clementz BA: The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Exp Brain Res 1997; 115:333–344Crossref, Medline, Google Scholar
38. Ross RG, Harris JG, Olincy A, Radant A, Adler LE, Freedman R: Familial transmission of two independent saccadic abnormalities in schizophrenia. Schizophr Res 1998; 30:59–70Crossref, Medline, Google Scholar
39. Diefendorf AR, Dodge R: An experimental study of the ocular reactions of the insane from photographic records. Brain 1908; 31:451–489Crossref, Google Scholar
40. Holzman PS, Proctor LR, Hughes DW: Eye tracking patterns in schizophrenia. Science 1973; 181:179–180Crossref, Medline, Google Scholar
41. Sweeney JA, Haas GL, Li S, Wieden PJ: Selective effects of antipsychotic medications on eye-tracking performance in schizophrenia. Psychiatry Res 1994; 54:185–198Crossref, Medline, Google Scholar
42. Crawford TJ, Lawden MC, Haegar B, Henderson L, Kennard C: Smooth pursuit eye movement abnormalities in patients with schizophrenia and focal cortical lesions, in Eye Movement Research: Mechanisms, Processes and Applications. Edited by Findlay JM, Walker R, Kentridge RW. New York, Elsevier, 1995, pp 281–289Google Scholar
43. Friedman L, Abel LA, Jesberger JA, Malki A, Meltzer HY: Saccadic intrusions into smooth pursuit in patients with schizophrenia or affective disorder and normal controls. Biol Psychiatry 1992; 31:1110–1118Crossref, Medline, Google Scholar
44. Friedman L, Jesberger JA, Meltzer HY: A model of smooth pursuit performance illustrates the relationship between gain, catch-up saccade rate, and catch-up saccade amplitude in normal controls and patients with schizophrenia. Biol Psychiatry 1991; 30:537–556Crossref, Medline, Google Scholar
45. Abel LA, Friedman L, Jesberger J, Malki A, Meltzer HY: Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenia and affective disorders. Biol Psychiatry 1991; 29:1063–1072Crossref, Medline, Google Scholar
46. Clementz BA, Sweeney JA, Hirt M, Haas G: Pursuit gain and saccadic intrusions in first-degree relatives of probands with schizophrenia. J Abnorm Psychol 1990; 99:327–335Crossref, Medline, Google Scholar
47. Holzman PS: Smooth pursuit eye movements in psychopathology. Schizophr Bull 1983; 9:33–36Crossref, Medline, Google Scholar
48. Mialet JP, Pichot P: Eye-tracking patterns in schizophrenia: an analysis based on the incidence of saccades. Arch Gen Psychiatry 1981; 38:183–186Crossref, Medline, Google Scholar
49. Cegalis JA, Sweeney JA: Eye movements in schizophrenia: a quantitative analysis. Biol Psychiatry 1979; 14:13–26Medline, Google Scholar
50. Henderson L, Crawford TJ, Kennard C: Neuropsychology of eye movement abnormalities in schizophrenia, in Schizophrenia: A Neuropsychological Perspective. Edited by Pantelis C, Nelson HE, Barnes TRE. New York, John Wiley & Sons, 1996, pp 259–277Google Scholar
51. Levin S: Frontal lobe dysfunctions in schizophrenia, I: eye movement impairments. J Psychiatr Res 1984; 18:27–55Crossref, Medline, Google Scholar
52. Sweeney JA, Clementz BA, Haas GL, Escobar MD, Drake K, Frances AJ: Eye tracking dysfunction in schizophrenia: characterization of component eye movement abnormalities, diagnostic specificity and the role of attention. J Abnorm Psychol 1994; 103:222–230Crossref, Medline, Google Scholar
53. Matsue Y, Osakabe K, Saito H, Goto Y, Ueno T, Matsuoka H, Chiba H, Fuse Y, Sato M: Smooth pursuit eye movements and express saccades in schizophrenic patients. Schizophr Res 1994; 12:121–130Crossref, Medline, Google Scholar
54. Sereno AB, Holzman PS: Express saccades and smooth-pursuit eye movement function in schizophrenic, affective-disorder, and normal subjects. J Cognitive Neurosci 1993; 5:303–316Crossref, Medline, Google Scholar
55. Rosenberg DR, Sweeney JA, Squires-Wheeler E, Keshavan MS, Cornblatt BA, Erlenmeyer-Kimling L: Eye-tracking dysfunction in offspring from the New York High-Risk Project: diagnostic specificity and the role of attention. Psychiatry Res 1997; 66:121–130Crossref, Medline, Google Scholar
56. Ross RG, Hommer D, Radant A, Roath M, Freedman R: Early expression of smooth pursuit eye movement abnormalities in children of schizophrenic parents. J Am Acad Child Adolesc Psychiatry 1996; 35:941–949Crossref, Medline, Google Scholar
57. Iocona WG, Lykken DT: Electro-oculographic recording and scoring of smooth-pursuit and saccadic eye tracking: a parametric study using monozygotic twins. Psychophysiology 1979; 16:94–107Crossref, Medline, Google Scholar
58. Allen JS, Lambert AJ, Johnson FYA, Schmidt K, Nero KL: Antisaccadic eye movements and attentional asymmetry in schizophrenia in three Pacific populations. Acta Psychiatr Scand 1996; 94:258–265Crossref, Medline, Google Scholar
59. Crawford TJ, Henderson L, Kennard C: Abnormalities in non-visually guided eye movements in Parkinson’s disease. Brain 1989; 112:1573–1586Crossref, Medline, Google Scholar
60. Crawford TJ, Goodrich S, Henderson L, Kennard C: Anticipatory responses to predictable visual signals in parkinson’s disease: manual keypresses and saccadic eye-movement. J Neurol Neurosurg Psychiatry 1989; 52:1033–1042Crossref, Medline, Google Scholar
61. Jacobsen LK, Hong WL, Hommer DW, Hamburger SD, Castellanos FX, Frazier JA, Giedd JN, Gordon CT, Karp BI, Mckenna K, Rapoport JL: Smooth-pursuit eye-movements in childhood-onset schizophrenia—comparison with attention-deficit hyperactivity disorder and normal controls. Biol Psychiatry 1996; 40:1144–1154Crossref, Medline, Google Scholar



