Increased Medial Orbitofrontal and Amygdala Activation: Evidence for a Systems-Level Endophenotype of Bipolar I Disorder
Abstract
Objective:
Bipolar I disorder is highly heritable, but endophenotypes of the disorder mediating genetic risk are only beginning to be defined. The authors investigate state- and trait-related neural mechanisms related to motivation in euthymic bipolar patients and unaffected first-degree relatives of bipolar patients to define the status of motivational processing as a neural systems-level endophenotype.
Method:
Our study comprised two samples; the first consisted of 19 euthymic bipolar patients and 19 matched comparison subjects, and the second included 22 relatives and 22 matched comparison subjects. Motivational processing was assessed with a probabilistic reversal learning task during event-related functional MRI. Data were analyzed using a region-of-interest approach restricting analysis to the medial and lateral orbitofrontal cortex, the amygdala, the anterior cingulate cortex, and the striatum.
Results:
The authors observed increased activation in response to reward and reward reversal contingencies in the left medial orbitofrontal cortex in patients with bipolar disorder and in the right medial orbitofrontal cortex in their relatives. Activation of the amygdala in response to reward reversal was increased in patients and relatives. In response to reward, activation of the amygdala was greater only in relatives, but there was a significant negative correlation between medication and amygdala activation in patients.
Conclusions:
These results identify increased activity of the orbitofrontal cortex and the amygdala, related to heightened sensitivity to reward and deficient prediction error signal, as a candidate endophenotype of bipolar disorder. The results support a role of motivational processing in the risk architecture of bipolar disorder and identify a new systems-level therapeutic target for the illness.
Bipolar disorder is a highly heritable disorder that is characterized by recurrent episodes of mania and depression. The core features of mania are heightened incentive motivation and compulsiveness toward positive cues despite negative consequences, whereas lack of interest in positive events is characteristic for depression. These symptoms can be seen as an expression of motivational dysregulation underlying bipolar disorder.
Motivation is defined as the process of initiating, controlling, and maintaining behavior with the goal of maximizing pleasant outcomes (1). Importantly, motivational regulation depends on intact reversal learning consisting of two processes: learning the stimulus-reinforcement contingencies and adapting behavior according to the changing stimulus-reinforcement contingencies. On a neural level, reversal learning is mediated by the medial orbitofrontal cortex associated with the representation of reward and the lateral orbitofrontal cortex known to suppress previously rewarded responses (2) and to code the representation of punishment (3). Furthermore, brain structures associated with the processing of positive and negative feedback, such as the amygdala, the anterior cingulate cortex, and the ventral striatum, are crucially involved in reversal learning (4).
Increased activation of the orbitofrontal cortex has been repeatedly observed in manic, depressed, and euthymic patients with bipolar disorder during tasks examining both emotional and cognitive processing (5, 6). Reversal learning studies have shown that manic, depressed, and euthymic patients with bipolar disorder commit more errors when reward contingencies change (7–11). Thus, increased orbitofrontal cortex activation and impaired reversal learning occur in bipolar disorder independent of the current mood state.
As intact reversal learning was shown to depend on the orbitofrontal cortex in healthy persons (2), it is plausible that altered orbitofrontal activation represents the neural correlate of impaired reversal learning in bipolar disorder, but findings from neuroimaging studies in bipolar patients to date provide no clear evidence for this assumption. In euthymic bipolar patients, the number of errors during a behavioral reversal learning task was negatively correlated with activity in the orbitofrontal cortex and the striatum that was recorded in a separate word production task (12). In contrast, no differences in neural activation were observed in a small sample of depressed patients with bipolar disorder when a reversal learning task was conducted during functional MRI (fMRI) (13). Furthermore, impaired behavioral adaptation in response to changing reward contingencies as well as increased activation in the parietal and frontal but not the orbitofrontal brain regions during reversal learning has been observed in pediatric bipolar patients (14).
In addition to being plausibly linked to the clinical symptoms of bipolar disorder, motivational dysregulation has been proposed as a potential endophenotype of bipolar disorder (15). Endophenotypes were initially conceptualized to facilitate genetic analyses through the identification of simpler phenotypes that show higher penetrance (16), but one of their most attractive aspects is that they nominate biological systems that contribute to the genetic risk architecture of mental illnesses, advancing pathophysiological understanding and potentially offering new therapeutic targets (17). However, not only must endophenotypes be associated with a disease, be heritable, be cosegregated with the disease within families, and be state independent, but they should also occur in unaffected relatives at a higher rate than in the general population (16).
In this study, we investigated whether the neural correlates of motivational dysregulation, assessed with a probabilistic reversal learning task (18) during event-related fMRI in euthymic patients with bipolar I disorder and their unaffected first-degree relatives, could be an endophenotype of bipolar I disorder. We hypothesized that both bipolar patients and their relatives would show increased activation in the orbitofrontal cortex, especially during reversal of reward contingencies, and show altered activation in brain regions associated with feedback processing (the anterior cingulate cortex, the amygdala, and the striatum).
Method
Participants
We invited patients with bipolar I disorder who had already participated in epidemiological studies at the Central Institute of Mental Health in Manheim, Germany, or who had frequented local support groups and unaffected first-degree relatives of patients with bipolar I disorder to participate in the study. To recruit healthy comparison subjects' matching patients and relatives, we drew a large random sample from the registry office of the city of Mannheim and contacted these persons by mail. All persons interested in participating were screened by telephone. Exclusion criteria for all participants were age under 18, lifetime alcohol or drug abuse or dependence, history of a neurological disorder or head trauma with unconsciousness, common MRI exclusion criteria, and lack of fluency in German. Relatives and comparison subjects were also excluded if they fulfilled the criteria for any lifetime or current DSM-IV axis I mental disorder (19) or took any psychotropic medication. Additional exclusion criteria for bipolar patients were having a diagnosis of rapid cycling or schizoaffective disorder and taking an antipsychotic medication known to influence reward processing (20). After the screening, 22 patients with bipolar I disorder, 22 relatives, and 43 healthy comparison subjects without a family history of psychotic or affective disorders were eligible for the study (see Figure 1 in the data supplement that accompanies the online edition of this article).
Sample 1.
From the original sample, three bipolar patients and two healthy comparison subjects had to be excluded from data analysis because they exceeded the minimum movement thresholds during their fMRI scans (2 mm or 2°) or showed abnormal brain morphology. In the final sample of 19 bipolar patients and 19 comparison subjects, the two groups did not significantly differ in gender, age, years of education, marital status, current employment, intelligence, Hamilton Depression Rating Scale (HAM-D) score (21), or consumption of caffeine, nicotine, and alcohol. Although the patients were euthymic, they scored significantly higher on subclinical scales of the Young Mania Rating Scale (22) and the Beck Depression Inventory (BDI; 23). Two patients were diagnosed with lifetime but currently remitted panic disorder. Demographic and clinical characteristics are summarized in Table 1.
| Bipolar Patients (N=19) | Healthy Subjects Matched to Relatives (N=19) | Relatives of Bipolar Patients (N=22) | Healthy Subjects Matched to Patients (N=22) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Median | Range | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 45 | 10 | 45 | 10 | 28 | 11 | 28 | 10 | ||
| Education (years) | 15 | 2 | 15 | 2 | 15 | 3 | 15 | 2 | ||
| Estimated IQ | 115 | 12 | 119 | 11 | 120 | 11 | 124 | 9 | ||
| Current symptoms | ||||||||||
| YMRS scorea | 0.9 | 1.1 | 0.3 | 0.6 | 0.3 | 0.5 | 0.1 | 0.4 | ||
| HAM-D scoreb | 1.0 | 1.5 | 0.4 | 0.7 | 0.5 | 0.9 | 0.4 | 0.7 | ||
| BDI scorec | 2.9 | 2.9 | 1.0 | 1.5 | 1.1 | 1.4 | 0.7 | 1.4 | ||
| History of illness | ||||||||||
| Age at illness onset | 29.6 | 9.7 | 27.0 | 16–48 | ||||||
| Age at first hospitalization | 31.8 | 10.5 | 33.0 | 17–48 | ||||||
| Previous hospitalizations | 3.0 | 2.6 | 2.0 | 1–10 | ||||||
| Past depressive episodes | 3.4 | 1.8 | 3.0 | 1–7 | ||||||
| Past manic episodes | 2.7 | 2.2 | 2.0 | 1–10 | ||||||
| Time in remission (months) | 53.0 | 77.8 | 20.0 | 3–300 | ||||||
| Ratings of medication loadd | 1.7 | 1.6 | 2.0 | 0–5 | ||||||
| N | % | N | % | N | % | N | % | |||
| Gender | ||||||||||
| Female | 11 | 11 | 11 | 11 | ||||||
| Male | 8 | 8 | 11 | 11 | ||||||
| Social status | ||||||||||
| Married (lifetime) | 13 | 68 | 13 | 68 | 12 | 55 | 9 | 41 | ||
| Currently employed | 13 | 68 | 17 | 89 | 16 | 73 | 17 | 77 | ||
| Regular substance consumption | ||||||||||
| Caffeine | 15 | 79 | 17 | 89 | 17 | 77 | 16 | 73 | ||
| Nicotine | 7 | 37 | 6 | 32 | 3 | 14 | 5 | 23 | ||
| Alcohol | 16 | 84 | 17 | 89 | 8 | 36 | 7 | 32 | ||
| Medication | ||||||||||
| None | 7 | 37 | ||||||||
| Lithium | 4 | 21 | ||||||||
| Valproic acid | 2 | 11 | ||||||||
| Clomipramine | 1 | 5 | ||||||||
| Duloxetine | 2 | 11 | ||||||||
| Sertraline and lithium | 1 | 5 | ||||||||
| Venlafaxine and pregabalin | 1 | 5 | ||||||||
| Opipramol and lithium | 1 | 5 | ||||||||
TABLE 1. Demographic and Clinical Characteristics of Participants in a Study of Medial Orbitofrontal and Amygdala Activation in Bipolar Disorder
Sample 2.
The relatives (N=22) did not differ significantly from comparison subjects (N=22) in gender, age, years of education, marital status, current employment, intelligence, or consumption of caffeine, nicotine, and alcohol. Current symptoms of depression and mania were comparable between groups (Table 1). Nine relatives were siblings, and 13 were children of patients with bipolar I disorder. Eleven relatives were from simplex families (one case in the family), and 11 were from multiplex families (two or more cases in the family).
Procedure
The Structured Clinical Interview for DSM-IV Axis I Disorders (25) was administered to all study participants and index cases of relatives to verify main diagnosis (for patients) and exclusion criteria (for relatives and comparison subjects). Residual mood symptoms were assessed with the Young Mania Rating Scale, the HAM-D, and the BDI. The cutoffs for euthymia were scores ≤4 on the Young Mania Rating Scale, ≤5 on the HAM-D, and ≤7 on the BDI. We ensured that patients had been euthymic for at least 2 months before testing.
We constructed a life chart with all of the patients to assess number of past depressive and manic episodes, age at illness onset, number of hospitalizations, age at first hospitalization, and time in remission. We also verified that the medication status of all patients had been stable during the past 6 months. We coded the dosage of each antidepressant and mood stabilizer (25) and calculated the composite measure of total psychotropic medication load reflecting both dosage and variety of different medications taken. Additionally, we collected data on caffeine, nicotine, and alcohol consumption and estimated the IQ of all participants using the Culture Fair Intelligence Test (26).
The ethics committee at the University of Heidelberg in Germany approved the study, and all participants gave written informed consent.
Reliability
For a subset of the sample (N=21), two clinicians (J.L., A.V.K.) evaluated participants with respect to current symptoms and diagnoses of mental disorders to determine interrater reliability. We had an interrater agreement of 100% for the main diagnosis of bipolar I disorder. For current symptoms, interrater reliability assessed by intraclass correlation was 0.87 for the HAM-D and 0.91 for the Young Mania Rating Scale.
Reversal Learning Task
The probabilistic reversal learning task has been described in detail elsewhere (4, 18). Participants had to learn which of two playing cards was the “better” one based on positive and negative feedback in terms of winning or losing a small amount of money (€0.10–€1.00). Choice of the better card was followed by either positive or negative probabilistic feedback with a ratio of 8:2, whereas the choice of the other card always led to negative feedback. After five to eight consecutive choices of the better card, the other card became the better one (rule reversal) and participants had to adapt their choice of card in order to maximize their gain. For fMRI analyses, we focused on three events: 1) positive feedback (“win”), 2) negative feedback not leading to a change in the choice of card (“lose/no shift”), and 3) negative feedback leading to a change in the choice of card (“lose/shift”). In contrast to other studies (5), we did not differentiate whether lose/shift and lose/no shift events occurred after a real rule reversal or after a probabilistic negative feedback following the correct choice of the better card, that is, because we believe that the same psychological process—adaptation of behavior in accordance with subjectively apparent changes in the environment—underlies both types of events as participants were blind to the experimental schedule.
In addition to these experimental trials, baseline trials were randomly interspersed. During baseline events, participants knew in advance which card to choose and received only a neutral feedback (“choice made”). The task was conducted in three separate functional runs with an average duration of 12 minutes. Details on timing parameters of the experiment, the types of trials, and statistical contrast are depicted in Figure 1.
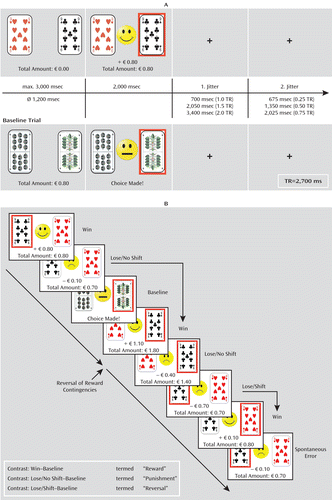
FIGURE 1. The Reward Reversal Learning Taska
a Panel A shows the timing parameters of experimental and baseline trials. Two stimuli are presented in each trial. During baseline trials, the participants know in advance that they have to choose the card with the green leaves, whereas during the experimental trials, the participants learn which card is correct with immediate positive and negative feedback. Panel B displays an example of all events of interest. Consecutive trials run from top left to bottom right. Changes in the choice of cards occurring after win events (termed “spontaneous errors”) were not analyzed because they might represent anticipation of rule reversal or simply a mistake, and they cannot be distinguished. Additionally, the figure contains a summary of the contrasts that were analyzed.
Functional Image Acquisition
All MRI sequences were performed on a 3-T whole body scanner (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany). We conducted one high-resolution T1-weighted three-dimensional MRI sequence (slice thickness=1.1 mm, field of view=256×240×176 mm3, matrix=256×240×160). For fMRI, we acquired 40 gradient-echo T2*-weighted slices (slice thickness=2.3 mm) per volume using a generalized autocalibrating partially parallel acquisition technique (acceleration factor 2) and the following parameters: TR=2,700 msec, flip angle=90°, TE=27 msec, field of view=220 mm2, matrix=96×96, slice gap=0.7 mm.
Data Analysis
Demographic characteristics, clinical data, and behavioral data were analyzed using Predictive Analytics SoftWare, version 18.0.0 (PASW Statistics 18) (27). For the reversal learning task, the following behavioral outcome variables were calculated: average number and reaction times of the different events, number of scans, and average amount of money won. We used Student's t test (between patients and comparison subjects and between relatives and comparison subjects) and analysis of covariance (ANCOVA) with age as covariate (patients compared with relatives) to determine group differences.
Imaging data were preprocessed and analyzed according to standard procedures using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Preprocessing involved realignment to the first scan of each run, slice-timing, coregistration, and normalization into Talairach and Tournoux stereotaxic space using Montreal Neurological Institute templates (28). During normalization, the images were resampled every 3 mm using sinc interpolation and smoothed with a 9×9×9 mm Gaussian kernel to decrease spatial noise. Statistical analyses were carried out in the context of the general linear model. Blood-oxygen-level-dependent (BOLD) signal changes were modeled to the onset of the feedback presentation, and movement parameters calculated during realignment were included as parameters of no interest.
We computed the following contrasts: wins minus baseline to assess the main effect of reward, “lose/no shift” minus baseline to assess the main effect of punishment, and “lose/shift” minus baseline to assess rule reversal. The contrast of wins, lose/no shift events, and lose/shift events against the baseline was important, as patients and relatives might differ from comparison subjects in processing positive and negative feedback (win and lose/no shift events) and reversal learning (lose/shift events) (2). Thus, contrasting against baseline was the only way to adequately assess the main effects of reward, punishment, and rule reversal.
For each contrast, the individual statistical parametric maps were entered in a second-level random-effects analysis to test for activation differences between bipolar patients and comparison subjects as well as between relatives and comparison subjects. We used an ANCOVA with money won, mania, and depression scores as nuisance variables to account for group differences between bipolar patients and comparison subjects in these variables. For comparison between relatives and comparison subjects, we used an ANCOVA with money won as the nuisance variable. We also compared bipolar patients and relatives using an ANCOVA with age as the covariate.
We used a region-of-interest approach (2, 4, 18) to test our hypotheses, restricting analyses to five bilateral regions of interest: the medial and lateral orbitofrontal cortex, the amygdala, the anterior cingulate cortex, and the striatum (including the nucleus caudate, the nucleus accumbens, and the putamen), derived from the Wake Forest University PickAtlas v2.0 (29). Hypotheses of altered activation in the five regions of interest were tested with a threshold of p<0.05, accounting for multiple comparisons by controlling for family-wise error rates. Additionally, we applied the Holm-Bonferroni method, a sequentially rejective version of the simple Bonferroni correction, which adjusts the p values that were already corrected for family-wise error rates within each region of interest according to the total number of regions of interest used in the analyses. This method resulted in increasing thresholds of p<0.010, p<0.013, p<0.017, p<0.025, and p<0.050 for the first to the fifth regions of interest in order to avoid false positive findings. For interpreting group differences, we extracted percent signal change for each event from a 3-mm3 sphere centered on the peak voxel of the cluster showing significant group differences with MarsBaR (30). To examine potential confounding effects of medication load, the mean contrast values were imported in PASW Statistics 18 and correlated with total medication load. We report only correlations that passed the threshold of p<0.006 (corresponding to p=0.05 after a Bonferroni correction for eight comparisons corresponding to the number of regions of interest, where group differences were observed in either sample).
Results
Behavioral Data
We did not observe significant differences in reaction times and frequency of any type of event during the reversal learning task between bipolar patients and comparison subjects, between relatives and comparison subjects, or between bipolar patients and relatives. However, relatives won significantly smaller amounts of money than comparison subjects, and bipolar patients tended to win less money than comparison subjects (Table 2).
| Sample 1 | Sample 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bipolar Patients (N=19) | Healthy Comparison Subjects (N=19) | Relatives (N=22) | Healthy Comparison Subjects (N=22) | Bipolar Patients vs. Comparison Subjects | Relatives vs. Comparison Subjects | Bipolar Patients vs. Relatives | ||||||||
| Characteristic | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ta | pb | tc | pb | Fd | pb |
| Money (€) | 12.51 | 7.13 | 17.15 | 7.22 | 15.15 | 9.11 | 19.70 | 5.29 | 1.99 | 0.054 | 2.03 | 0.049 | 0.12 | |
| Number of acquired scans | 764 | 78 | 740 | 64 | 736 | 81 | 711 | 58 | –1.06 | –1.19 | 0.00 | |||
| Event frequencies | ||||||||||||||
| Baseline events | 36 | 7 | 39 | 4 | 36 | 7 | 37 | 4 | 1.26 | 0.89 | 0.45 | |||
| Win events | 145 | 5 | 147 | 7 | 146 | 8 | 148 | 5 | 0.63 | 0.98 | 0.01 | |||
| Lose/shift events | 57 | 10 | 51 | 12 | 52 | 14 | 53 | 16 | –1.53 | 0.16 | 2.09 | |||
| Lose/no shift events | 18 | 9 | 21 | 11 | 23 | 9 | 22 | 11 | 0.94 | –0.41 | 2.89 | 0.097 | ||
| Spontaneous errors | 44 | 19 | 41 | 20 | 40 | 27 | 30 | 15 | –0.53 | –1.67 | 0.01 | |||
| Reaction times (ms) | ||||||||||||||
| Baseline events | 897 | 106 | 850 | 123 | 839 | 194 | 765 | 84 | –1.29 | –1.63 | 0.13 | |||
| Win events | 739 | 127 | 702 | 87 | 638 | 118 | 643 | 102 | –1.04 | 0.14 | 1.22 | |||
| Lose/shift events | 736 | 127 | 703 | 102 | 635 | 146 | 645 | 123 | -0.89 | 0.25 | 1.03 | |||
| Lose/no shift events | 705 | 117 | 665 | 91 | 617 | 118 | 613 | 115 | –1.15 | –0.13 | 0.44 | |||
| Spontaneous error trials | 790 | 147 | 771 | 136 | 714 | 150 | 748 | 195 | –0.41 | 0.64 | 0.01 | |||
TABLE 2. Behavioral Data of the Reversal Learning Paradigm in a Study of Medial Orbitofrontal and Amygdala Activation in Bipolar Disorder
Functional Neuroimaging Data
Bipolar patients versus comparison subjects.
Relative to comparison subjects, euthymic bipolar patients showed significantly higher activation of the medial orbitofrontal cortex during reward and rule reversal but not during punishment. In addition, rule reversal was associated with higher activation of the right lateral orbitofrontal cortex, the right amygdala, the dorsal anterior cingulate cortex, and the putamen in euthymic bipolar patients relative to comparison subjects (see Table 3 and Figure 2). We observed a significant negative correlation between medication load and mean activation of the right amygdala in response to reward in patients with bipolar disorder (r=–0.46, p=0.002; see Figure 2 in the online data supplement). No other statistically significant correlations between medication load and BOLD response changes were observed.
| Bipolar Patients vs. Healthy Comparison Subjects (N=38) | Relatives vs. Healthy Comparison Subjects (N=44) | Bipolar Patients vs. Relatives (N=41) | Relatives vs. Bipolar Patients (N=41) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinatesa | Coordinatesa | Coordinatesa | Coordinatesa | ||||||||||||||||||||||
| Brain Region | Left/Right | x | y | z | z | Cluster Sizec | Left/Right | x | y | z | z | Cluster Sizec | Left/Right | x | y | z | z | Cluster Sizec | Left/Right | x | y | z | z | Cluster Sizec | |
| Reward: “win”–baselineb | |||||||||||||||||||||||||
| Medial orbitofrontal cortex | L | –6 | 24 | –15 | 3.64 | 5 | R | 6 | 27 | –15 | 3.66 | 5 | |||||||||||||
| Amygdala | R | 27 | 3 | –21 | 3.22 | 6 | R | 24 | –3 | –12 | 2.76 | 4 | |||||||||||||
| Punishment: “lose/no shift”–baselineb | |||||||||||||||||||||||||
| Medial orbitofrontal cortex | L | –3 | 30 | –12 | 3.64 | 11 | |||||||||||||||||||
| Rule reversal: “lose/shift”–baselineb | |||||||||||||||||||||||||
| Medial orbitofrontal cortex | L | –3 | 30 | –15 | 3.60 | 9 | R | 6 | 33 | –9 | 3.70 | 11 | |||||||||||||
| Lateral orbitofrontal cortex | R | 27 | 33 | –12 | 3.64 | 6 | R | 30 | 39 | –9 | 3.14 | 6 | |||||||||||||
| Dorsal anterior cingulate cortex | R | 3 | 42 | 21 | 3.66 | 6 | |||||||||||||||||||
| Amygdala | R | 24 | 3 | –12 | 3.57 | 6 | R | 21 | –3 | –18 | 3.68 | 8 | |||||||||||||
| 30 | –9 | –12 | 2.95 | 2 | L | –24 | –3 | –15 | 3.03 | 8 | |||||||||||||||
| Striatum (putamen) | R | 24 | 6 | –9 | 3.74 | 21 | R | 24 | 15 | –9 | 3.58 | 9 | |||||||||||||
| L | –27 | –9 | 0 | 3.59 | 7 | ||||||||||||||||||||
TABLE 3. Brain Regions Showing Main Effect for Reward, Punishment, Rule Reversal, and Behavioral Change in Euthymic Patients With Bipolar Disorder and Comparison Subjects
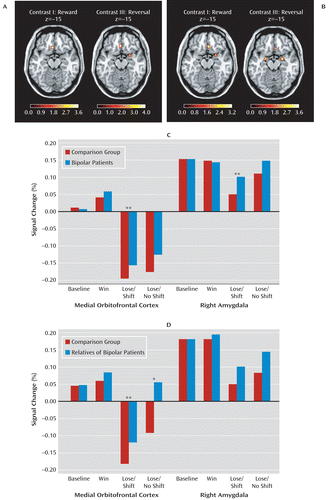
FIGURE 2. Illustration of Activation Differences During Reward and Reversal of Reward Contingenciesa
a Panel A depicts the significantly greater activation of the orbitofrontal cortex and the amygdala in patients with bipolar disorder relative to healthy comparison subjects. Panel B depicts the significantly greater activation of the orbitofrontal cortex and the amygdala in the relatives of bipolar patients relative to healthy comparison subjects. Axial slices are overlaid on the MRIcron ch2 template. The left hemisphere is displayed on the left, and the color scale represents the t scores. Panel C depicts the associated percentage signal changes from baseline for the medial orbitofrontal cortex and the right amygdala for patients with bipolar disorder and healthy comparison subjects. Panel D depicts the associated percentage signal changes from baseline for the relatives of bipolar patients and healthy comparison subjects.
*p<0.01. **p<0.1.
Relatives versus comparison subjects.
The relatives of the bipolar I patients showed significantly higher activation of the medial orbitofrontal cortex in response to reward, punishment, and rule reversal relative to comparison subjects. Additionally, we observed significantly higher activation in the right amygdala in response to reward and rule reversal in the relatives compared with healthy comparison subjects (see Table 3 and Figure 2). Relatives who had passed the peak onset age (31) did not differ significantly from the younger ones, and we observed no differences between relatives stemming from multiplex or simplex families.
Bipolar patients versus relatives.
In response to reward, we observed increased activation in the right amygdala in relatives compared with bipolar patients, whereas rule reversal was related to higher activation of the right lateral orbitofrontal cortex and the putamen in patients compared with relatives (Table 3).
Discussion
To our knowledge, this is the first study to show dysfunctional activation in neural systems related to motivation and reward in euthymic bipolar I patients and unaffected first-degree relatives of bipolar I patients, and it identifies a potential neural intermediate phenotype of bipolar disorder. Specifically, increased activity of the medial orbitofrontal cortex in response to reward and rule reversal was found in both euthymic bipolar patients and their first-degree relatives compared with healthy comparison subjects. In addition, we observed increased activation in the right amygdala during rule reversal in both euthymic bipolar patients and their relatives compared with healthy comparison subjects. Such similar findings in medicated patients and unmedicated high-risk persons are of particular importance, because they suggest that the results are unlikely to be affected by medication. Interestingly, our data also suggest that increased amygdala activation in response to reward, which was observed in relatives only, may have been suppressed by psychotropic medication.
Previous studies suggest that the medial orbitofrontal cortex codes the expectation of reinforcement, which is acquired via input from the amygdala and is used to guide behavior (32). In the present study, the amygdala and the medial orbitofrontal cortex showed greater neural activation in response to reward and decreased neural activation in response to negative feedback in all groups. It has been suggested that reduced activity of the amygdala and the medial orbitofrontal cortex in response to unexpected negative feedback occurring during rule reversal represents a prediction error signaling that a change in behavior is required (3). Thus, we propose that heightened activation of the medial orbitofrontal cortex and the amygdala during wins in bipolar patients and unaffected relatives probably represents heightened sensitivity toward reward, whereas greater activation of the amygdala and reduced deactivation of the medial orbitofrontal cortex during rule reversal in bipolar patients and their relatives represents an attenuated prediction error signal. This attenuated prediction error signal, indicated by increased medial orbitofrontal cortex activation, was particularly pronounced in unaffected relatives during negative feedback that was not followed by a behavioral change (“lose/no shift” events). This result suggests clinical manifestations in manic bipolar patients, who continue to pursue immediate rewards despite negative consequences (19).
Similar activation patterns were observed in patients and relatives, although some relatives had a lower risk of developing the disorder than others as they had already passed the peak age of illness onset or came from simplex rather than multiplex families. However, additional analyses revealed no differences between these subgroups. This raises the question of how the observed abnormalities might contribute to an increased vulnerability for bipolar disorder. The behavioral activation system dysregulation theory (33) suggests that a hypersensitive behavioral activation system, which regulates approach motivation and goal-directed behavior and is dependent on the orbitofrontal cortex among other regions (34), mediates vulnerability for bipolar disorder. This system is triggered by reward or obstructed reward, eventually leading to manic or depressive episodes. Indeed, exploratory analyses revealed positive correlations between the score on the behavioral activation system scale (35) and neural activation in the medial orbitofrontal cortex in response to reward (r=0.36, p=0.012) and rule reversal (r=0.37, p=0.008) in bipolar patients and relatives (see Table 1 in the online data supplement).
Apart from the reported similar activation patterns in response to reward and rule reversal in bipolar patients and relatives, bipolar patients exhibited increased activity in the ventral putamen and the lateral orbitofrontal cortex during rule reversal in contrast to comparison subjects and relatives. We speculate that these neural abnormalities do not represent vulnerability but a state marker. In this context, increased activity in the lateral orbitofrontal cortex signaling punishment could represent a compensatory mechanism that aids in suppressing previously rewarded responses (2) and potentially enables adequate performance during euthymia.
Our results are in line with studies showing impaired reversal learning on a behavioral level in bipolar patients (7–11), altered functioning of the amygdala and orbitofrontal cortex during emotional processing in bipolar patients (36–37), and the behavioral activation system dysregulation model for bipolar disorder (33), suggesting a hypersensitive behavioral activation system as a vulnerability in bipolar disorder. Moreover, our findings extend those of previous studies by identifying the neural correlates of altered reversal learning in bipolar disorder and showing that heightened emotional reactivity in bipolar disorder is not limited to primary emotional cues but also occurs for positive and negative feedback, leading to emotional and motivational dysregulation. Most importantly, the results from patients and their relatives suggest that increased activation of the amygdala and the medial orbitofrontal cortex constitutes an endophenotype of bipolar disorder. These results are further supported by another study by our group (38) that showed increased amygdala activation in response to reward in carriers of the risk allele of CACNA1C rs1006737, a genome-wide supported risk variant for bipolar disorder. It is worth noting that in schizophrenia, neural responses to reward are variable (39–40) and are related to disease state (39). Therefore, reward-related processing could represent a neural “point of rarity” distinguishing between these two genetically related yet clinically different disorders.
This study of motivation-related circuits as a candidate endophenotype was limited by sample sizes and sample characteristics that preclude generalizability to other ethnicities and countries. Moreover, it is possible that our use of tight exclusion criteria, designed to minimize confounds inherent in bipolar research (e.g., substance abuse, medication, and residual mood symptoms), may limit the generalizability of our findings. Further work to evaluate this possibility is needed.
Conclusion
Our results identify increased activation of the medial orbitofrontal cortex and the amygdala that was related to heightened reward sensitivity and deficient prediction error signal as a candidate brain functional endophenotype of bipolar disorder. In sum, our results support a role of motivational processing in the risk architecture of bipolar disorder and identify a new systems-level therapeutic target for the illness.
1. : Does psychology make a significant difference in our lives? Am Psychol 2004; 59:339–351Crossref, Medline, Google Scholar
2. : Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci 2002; 22:4563–4567Crossref, Medline, Google Scholar
3. : Neural correlates of response reversal: considering acquisition. Neuroimage 2007; 34:1754–1765Crossref, Medline, Google Scholar
4. : Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage 2005; 26:609–618Crossref, Medline, Google Scholar
5. : A longitudinal fMRI study of the manic and euthymic states of bipolar disorder. Bipolar Disord 2010; 12:344–347Crossref, Medline, Google Scholar
6. : Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry 2007; 164:638–646Link, Google Scholar
7. : Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med 2010; 40:1089–1100Crossref, Medline, Google Scholar
8. : Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry 2005; 162:1975–1977Link, Google Scholar
9. : Set shifting and reversal learning in patients with bipolar disorder or schizophrenia. Psychol Med 2009; 39:1289–1293Crossref, Medline, Google Scholar
10. : Hot and cold cognition in unmedicated depressed subjects with bipolar disorder. Bipolar Disord 2009; 11:178–189Crossref, Medline, Google Scholar
11. : Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol Psychiatry 2008; 64:162–168Crossref, Medline, Google Scholar
12. : Prefrontal function and activation in bipolar disorder and schizophrenia. Am J Psychiatry 2008; 165:378–384Link, Google Scholar
13. : Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage 2008; 42:1118–1126Crossref, Medline, Google Scholar
14. : Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord 2010; 12:707–719Crossref, Medline, Google Scholar
15. : Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry 2006; 60:93–105Crossref, Medline, Google Scholar
16. : The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 2003; 160:636–645Link, Google Scholar
17. : From maps to mechanisms through neuroimaging of schizophrenia. Nature 2010; 468:194–202Crossref, Medline, Google Scholar
18. : Motivational orientation modulates the neural response to reward. Neuroimage 2010; 49:2618–2625Crossref, Medline, Google Scholar
19.
20. : Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology 2007; 191:823–833Crossref, Medline, Google Scholar
21. : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
22. : A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
23. : Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry 1974; 7:151–169Crossref, Medline, Google Scholar
24. : The definition and meaning of treatment-resistant depression. J Clin Psychiatry 2001; 62(suppl 16):10–17Medline, Google Scholar
25. : SKID-I: Strukturiertes Klinisches Interview für DSM-IV (
26. : Grundintelligenztest Skala 2 (CFT-20) mit Wortschatztest (WS) und Zahlenfolgetest (ZF), 4th ed. Göttingen, Westermann Test, 1998Google Scholar
27.
28. : Co-Planar Stereotaxic Atlas of the Human Brain, 1st ed. New York, Thieme Medical, 1988Google Scholar
29. : An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239Crossref, Medline, Google Scholar
30. : Region of interest analysis using an SPM toolbox. Neuroimage 2002; 16:497Google Scholar
31. : Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med 2005; 35:855–863Crossref, Medline, Google Scholar
32. : Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem 2003; 10:129–140Crossref, Medline, Google Scholar
33. : The role of the Behavioral Approach System (BAS) in bipolar spectrum disorders. Curr Dir Psychol Sci 2010; 19:189–194Crossref, Medline, Google Scholar
34. : Neurobehavioral aspects of affective disorders. Annu Rev Psychol 1989; 40:457–492Crossref, Medline, Google Scholar
35. : Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol 1994; 67:319–333Crossref, Google Scholar
36. : Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry 2009; 66:451–459Crossref, Medline, Google Scholar
37. : Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry 2010; 67:422–431Crossref, Medline, Google Scholar
38. : The CACNA1C risk variant for bipolar disorder influences limbic activity. Mol Psychiatry 2010; 15:1126–1127Crossref, Medline, Google Scholar
39. : Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 2006; 29:409–416Crossref, Medline, Google Scholar
40. : Neural correlates of reward processing in schizophrenia: relationship to apathy and depression. Schizophr Res 2010; 118:154–161Crossref, Medline, Google Scholar



