Lower β2*-Nicotinic Acetylcholine Receptor Availability in Smokers With Schizophrenia
Abstract
Objective:
There is a strong association between cigarette smoking and schizophrenia. Nicotine's actions in the brain are mediated through nicotinic acetylcholine receptors. Those containing α4 and β2 subunits are the most abundant ones in the brain, have the highest affinity for nicotine, and are critical in mediating nicotine's reinforcing properties. Healthy tobacco smokers have significantly higher levels of β2*-nicotinic acetylcholine receptors than do nonsmokers. However, in postmortem studies, smokers with schizophrenia do not show these higher levels. The purpose of this study was to measure β2*-nicotinic acetylcholine receptors in vivo and to relate levels to concurrent behavioral measures of smoking and schizophrenia.
Method:
By using single-photon emission computed tomography with the β2*-nicotinic acetylcholine receptor agonist radiotracer [123I]5-IA-85380, the availability of receptors was measured in smokers with schizophrenia (11 men) and matched comparison smokers after 1 week of confirmed smoking abstinence.
Results:
Smokers with schizophrenia showed significantly lower (21%–26%) β2*-nicotinic acetylcholine receptor availability relative to comparison smokers in the frontal cortex, parietal cortex, and thalamus (in descending order). There was a specific and robust negative correlation between regional β2*-nicotinic acetylcholine receptor availability and negative symptoms.
Conclusions:
These are the first in vivo findings of lower β2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. The relationship between β2*-nicotinic acetylcholine receptor availability and negative symptoms may explain the high rates of smoking in schizophrenia and the relationship between smoking and negative symptoms. Findings support the development of medications targeting the β2*-nicotinic acetylcholine receptor system for the treatment of negative symptoms.
Individuals with schizophrenia have high rates of comorbid cigarette smoking (1, 2) and are more dependent on nicotine than other smokers (2, 3). The risk for developing schizophrenia is associated with higher rates of smoking (2, 4, 5). Furthermore, smoking cessation rates for smokers with schizophrenia are significantly lower than those for the general population and smokers with other mental illnesses (2).
Nicotinic acetylcholine receptors containing β2 subunits are abundant in the brain and have the highest affinity for nicotine. They are the most common subtype in striatal reward pathways and are critical in mediating the reinforcing effects of nicotine (6). Repeated exposure to nicotine or smoking has been consistently shown to result in the up-regulation of these receptors, i.e., increased availability, throughout the brain (7–11). Nicotinic acetylcholine receptors containing two α4 and three β2 subunits are believed to be the primary ones that up-regulate in response to nicotine. However, other subunits (α5, α6, β3) may combine with the α4 and β2 subunits to result in alternate forms that have distinct characteristics (12) and may contribute to receptor up-regulation (13). To account for the existence of other β2-containing subunit combinations, the term “β2*-nicotinic acetylcholine receptors” is used, where the asterisk represents other subunits that may also be part of the receptor. The increase in binding sites for β2*-nicotinic acetylcholine receptors following chronic nicotine treatment has been shown to result from an increase in the number of receptors containing β2 subunit proteins (14).
In postmortem studies, smokers with schizophrenia showed lower numbers of β2*-nicotinic acetylcholine receptors in several brain regions, including the hippocampus, cortex, and striatum, relative to comparison smokers (15, 16). Furthermore, there was no difference in receptor numbers in these same regions between smokers and nonsmokers with schizophrenia (15), suggesting a lack of up-regulation in smokers with schizophrenia. In addition, there is a genetic link between the genes that encode the α4 and β2 subunits and heavy smoking in individuals with schizophrenia (17).
The availability of β2*-nicotinic acetylcholine receptors in vivo can be measured with [123I]5-IA-5380 ([123I]5-IA) and single-photon emission computed tomography (SPECT), as described elsewhere (13, 18, 19). Using [123I]5-IA SPECT, we showed significantly higher availability of β2*-nicotinic acetylcholine receptors in the striatum and cerebellum and throughout the cortex in recently abstinent smokers than in nonsmokers.
The goals of this study were to compare β2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia and comparison smokers and to examine the relationships of availability to the symptoms of schizophrenia and to tobacco smoking, craving, and withdrawal.
Method
Subjects
Tobacco smokers with a primary diagnosis of DSM-IV schizophrenia, confirmed by the Structured Clinical Interview for DSM-IV, were compared to age- and gender-matched comparison smokers selected from a group of healthy tobacco smokers who were being studied concurrently. Nicotine dependence was evaluated with the Fagerström Test for Nicotine Dependence (20). The comparison smokers were medically and psychiatrically healthy and did not have any lifetime diagnosis of substance abuse or dependence (excluding nicotine and caffeine). The process of obtaining consent from the subjects with schizophrenia involved several steps, as described in the supplemental text accompanying the online version of this article.
The subjects with schizophrenia underwent a medical examination by a research physician to exclude any major, unstable medical or neurological disorders. Those with a diagnosis of substance abuse within the past month or substance dependence (with the exception of nicotine or caffeine use) within the 6 months prior to the screening evaluation were excluded. Smoking status was confirmed by a plasma cotinine level above 150 ng/ml, urine cotinine level above 100 ng/ml, and carbon monoxide level above 11 ppm at baseline. Only clinically stable patients taking a stable dose of antipsychotics for the past 12 weeks were included. Benzodiazepines were permitted on an as-needed basis. Subjects taking tricyclic antidepressants, anticholinergics, or selective serotonin reuptake inhibitors were excluded because there is some evidence that these drugs interfere with [123I]5-IA binding. Subjects were screened for any characteristics that would exclude them from magnetic resonance imaging (MRI).
Further details about regulatory approvals, the consent process, inclusion and exclusion criteria, screening, and contingency management are described in the supplemental text accompanying the online version of this article.
Smoking Cessation
To help the subjects abstain from smoking for at least 5 days before the SPECT scan day, they were given brief behavioral counseling based on clinical practice guidelines and contingency management. Approximately 1 week of abstinence from tobacco smoking is required for nicotine to clear from the brain such that it will not interfere with [123I]5-IA binding (21). Eligible smokers with schizophrenia were hospitalized on a smoke-free unit and were not allowed to use any drugs that could facilitate smoking abstinence. Trained research staff counseled the subjects daily to help them cope with abstinence. Counseling was paired with contingency management: subjects were paid $25 for the first day of inpatient abstinence, and payments were escalated by $25 every inpatient day (as described in supplemental text). If subjects were able to complete the inpatient abstinence period before the scan, they were eligible to receive $825. Abstinence from smoking and other nicotine products was confirmed by daily monitoring of breath carbon monoxide and dipstick measurement of urinary cotinine.
Nicotine craving and withdrawal were evaluated by using the Tiffany Urge to Smoke Questionnaire (22) and the Minnesota Nicotine Withdrawal Scale (23), respectively, at intake, during smoking cessation, and on the SPECT scan day. Positive, negative, and general symptoms of schizophrenia were measured by the Positive and Negative Syndrome Scale (PANSS) (24) and the Scale for the Assessment of Negative Symptoms (SANS) (25). Depressive symptoms were assessed by the Montgomery-Åsberg Depression Scale (MADRS) (26), and involuntary movements were evaluated with the Abnormal Involuntary Movements Scale (AIMS) (27).
[123I]5-IA SPECT and MRI
After achieving abstinence for at least 5 days, each subject had one [123I]5-IA SPECT scan and one MRI scan. MRI was performed on a Signa 1.5-T system (General Electric, Milwaukee, Wis.) as described previously (18). SPECT imaging was conducted as described previously (21). In brief, [123I]5-IA was administered over 8 hours by using a bolus plus constant infusion at a ratio of 7.0 hours (i.e., with a bolus worth 7 hours of infusion). Using a simultaneous transmission and emission protocol (STEP), we obtained three 30-minute emission scans and one 15-minute transmission scan 6–8 hours after infusion on a Picker PRISM 3000 XP SPECT camera (Picker International, Cleveland). Plasma samples were collected in the middle of the second scan to quantify the total amount of parent tracer and the free fraction (fp) of the parent tracer in plasma (28, 29) and to correct for individual differences in metabolism and protein binding of [123I]5-IA) (30). A 57Co-distributed source was measured with each experiment to control for day-to-day variation in camera sensitivity.
Image Analysis and Outcome Measures
SPECT emission images were analyzed as described previously (31). A coregistered image from the MRI scan was used to guide the placement of standard two-dimensional region of interest templates with MEDx software (Medical Numerics, Germantown, Md.). A three-dimensional volume of interest was generated for each region and transferred to the coregistered SPECT image to determine regional radioactive densities. The chosen regions were those known to contain β2*-nicotinic acetylcholine receptors and included the frontal, parietal, anterior cingulate, temporal, and occipital cortices, the thalamus, the striatum (an average of caudate and putamen), hippocampus, amygdala, brainstem, and cerebellum. Regional [123I]5-IA uptake was determined as VT/fp, where VT is the volume of distribution and fp is the free plasma fraction. Each case was analyzed by two raters, and the mean of the values calculated by the two raters was used. The interrater variability for VT/fp was less than 10% across all regions.
Statistical Analyses
Statistical analyses were conducted by using SAS version 9.1 (SAS Institute, Cary, N.C.) and SPSS version 16.0 (SPSS, Chicago). Independent t tests were used to compare baseline demographic and clinical variables (e.g., smoking characteristics) in the two diagnostic groups. Multivariate analysis of variance (MANOVA) models were used to examine differences in receptor availability (VT/fp) between the patients and comparison smokers in brain regions (frontal and parietal cortices, striatum, hippocampus, and thalamus) chosen a priori on the basis of the postmortem literature.
PANSS, MADRS, SANS, and AIMS data in the smokers with schizophrenia were examined descriptively by using means, standard deviations, and graphs. Each outcome was tested for normality by using Kolmogorov-Smirnov test statistics and normal probability plots. The outcomes were approximately normal and were assessed by means of linear mixed models with time (baseline day and scan day) included as a within-subjects explanatory factor. Spearman's rho correlation coefficients were used to examine correlations of regional availability of β2*-nicotinic acetylcholine receptors with smoking characteristics (packs per day, Fagerström score, years of smoking, craving [desire/relief], withdrawal) in both groups and with measures of schizophrenia (PANSS, SANS) and depression (MADRS) in the smokers with schizophrenia. All results were considered statistically significant at p<0.05.
Results
Fourteen smokers with schizophrenia were enrolled, but two were not scanned; one did not achieve smoking abstinence, and one could not tolerate inpatient hospitalization. Demographic characteristics of the remaining 12 are presented in Table 1. Though both male and female smokers were recruited, the only female subject who enrolled was unable to remain abstinent for the scan.
| Patient | Age (years) | Cigarettes per Day | Score on Fagerström Test for Nicotine Dependence | Race | Education (years) | Antipsychotic Medication | Chlorpromazine-Equivalent Dose (mg/day) |
|---|---|---|---|---|---|---|---|
| 1b | 60 | 18 | 6 | Caucasian | 15 | Aripiprazole, 30 mg/day | 400 |
| 2 | 53 | 30 | 8 | Caucasian | 14 | Long-acting risperidone, 50 mg i.m. every 2 weeks | 800 |
| 3 | 38 | 20 | 8 | Caucasian | 12 | Olanzapine, 30 mg/day | 600 |
| 4 | 48 | 20 | 4 | African American | 12 | Risperidone, 8 mg/day | 800 |
| 5 | 25 | 20 | 8 | African American | General equivalency diploma | Haloperidol decanoate, 160 mg every 2 weeks | 800 |
| 6 | 41 | 20 | 7 | African American | 12 | Aripiprazole, 30 mg h.s. | 400 |
| 7 | 46 | 35 | 3 | African American | 13 | Olanzapine, 15 mg h.s. | 300 |
| 8 | 42 | 15 | 4 | African American | 9 | Risperidone, 3 mg in the morning, 1 mg at noon | 400 |
| 9 | 24 | 10 | 7 | African American | 14 | Haloperidol decanoate, 150 mg i.m. every 4 weeks; oral haloperidol, 1 mg/day | 800 |
| 10 | 61 | 40 | 6 | African American | 13 | Clozapine, 300 mg/day | 300 |
| 11 | 48 | 20 | 7 | African American | 13 | Haloperidol, 5 mg in the morning, 10 mg h.s. | 750 |
| 12 | 28 | 20 | 8 | African American | 14 | Long-acting risperidone, 25 mg i.m. every 2 weeks | 400 |
| Mean | 42.83 | 22.33 | 6.33 | 12.73 | 562.5 | ||
| SD | 12.47 | 8.47 | 1.77 | 1.57 | 214.4 |
TABLE 1. Demographic and Clinical Characteristics of Smokers With Schizophrenia in a Study of β2*-Nicotinic Acetylcholine Receptorsa
The period of smoking abstinence varied from 5 to 7 days (mean=5.57, SD=0.65) because of scan and inpatient scheduling constraints. An additional subject was not included in the analysis because his data were more than 3 SD above the mean. The data from the remaining 11 subjects were compared to those of age- and gender-matched comparison smokers who were selected from a group of subjects who were being concurrently scanned with [123I]5-IA SPECT. As shown in Table 2, at intake the smokers with schizophrenia reported greater nicotine dependence and smoked more cigarettes per day than the comparison smokers, although these differences did not reach statistical significance (Fagerström score: t=1.96, df=20, p=0.06; cigarettes per day: t=1.71, df=20, p=0.10).
| Comparison Smokers (N=11) | Smokers With Schizophrenia (N=11) | |||
|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD |
| Age (years) | 39 | 12 | 39 | 13 |
| Duration of smoking (years) | 17 | 10 | 24 | 16 |
| Number of cigarettes smoked per day | 16.9 | 4.9 | 21.9 | 8.4 |
| Score on Fagerström Test for Nicotine Dependence | 4.2 | 2.9 | 6.3 | 2.1 |
| Carbon monoxide level on day of SPECT scan (ppm)a | 4.1 | 2.4 | 2.3 | 1.5 |
| Duration of abstinence (days)b | 8.0 | 2.2 | 5.6 | 0.7 |
| Plasma cotinine level on day of SPECT scan (ng/ml) | 23.3 | 38.7 | 12.9 | 9.7 |
TABLE 2. Smoking Characteristics of Smokers With Schizophrenia and Comparison Smokers
Confirmation of Smoking Abstinence
Breath carbon monoxide (F=26.35, df=1, 10, p=0.0004) and urine cotinine (F=87.91, df=1, 10, p<0.0001) levels declined over time, confirming that all smokers with schizophrenia were abstinent from tobacco smoking for at least 5 days before scanning (see supplemental Figure 1 accompanying the online version of this article). Furthermore, both plasma and urine cotinine levels declined over time, and there were no differences between smokers with schizophrenia and comparison smokers in either urine cotinine levels (group effect: F=0.22, df=1, 20, p=0.65; time effect: F=252.30, df=1, 12, p<0.0001; group-by-time interaction: F=0.08, df=1, 20, p=0.78) or plasma cotinine levels (group effect: F=0.71, df=1, 17, p=0.41; time effect: F=36.50, df=1, 17, p<0.0001; group-by-time interaction: F=1.25, df=1, 17, p=0.28) (see supplemental Figure 2).
Availability of β2*-Nicotinic Acetylcholine Receptors
There was a significant omnibus MANOVA effect of group (Hotelling's trace=1.09, p=0.03). Smokers with schizophrenia had significantly lower β2*-nicotinic acetylcholine receptor availability in the parietal cortex (21%), frontal cortex (26%), and thalamus (21%). However, there were no significant differences in the striatum (16%) and hippocampus (13%) (Figure 1, Table 3).
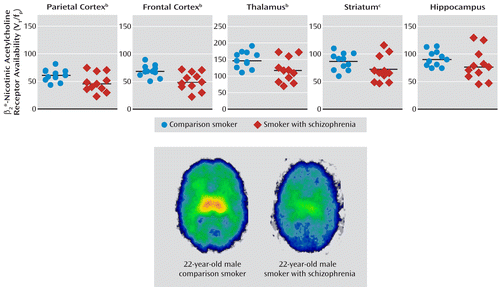
FIGURE 1. Individual Values for Regional Availability of β2*-Nicotinic Acetylcholine Receptors in Smokers With Schizophrenia and Comparison Smokersa
a Determined with SPECT imaging after 5–7 days of abstinence. VT is the volume of distribution, and fp is the free plasma fraction. The horizontal line in each scatter plot represents the group mean value.
b Significant group difference (p<0.05, MANOVA).
c Average of the values for the caudate and putamen.
| β2*-Nicotinic Acetylcholine Receptor Availability (VT/fp) | |||||||
|---|---|---|---|---|---|---|---|
| Comparison Smokers (N=11) | Smokers With Schizophrenia (N=11) | MANOVA (df=1, 20) | |||||
| Brain Region | Mean | SD | Mean | SD | F | p | Effect Size (Cohen's d) |
| Frontal cortex | 67.85 | 11.30 | 50.20 | 16.25 | 9.41 | 0.006 | 0.32 |
| Parietal cortex | 60.83 | 11.47 | 47.79 | 17.18 | 4.73 | 0.04 | 0.19 |
| Thalamus | 145.00 | 25.63 | 114.97 | 36.26 | 5.50 | 0.03 | 0.22 |
| Striatumb | 85.96 | 15.89 | 71.96 | 23.53 | 2.91 | 0.10 | 0.13 |
| Hippocampus | 89.50 | 14.11 | 77.60 | 27.60 | 1.62 | 0.22 | 0.08 |
TABLE 3. Regional Availability of β2*-Nicotinic Acetylcholine Receptorsa in Smokers With Schizophrenia and Comparison Smokers
Smoking Behaviors
In the smokers with schizophrenia, there were no statistically significant changes in scores on the Tiffany Urge to Smoke Questionnaire subscale for the desire to smoke (F=0.72, df=6, 54, p=0.63), the Tiffany relief from smoking subscale (F=1.23, df=6, 54, p=0.30), or the Minnesota Nicotine Withdrawal Scale (F=0.20, df=6, 54, p=0.97) from the baseline state (smoking as usual) to the day of the SPECT scan (supplemental Figure 3). On the day the subjects were scanned, the smokers with schizophrenia had higher ratings than the comparison smokers for the desire to smoke cigarettes (t=–3.56, df=20, p=0.002) and for expected relief from smoking (t=–5.90, df=20, p<0.001) (supplemental Figure 4). There were no significant differences (t=–0.92, df=20, p=0.37) in withdrawal symptoms between the smokers with schizophrenia (Minnesota Nicotine Withdrawal Scale score: mean=4.6, SD=4.9) and comparison smokers (mean score=2.7, SD=4.8). All but one smoker with schizophrenia resumed smoking within minutes of completing all the study procedures.
Symptoms and Dyskinesia
The smokers with schizophrenia had no statistically significant change in the total score on the PANSS (F=2.14, df=1, 10, p=0.17), MADRS (F=0.58, df=1, 10, p=0.46), or SANS (F=0.14, df=1, 10, p=0.72) (supplemental Figure 5, supplemental Table 1) between baseline (smoking as usual) and the day of the SPECT scan (5 or more days of smoking abstinence). Furthermore, while there was no change in the PANSS score for positive symptoms (F=2.91, df=1, 10, p=0.12), there was a reduction in the PANSS score for negative symptoms (F=5.71, df=1, 10, p=0.04) and an increase in the PANSS score for general symptoms (F=6.10, df=1, 10, p=0.04) with smoking abstinence. The total AIMS score increased significantly over time (F=5.71, df=1, 10, p=0.04).
Relationship of β2*-Nicotinic Acetylcholine Receptor Availability to Nicotine Dependence
There were no significant correlations between the number of cigarettes smoked per day or the score on the Fagerström Test for Nicotine Dependence and β2*-nicotinic acetylcholine receptor availability in either the smokers with schizophrenia or comparison smokers.
Relationship of β2*-Nicotinic Acetylcholine Receptor Availability to Negative Symptoms
In the schizophrenia group, there were significant negative correlations between the availability of β2*-nicotinic acetylcholine receptors in the parietal cortex, frontal cortex, thalamus, striatum, and hippocampus and negative symptoms after smoking abstinence (Figure 2). This was evident with the negative symptoms subscale of the PANSS, the SANS, or both (Table 4). Negative symptoms assessed at baseline also negatively correlated with β2*-nicotinic acetylcholine receptor availability in the parietal cortex (r=–0.61, N=11, p<0.05). Negative symptoms at baseline were negatively correlated with receptor availability in the frontal cortex, temporal cortex, striatum, and thalamus (r values, –0.57 to –0.52), but these relationships did not reach statistical significance (p=0.05–0.09).
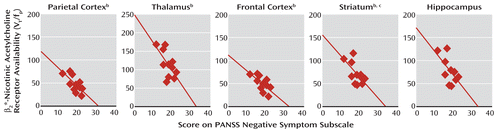
FIGURE 2. Relationships Between Regional β2*-Nicotinic Acetylcholine Receptor Availability and Negative Symptoms in 11 Smokers With Schizophreniaa
a Receptor availability was determined with SPECT imaging after 5–7 days of abstinence. VT is the volume of distribution, and fp is the free plasma fraction. The line in each scatter plot represents the correlation line of best fit.
b Significant correlation (p<0.05, Pearson correlation).
c Average of the values for the caudate and putamen.
| Correlation Between Negative Symptoms and Availability of β2*-Nicotinic Acetylcholine Receptors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Symptoms Rated With PANSS Negative Symptom Subscale | Symptoms Rated With Scale for the Assessment of Negative Symptoms | |||||||
| Baseline | Day of SPECT Scana | Baseline | Day of SPECT Scana | |||||
| Brain Region | r | p | r | p | r | p | r | p |
| Overall cortex | –0.57 | 0.07 | –0.67 | 0.03 | –0.48 | 0.13 | –0.59 | 0.05 |
| Parietal cortex | –0.61 | 0.05 | –0.71 | 0.01 | –0.50 | 0.12 | –0.56 | 0.07 |
| Frontal cortex | –0.56 | 0.08 | –0.68 | 0.02 | –0.41 | 0.21 | –0.46 | 0.16 |
| Anterior cingulate cortex | –0.53 | 0.09 | –0.64 | 0.03 | –0.46 | 0.15 | –0.59 | 0.06 |
| Temporal cortex | –0.57 | 0.07 | –0.65 | 0.03 | –0.51 | 0.11 | –0.64 | 0.04 |
| Occipital cortex | –0.51 | 0.12 | –0.58 | 0.06 | –0.47 | 0.15 | –0.62 | 0.04 |
| Thalamus | –0.56 | 0.07 | –0.66 | 0.03 | –0.46 | 0.15 | –0.62 | 0.04 |
| Cerebellum | –0.46 | 0.15 | –0.57 | 0.07 | –0.44 | 0.18 | –0.65 | 0.03 |
| Striatum | –0.54 | 0.08 | –0.63 | 0.04 | –0.41 | 0.21 | –0.60 | 0.05 |
| Hippocampus | –0.49 | 0.12 | –0.60 | 0.05 | –0.42 | 0.19 | –0.63 | 0.04 |
| Amygdala | –0.43 | 0.18 | –0.61 | 0.05 | –0.25 | 0.45 | –0.47 | 0.14 |
TABLE 4. Relationship Between Regional Availability of β2*-Nicotinic Acetylcholine Receptors and Negative Symptoms in 11 Smokers With Schizophrenia
Discussion
To our knowledge, this report provides the first in vivo evidence of lower β2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia relative to comparison smokers during early abstinence. The magnitude of group differences ranged from 13% to 26% (effect sizes of 0.08–0.32), with the largest differences, in descending order, observed in the frontal cortex, parietal cortex, thalamus, striatum, and hippocampus. These in vivo findings are consistent with the postmortem findings of lower β2*-nicotinic acetylcholine receptor levels in smokers with schizophrenia than in comparison smokers (15, 16), except for the thalamus, which did not differ significantly between groups in the postmortem studies. An important finding is the inverse correlation between negative symptoms and β2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia; i.e., those with lower receptor availability had higher negative symptom levels.
Lower availability of β2*-nicotinic acetylcholine receptors in smokers with schizophrenia may reflect altered receptor functionality. Specifically, greater availability in tobacco smokers likely represents greater numbers of desensitized and inactivated nicotinic acetylcholine receptors (29, 32). The lower β2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia may be related to abnormal desensitization or turnover of nicotinic acetylcholine receptors. It should be noted that nicotine-induced desensitization is one of the major mechanisms for nicotine addiction (33). Since maintenance of receptor desensitization may be important for relieving nicotine withdrawal in human smokers (34), the lower availability of β2*-nicotinic acetylcholine receptors observed in smokers with schizophrenia might explain why they are more likely to be addicted to nicotine.
The lower availability in smokers with schizophrenia may also reflect a deficit in nicotine-induced up-regulation of high-affinity nicotinic acetylcholine receptors, as suggested by postmortem studies. However, that conclusion cannot be drawn from the current study. In vivo studies of schizophrenia patients that compare smokers to nonsmokers and former smokers need to be conducted.
Underlying differences in the β2 gene (CHRNB2) may also explain lower nicotinic receptor availability in smokers with schizophrenia. In a family-based association study of a Canadian sample, CHRNB2 combined with the α4 gene (CHRNA4) was shown to be linked to schizophrenia (35). However, there was no significant association between schizophrenia and either CHRNA4 or CHRNB2 in a Japanese study (36). CHRNA4 and CHRNB2 have also been associated with smoking among people with schizophrenia (17, 37).
Relationship of Negative Symptoms to β2*-Nicotinic Acetylcholine Receptor Availability
The relationship between β2*-nicotinic acetylcholine receptor availability and negative symptoms was specific and robust. Individuals with lower receptor availability had higher levels of negative symptoms. This relationship was consistent across two separate measures of negative symptoms: the SANS and PANSS. There was no such relationship with depression or positive symptoms. The current finding of a negative correlation between β2*-nicotinic acetylcholine receptor availability and negative symptoms is consistent with the observation in some studies that the schizophrenia patients who were the heaviest smokers had the fewest negative symptoms (38). The literature on the relationship between nicotine dependence and symptoms is mixed (39–41). Smoking high-nicotine cigarettes has been shown to decrease negative symptoms more than denicotinized cigarettes; these effects were specific, since no effects on positive symptoms, depression, or anxiety were observed (42). Nicotine, which has been shown to increase burst firing of dopaminergic neurons and dopamine release (43–45), may reduce negative symptoms by correcting the cortical hypodopaminergia that possibly mediates negative symptoms (46). Thus, the present finding of a negative association between receptor availability and negative symptoms may contribute to understanding the high smoking rates in schizophrenia and the relationship between smoking and negative symptoms in this population. Finally, in the absence of any approved treatments for negative symptoms, the results of this study should provide the impetus to test treatments based on a β2*-nicotinic acetylcholine receptor mechanism.
Effect of Smoking Cessation on Symptoms
There was a small increase in dyskinesia that was not considered clinically significant and is well within the margin of test-retest variability of the AIMS. Smoking cessation and short-term abstinence had no significant effects on positive symptoms, negative symptoms, or depression ratings. The data from this small study group do not support the self-medication hypothesis, according to which individuals with schizophrenia smoke to alleviate positive and negative symptoms and depression. Nevertheless, most of the smokers with schizophrenia in this study resumed smoking immediately after completing the SPECT scan. Perhaps they resumed smoking to self-medicate feeling states or needs distinct from positive or negative symptoms or mood states or because their drive to smoke is different from that of the comparison smokers.
Relationship of β2*-Nicotinic Acetylcholine Receptor Availability to Baseline Smoking
As in “healthy” smokers observed previously (21), there was no significant correlation between the number of cigarettes smoked per day or nicotine dependence and receptor availability in several brain regions in the smokers with schizophrenia.
Alternative Explanations
Besides a true difference between groups, there are several other explanations for the finding of lower β2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. The two groups were not matched for antipsychotic treatment, and it is possible that this factor contributed to the finding of a group difference. However, the region-specific difference in receptor availability observed in the current in vivo study argues against a generalized effect of antipsychotic exposure. Furthermore, preclinical studies suggest that chronic exposure to clinically relevant doses of several antipsychotics does not affect high-affinity nicotinic receptors in the brain (47). Similarly, human postmortem studies suggest that the lower numbers of β2*-nicotinic acetylcholine receptors observed in smokers with schizophrenia are most likely independent of antipsychotic exposure. Ongoing in vivo studies comparing medicated and unmedicated smokers with schizophrenia will conclusively address this issue.
Smokers with schizophrenia and comparison smokers may differ in metabolism of nicotine and its metabolite cotinine. However, analysis of plasma and urine nicotine and cotinine levels, which are indications of nicotine clearing the brain (21), did not reveal significant differences in these levels between the two groups on the SPECT scan day.
Endogenous acetylcholine may compete with the [123I]5-IA radioligand binding at the receptor site. In fact, increased endogenous acetylcholine after administration of a high dose of the acetylcholinesterase inhibitor physostigmine to nonhuman primates resulted in an approximately 15% displacement of [123I]5-IA in the thalamus (48). However, the postmortem finding (15) of a lower number of receptors where acetylcholine is washed out, i.e., not a confound, argues against the explanation that endogenous acetylcholine lowered receptor availability in this study.
It is unlikely that the lower β2*-nicotinic acetylcholine receptor availability in the smokers with schizophrenia was due to a change in receptor affinity. Breese et al. conducted Scatchard analyses in their postmortem study and suggested that the differences in nicotinic acetylcholine receptor levels between schizophrenia and comparison subjects were due to differences in the total number of receptors (Bmax) and not to a change in receptor affinity (Kd) (15).
Limitations
Whether these findings are a result of schizophrenia, smoking, or some interaction between the two cannot be confirmed unless nonsmoking schizophrenia patients are studied. In the aforementioned postmortem study (15), β2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia and subjects with schizophrenia who had never smoked differed only in the cortex. Furthermore, while nicotine dose (cigarettes smoked per day) was not correlated with receptor availability, the limited range of nicotine exposure in the current study participants (most were heavy smokers) was not ideal for establishing the relationship between dose and receptor availability. Future studies that include subjects with a wider range of nicotine exposure might be useful in determining whether there is any relationship between nicotine dose and receptor availability. Other study limitations include the lack of a psychiatric comparison group and the medication received by the smokers with schizophrenia. Future work should include imaging of an unmedicated cohort of subjects. Last, whether these changes are present at the onset of the illness or develop with time is not known. Subjects early in the course of their illness will need to be studied.
Conclusions
This study provides the first in vivo evidence to our knowledge of lower β2*-nicotinic acetylcholine receptor availability and an inverse correlation between receptor availability and negative symptoms in smokers with schizophrenia. Given the absence of any effective treatments for negative symptoms, the findings of this study suggest that medications targeting the β2*-nicotinic acetylcholine receptor system may be effective. Future studies will need to be conducted in unmedicated schizophrenia patients, nonsmoking schizophrenia patients, patients with first-episode psychosis, and prodromal patients to clarify the effects of antipsychotics and stage of illness on the availability of nicotinic acetylcholine receptors and to determine whether there is less nicotine-induced up-regulation of nicotinic acetylcholine receptors in schizophrenia, as suggested by postmortem data.
1. : Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry 1998; 155:1490–1501Link, Google Scholar
2. : A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 2005; 76:135–157Crossref, Medline, Google Scholar
3. : Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr Res 1998; 33:113–118Crossref, Medline, Google Scholar
4. : The association between high nicotine dependence and severe mental illness may be consistent across countries. J Clin Psychiatry 2002; 63:812–816Crossref, Medline, Google Scholar
5. : Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry 1999; 156:1751–1757Link, Google Scholar
6. : Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 1998; 391:173–177Crossref, Medline, Google Scholar
7. : Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther 1997; 282:7–13Medline, Google Scholar
8. : Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci 1992; 12:2765–2784Crossref, Medline, Google Scholar
9. : A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 1992; 41:31–37Medline, Google Scholar
10. : Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science 1983; 220:214–216Crossref, Medline, Google Scholar
11. : Beta2-nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry 2009; 66:666–676Crossref, Medline, Google Scholar
12. : Alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol 2006; 70:755–768Crossref, Medline, Google Scholar
13. : Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol 2008; 74:132–143Crossref, Medline, Google Scholar
14. : Increased nicotinic acetylcholine receptor protein underlies chronic nicotine-induced up-regulation of nicotinic agonist binding sites in mouse brain. J Pharmacol Exp Ther 2011; 337:187–200Crossref, Medline, Google Scholar
15. : Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology 2000; 23:351–364Crossref, Medline, Google Scholar
16. : Human postmortem striatal alpha4beta2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson's syndrome. Neurosci Lett 2000; 287:109–112Crossref, Medline, Google Scholar
17. : Association of alpha4beta2 nicotinic receptor and heavy smoking in schizophrenia. J Psychiatry Neurosci 2007; 32:412–416Medline, Google Scholar
18. : Quantification of smoking-induced occupancy of beta2-nicotinic acetylcholine receptors: estimation of nondisplaceable binding. J Nucl Med 2010; 51:1226–1233Crossref, Medline, Google Scholar
19. : In vivo imaging of brain nicotinic acetylcholine receptors with 5-[123I]iodo-A-85380 using single photon emission computed tomography. Life Sci 1998; 63(25):PL355–PL360Crossref, Google Scholar
20. : The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addiction 1991; 86:1119–1127Crossref, Medline, Google Scholar
21. : Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci 2006; 26:8707–8714Crossref, Medline, Google Scholar
22. : The development and initial validation of a questionnaire on smoking urges. Br J Addiction 1991; 86:1467–1476Crossref, Medline, Google Scholar
23. : Tobacco withdrawal symptoms: an experimental analysis. Psychopharmacology (Berl) 1984; 84:231–236Crossref, Medline, Google Scholar
24. : The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl 1989; 7:59–67Crossref, Medline, Google Scholar
25. : The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl 1989; 7:49–58Crossref, Medline, Google Scholar
26. : Depressive symptoms in acute schizophrenia. Prog Neuropsychopharmacol 1979; 3:429–433Crossref, Medline, Google Scholar
27. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology:
28. : Measurement of plasma metabolites of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-IA-85380), a nicotinic acetylcholine receptor imaging agent, in nonhuman primates. Nucl Med Biol 2001; 28:91–96Crossref, Medline, Google Scholar
29. : The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci 1990; 11:216–219Crossref, Medline, Google Scholar
30. : 123I-5-IA-85380 SPECT imaging of nicotinic acetylcholine receptor availability in nonsmokers: effects of sex and menstrual phase. J Nucl Med 2007; 48:1633–1640Crossref, Medline, Google Scholar
31. : 123I-5-IA-85380 SPECT measurement of nicotinic acetylcholine receptors in human brain by the constant infusion paradigm: feasibility and reproducibility. J Nucl Med 2005; 46:1466–1472Medline, Google Scholar
32. : Molecular and cellular aspects of nicotine abuse. Neuron 1996; 16:905–908Crossref, Medline, Google Scholar
33. : Rapid desensitization of the acute stimulatory effects of nicotine on rat plasma adrenocorticotropin and prolactin. J Pharmacol Exp Ther 1986; 238:486–491Medline, Google Scholar
34. : Cigarette smoking saturates brain α4β2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 2006; 63:907–915Crossref, Medline, Google Scholar
35. : Genetic interaction between alpha4 and beta2 subunits of high affinity nicotinic receptor: analysis in schizophrenia. Exp Brain Res 2006; 174:292–296Crossref, Medline, Google Scholar
36. : Genetic association analysis of tagging SNPs in alpha4 and beta2 subunits of neuronal nicotinic acetylcholine receptor genes (CHRNA4 and CHRNB2) with schizophrenia in the Japanese population. J Neural Transm 2008; 115:1457–1461Crossref, Medline, Google Scholar
37. : A novel permutation testing method implicates sixteen nicotinic acetylcholine receptor genes as risk factors for smoking in schizophrenia families. Hum Hered 2004; 57:59–68Crossref, Medline, Google Scholar
38. : Nicotine dependence and schizophrenia. Hosp Community Psychiatry 1994; 45:204–206Abstract, Google Scholar
39. : Cigarette smoking among patients with schizophrenia and bipolar disorders. Psychiatry Clin Neurosci 2004; 58:434–437Crossref, Medline, Google Scholar
40. : Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis 2002; 190:604–610Crossref, Medline, Google Scholar
41. : Smoking and cognitive deficits in schizophrenia: a pilot study. Addict Behav 1998; 23:263–266Crossref, Medline, Google Scholar
42. : Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology 2002; 27:479–497Crossref, Medline, Google Scholar
43. : Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand 1986; 128:351–358Crossref, Medline, Google Scholar
44. : Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol 2000; 393:295–314Crossref, Medline, Google Scholar
45. : Nicotine-induced dopamine release in primates measured with [C-11]raclopride PET. Neuropsychopharmacology 2004; 29:259–268Crossref, Medline, Google Scholar
46. : Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148:1474–1486Link, Google Scholar
47. : Chronic treatment with first or second generation antipsychotics in rodents: effects on high affinity nicotinic and muscarinic acetylcholine receptors in the brain. Neuroscience 2006; 140:1277–1287Crossref, Medline, Google Scholar
48. : Influence of acetylcholine levels on the binding of a SPECT nicotinic acetylcholine receptor ligand [123I]5-I-A-85380. Synapse 2003; 48:116–122Crossref, Medline, Google Scholar



