Optimistic Bias in the Perception of Personal Risk: Patterns in Schizophrenia
Abstract
OBJECTIVE: Biases in the perception of personal vulnerability to risk could influence how individuals make decisions in many contexts. Schizophrenia patients, because of neurocognitive deficits and psychiatric symptoms, are often seen as compromised in their ability to appreciate risk information and in their decision-making capacity. The authors investigated whether schizophrenia patients share the same optimistic biases frequently demonstrated by non-ill adults in their perceptions of personal risk. METHOD: Twenty-five schizophrenic outpatients and 23 healthy comparison subjects completed a risk perception questionnaire on which they compared their own likelihood of experiencing adverse events to that of other adults. Questionnaire items were adverse events of three types: controllable, uncontrollable, and neutral. The degree to which subjects rated their own likelihood of experiencing adverse events as lower than others’ was an index of optimistic bias. RESULTS: Although both groups showed an optimistic bias in general, healthy comparison subjects demonstrated a greater level of optimism than did schizophrenia patients, especially for events typically perceived as controllable. Psychiatric symptoms rated with the Brief Psychiatric Rating Scale and the Scale for the Assessment of Negative Symptoms bore little relationship to patients’ ratings on the risk questionnaire. CONCLUSIONS: Results showed that an unrealistically optimistic bias in the perception of personal risk was at least as evident in a healthy comparison group as in a schizophrenia group. Such a bias could influence decision making. By identifying and responding to such biases, clinicians and researchers can promote more fully informed and rational decisions in patients and healthy adults.
Schizophrenia patients’ decision-making capabilities are often considered suspect in part because pervasive cognitive deficits, frequent lack of insight, and paranoid and delusional tendencies could make them particularly vulnerable to distortions in their perceptions of risk (1–7). Although there is an extensive literature describing healthy adults’ biases in risk perception and the variables that can affect them (8–12), we know little about these areas in schizophrenia patients. In this study, we asked whether adult schizophrenia patients share the same biases as nonill adults in their perceptions of personal risk. These biases are relevant to a variety of patients’ daily activities, such as making decisions about medical treatment options or treatment compliance. With a greater number of novel antipsychotics available on the market, patients are increasingly faced with the task of making treatment decisions based on an analysis of risk and benefit information. Likewise, patients who are invited to participate in pharmacological research face similar decisions. Outside the clinical realm, patients make choices every day in sometimes risky social situations. Distortions in the perception of personal vulnerability have the potential to influence one’s approach to any of these activities.
Risk perception research in healthy adults shows that although we treat this group as “normal,” they frequently exhibit a bias known as “unrealistic optimism” in which individuals feel they are less likely than other people to experience unpleasant or harmful events in their lives but more likely to experience pleasant or beneficial events (13–16). Whether this bias is rooted in motivational distortions, such as an unwillingness to admit vulnerability, or in cognitive perceptual errors, such as a failure to grasp or apply probabilistic principles, has been debated in the literature (17, 18).
Perceived controllability is among the most widely studied variables to have demonstrated an influence on people’s optimistic perception of risk and was the focus of the manipulations in the current study. Specifically, unrealistic optimism is typically greater for events perceived as under one’s personal control than for uncontrollable events (18–20). For negative events, this effect appears to stem in large part from an overestimation of how one’s own preventative behavior mitigates vulnerability in combination with an underestimation of the same behavioral influence in other people (18, 21, 22). Indeed, when asked to conjure an “average” person or group (a “target”) for the purposes of self-other comparison, people tend to imagine targets that are especially susceptible to the risk at hand or are stereotypical members of a high-risk group (18, 21). This illusion serves to encourage an unrealistically optimistic perspective of one’s own relative risk of controllable events.
Empirical research on risk perception in schizophrenia is sparse, primarily reporting on behaviors and decisions in the laboratory that likely are influenced by risk perception, such as performance on gambling tasks (5–7). A line of research that more closely examines the real-life implications of these patients’ attitudes toward risk is found in the growing number of studies assessing their capacity to provide informed consent for treatment or research participation.
The question of whether individuals with mental illness are able to provide valid informed consent has been controversial (2, 23–25) and hinges in part on whether these individuals are able to understand the nature of the risks (and benefits) they would face if they agreed to participate (1, 2, 4, 26). Also critical to valid informed consent are the abilities to appreciate the personal consequences of possible treatment or research decisions and to use that appreciation to rationally weigh the options (26). Much of the information necessary to meet these qualifications is typically conveyed during the informed consent interview. But even if a potential research subject adequately understands, for example, that the drug under study causes high blood pressure in 20% of the people who take it, the potential subject still needs to make some sort of judgment about whether he or she is likely to be in the affected 20% or the unaffected 80%. To the extent that individuals bring to the decision-making process any biases in their perception of personal risk, this judgment may also be biased.
The present study employed a risk perception questionnaire similar to those used in the general risk perception literature to address the previously unstudied matter of whether schizophrenia patients demonstrate optimistic risk perception biases similar to those seen in healthy comparison subjects and whether that optimism is greater for events typically perceived as controllable. Schizophrenia patients and healthy comparison subjects compared their own likelihood of experiencing certain adverse events to that of other adults of their own age and gender. The degree to which participants judged themselves less likely than others to experience the unpleasant events in the questionnaire determined their degree of optimism. Weinstein (14) pointed out that it is nearly impossible to say with any confidence that a person’s optimism about the future is unrealistic. Individuals might, in fact, be less likely than others to have certain experiences. It is when a group (or even a person) has a systematic or persistent optimism that we can more reliably call it unrealistic. By examining this potential systematic bias, we sought to characterize an attitude that is likely brought to bear on any number of personal circumstances and decisions of which informed consent, our own research interest, is but one example.
Method
Participants
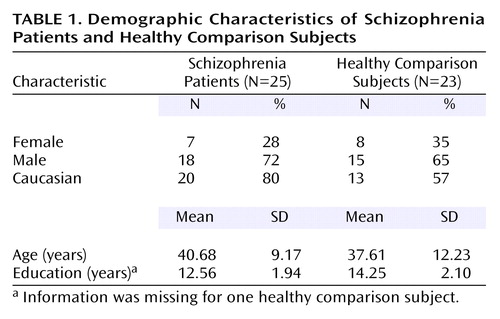
Twenty-five adult schizophrenic outpatients and 23 adult healthy comparison subjects participated in the study (Table 1). Of the 25 patients, 22 were diagnosed as schizophrenic and three as schizoaffective, according to the Structured Clinical Interview for DSM-IV, and all were symptomatically stable for a period of at least 4 weeks before testing, according to their primary therapists or physicians. All patients had stable treatment with antipsychotic medications for a period of at least 4 weeks at the time of participation. The healthy comparison subjects were recruited from the greater Baltimore area primarily through newspaper advertisements and were thoroughly screened for personal and family medical and psychiatric histories, as well as present and past substance use and abuse. Although the two groups were matched on age (F=0.98, df=1, 47, p=0.33), the healthy comparison subjects had completed more education than the patients (F=8.24, df=1, 46, p<0.01). Ethnicity and gender ratios were not significantly different between the two groups.
After a complete description of the study was presented to the subjects, written informed consent was obtained. In addition, after reviewing the consent form and before signing it, the patients completed an Evaluation to Sign Consent (27) to ensure a clear understanding of the consent information. All of the patients were able to complete the consent form successfully and were entered into the study.
Materials
The risk perception questionnaire consisted of 40 different events, each accompanied by a 7-point response-option scale. For each event, the participants used the scale to answer the question, “Compared to other adults of your age and gender, how likely is it that you will <fill in event here> at some point in your lifetime?” The response options were numbers between –3 and 3, in which 0 represented “equally likely”; –1, –2, and –3 represented “a little less likely,” “less likely,” and “much less likely,” respectively; and 1, 2, and 3 represented “a little more likely,” “more likely,” and “much more likely,” respectively. Both the numbers and the operational phrases they represented were provided on the scale. The 40 items and scales were printed in a 6-page booklet, the first page of which featured task instructions and practice items.
The 40 events differed with regard to controllability. The events were of three types: controllable, uncontrollable, and neutral. The events’ types were determined in a small pilot study of 19 healthy adults (different from those in the larger study described here) in which the participants were asked to make controllability judgments for 68 different adverse events. The participants used a 4-point scale in which scores of 1, 2, 3, and 4 represented responses “entirely out of my control,” “mostly out of my control,” “mostly in my control,” and “entirely in my control,” respectively. Of the 68 total events, the 14 rated most frequently at 3 or 4 (averaging 94% of the time) were labeled “controllable,” and the 14 rated most frequently at 1 or 2 (also averaging 94% of the time) were labeled “uncontrollable.” Twelve items with a near-50/50 ratio of ratings in these two categories were labeled “neutral.” Some examples of items of each type are the following: controllable—“Be pressured by friends or family to commit a crime”; uncontrollable—“Have appendicitis”; neutral—“Become anemic.” All 40 events presented on the questionnaire are shown in Appendix 1.
Procedure
During a brief introduction and practice phase, the participants were given instructions as to how to complete the questionnaire. They were informed that they would be asked how likely they thought they were, compared to other people, to experience certain events and that they would respond by circling one of the options on a 7-point scale. Two practice events were completed with the help of the interviewer before the participants began to answer the questionnaire. When the practice trials were completed, the interviewer read each event on the questionnaire aloud to each participant to control for differences in reading ability and to provide opportunities for the participants to ask questions about the events. The participants were assured they did not have to report their responses aloud and they did not have to answer any questions that made them feel uncomfortable. All of the patients and healthy comparison subjects received a small monetary payment for their participation.
Data Analysis
Analyses of questionnaire data were based on the mean value of the participants’ responses (ranging from –3 to 3) to each of the three event types (controllable, uncontrollable, and neutral). A two-by-three repeated-measures analysis of variance (ANOVA) was used to assess the effects of group and event type, as well as their interaction. Post hoc analyses were carried out with one-way (when differences were in a known direction), paired, and independent-sample t tests. Although it was not a primary goal of the study, to make a preliminary assessment of the role of psychiatric symptoms in patients’ perception of risk, we calculated Spearman’s correlations (rs) in a subset of patients for questionnaire responses and Brief Psychiatric Rating Scale (BPRS) (28) total score (20 items; 140 possible points), as well as a BPRS psychosis factor (a sum of scores for conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content; 28 possible points) and an anxiety factor (sum of scores for somatic concern, anxiety, guilt feelings, and depression; 28 possible points). The same analysis was carried out with a version of the Scale for the Assessment of Negative Symptoms (SANS) (29) total score (15 items; 75 possible points). BPRS ratings were available for 17 patients, and SANS ratings were available for 16 of those 17 patients.
Results
Figure 1 illustrates patients’ and healthy comparison subjects’ patterns of questionnaire responses. The horizontal line represents a 0 rating, indicating an “equally likely” judgment. All values below the 0 line indicate responses in the “less likely” range, whereas values above 0 represent responses in the “more likely” range.
The patients’ data are shown on the left side of Figure 1 and those of the healthy comparison subjects on the right. A two-by-three repeated-measures ANOVA showed the main effects of group (F=4.57, df=1, 46, p<0.05; partial η2=0.09), indicating lower (i.e., more optimistic) overall ratings among healthy comparison subjects, and of event type (F=93.80, df=2, 92, p<0.001; partial η2=0.67), as well as a significant group-by-event-type interaction (F=9.94, df=2, 92, p<0.001; partial η2=0.18). This interaction is due, as is evident in Figure 1, to strongly negative ratings among the healthy comparison subjects for controllable events.
In both groups, when the three event types were averaged together, the overall mean ratings were significantly less than 0 (patients: t=3.92, df=24, p=0.001; healthy comparison subjects: t=7.72, df=22, p<0.001). Additional one-way t tests showed that in both groups, ratings for each of the three event types were also independently significantly less than 0 at p<0.01, with the exception of the uncontrollable events in the patient group, where p<0.05. These results indicate that both the patients and comparison subjects believed they were significantly less likely than others to experience a variety of adverse events in their lifetimes. Nevertheless, post hoc independent-sample comparisons confirmed that although the ratings for uncontrollable and neutral events were not different between the groups (t=0.92, df=46, p=0.36, and t=1.60, df=46, p=0.12, respectively), the healthy comparison subjects had significantly lower ratings than the patients for controllable events (t=3.53, df=46, p=0.001).
These results illustrate that the patients and comparison subjects reported optimistic beliefs about their vulnerability to adverse events, and although this optimism was more pronounced for controllable events in both groups, the healthy comparison subjects reported a significantly greater sense of invulnerability to controllable events than the patients. Results from post hoc analyses covarying for education (on which the two groups differed) and age did not differ from those reported here.
Analyses of psychiatric symptoms’ relationship to biases in risk perception revealed no significant correlations. The group’s SANS total score (mean=22.13, SD=11.55) and BPRS total score (mean=34.71, SD=7.53) indicated that the patients had relatively low symptom severity, and neither score was significantly correlated with degree of optimism. Furthermore, neither the psychosis factor (mean=8.76, SD=4.66) nor the anxiety factor (mean=6.71, SD=2.57) from the BPRS was significantly correlated with degree of optimism. However, in light of the complex literature on depressive realism (30) and the hopelessness associated with depression, we investigated the relationship between patients’ BPRS ratings of depressed mood and the degree of optimism demonstrated on the questionnaire. Correlational analyses showed that higher ratings of depressed mood were moderately associated with lower levels of optimism for all three event types but only the uncontrollable events approached a statistically significant relationship (rs=0.48, p=0.053). These data are preliminary and should be interpreted with caution for several reasons: the group was small, the patients in the group had relatively low symptom severity in general, and the ranges of scores on the individual SANS and BPRS items were narrow.
Discussion
Our data illustrate a considerable optimistic bias in both healthy adults and schizophrenia patients regarding the personal risk of experiencing adverse events. The bias was greater in the healthy comparison subjects than in the patients, particularly for adverse events over which one typically feels one has personal control. Also, although the data are preliminary, there was no apparent relationship between psychosis, which one might expect to be an important contributor to the group’s vulnerability to decisional incapacity (26, 31, 32), and the extent of bias. The moderate correlation between depressed mood and reduced optimism among the patients is interesting and worthy of further study. This relationship is commensurate with the theory that unrealistic optimism may be rooted in an overestimation of the effectiveness of one’s own preventative behaviors, a confidence likely to be lower in patients reporting a depressed mood.
The controllability manipulation in our study revealed that the healthy adults, relative to the schizophrenia patients, were disproportionately optimistic about their risk of experiencing controllable adverse events. The strength of this controllability effect relies on the assumption that patients and comparison subjects share similar opinions about which events are controllable and which are not. It is possible that in defining our event types, where we used controllability ratings from healthy adults, we created categories that are not in alignment with those that patients would create themselves. A more likely interpretation of our data is that although patients view a particular event as one that can be controlled by individuals, they are less likely to count themselves among those who have the power to do so. That is, they see their own behavior as less effective than others’. This interpretation is supported by the similarity in the two groups’ overall patterns of ratings, in which the greatest optimism was reported for controllable events, followed by neutral and then uncontrollable events. That the perceived relationships among the three items types were the same in both groups suggests that a mismatch in the groups’ event classifications is unlikely.
In the context of informed consent and making decisions about medical treatment or research participation, one can imagine the potential influence of an optimistic bias such as that observed in our subjects. Researchers would like to be sure that whenever someone agrees to participate in a study it is because, for example, he or she understands clearly that the noxious side effects frequently associated with the study drug, although likely to affect him or her, are not usually severe or long-lasting in the people who take it. However, an unrealistically optimistic bias such as that demonstrated in our study could lead someone to make the same decision based on potentially troubling reasoning. It is, for example, possible that the act of agreeing to participate in a research study could lead a participant to feel he or she is “taking control” of the situation, consequently rendering his or her perception of the risks inherent to the study susceptible to a particularly optimistic bias. The possibility is of particular interest when the decision concerns a higher-risk study.
Overall, our results are positive with regard to risk perception in schizophrenia patients. In deviating from the “normative” pattern of optimistic bias, as defined by the comparison group, patients are at an uncharacteristic advantage over healthy comparison subjects who appear to have a deeper bias. In applying our findings, which characterize schizophrenia patients’ general attitudes about their own vulnerability to negative events, to the narrower context of decision making in a clinical context, we make a small interpretive leap. A potential weakness in our interpretation of the data is that we have not produced evidence for or against a particular risk-related attitude among patients making specific treatment or research decisions. However, in establishing that there is not a disproportionate broad-based optimistic distortion in patients’ perceptions of their own vulnerability, we have taken an important first step toward gaining a clearer understanding of how these patients use risk information to make a variety of life choices, including medical treatment and research decisions. We recognize that there are many factors that will determine how one makes these types of decisions, both internal, such as neurocognitive capability and altruistic motivations, and external, such as family influence and potential personal gain (2, 9, 33, 34). All of these factors and others could be related to—or even influenced by—risk perception. Future research can address the relative contributions of these variables to the research decision process.
We find support for our positive results in a study reporting strengths in schizophrenia patients’ treatment of research-based risk. Roberts et al. (3) showed that when asked to rank-order four hypothetical clinical protocols according to their potential harmfulness, patients’ rank orders were similar to those provided by psychiatrists. The study also demonstrated that patients would use indicators of potential harm as factors in a decision about study participation. Furthermore, although the patients of Roberts et al. were able to recognize that studies featuring washout periods or placebo conditions are riskier than a simple blood draw or a trial of a known medication, a nontrivial number of them agreed to participate in the riskier hypothetical trials. The results reported by Roberts et al. are important because they tell us schizophrenia patients are able to recognize the potential for harm when they see it and are aware it should play a role in their decision making. To the extent that our data are generalizable to the consent context, one might expect that because patients consider the potential harmful consequences of study participation, they are not any more likely than healthy adults to perceive themselves as invulnerable to that harm but may in fact be less likely to do so. Our results leave open for further study important questions about schizophrenia patients’ treatment of specific types of risk information, including treatment- and research-related risk, as well as social and behavioral risks.
In the clinical domain, it is likely that by knowing about research participants’ biases, researchers and clinicians can make efforts to minimize their potential effects. For example, when working with local review boards to identify the best wording for a consent form, researchers should try to be sensitive to the effects of wording subtleties on potential subjects’ interpretations of risk information. Thorough inquiries into the decision-making processes employed by study participants can help identify false beliefs or perceptions of risk. Given our data and those reported by others, it appears that those who are most susceptible to optimistic bias effects will not be found only among traditionally protected groups, such as the mentally ill, but among the general adult population. By predicting and responding to potential biases in risk perception, we have an opportunity to encourage more fully informed decisions in patients and healthy adults alike.
 |
Received Nov. 20, 2003; revision received March 22, 2004; accepted April 12, 2004. From Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine. Address correspondence and reprint requests to Dr. Prentice, Maryland Psychiatric Research Center, P.O. Box 21247, Baltimore, MD 21228; [email protected] (e-mail). Supported by NIMH grants P30 MH-068580 (Advanced Center for Intervention and Services Research) to Dr. Carpenter and MH-58898 to Dr. Carpenter and a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression to Dr. Prentice.
 |
Appendix 1.
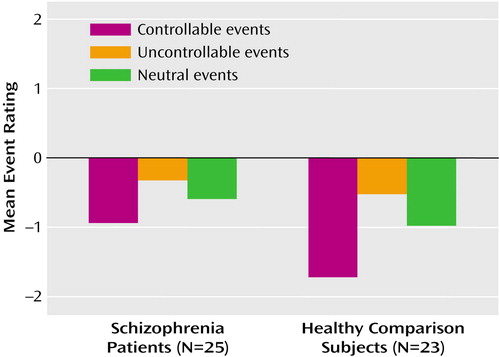
Figure 1. Mean Event Ratings for Schizophrenia Patients and Healthy Comparison Subjectsa
aRatings were the following: 3=much more likely, 0=equally likely, –3=much less likely.
1. Dunn LB, Jeste DV: Problem areas in the understanding of informed consent for research: study of middle-aged and older patients with psychotic disorders. Psychopharmacology (Berl) 2003; 171:81–85Crossref, Medline, Google Scholar
2. Roberts LW, Roberts B: Psychiatric research ethics: an overview of evolving guidelines and current ethical dilemmas in the study of mental illness. Biol Psychiatry 1999; 46:1025–1038Crossref, Medline, Google Scholar
3. Roberts LW, Warner TD, Brody JL, Roberts B, Lauriello J, Lyketsos C: Patient and psychiatrist ratings of hypothetical schizophrenia research protocols: assessment of harm potential and factors influencing participation decisions. Am J Psychiatry 2002; 159:573–584Link, Google Scholar
4. Van Staden CW, Kruger C: Incapacity to give informed consent owing to mental disorder. J Med Ethics 2003; 29:41–43Crossref, Medline, Google Scholar
5. Hutton SB, Murphy FC, Joyce EM, Rogers RD, Cuthbert I, Barnes TR, McKenna PJ, Sahakian BJ, Robbins TW: Decision making deficits in patients with first-episode and chronic schizophrenia. Schizophr Res 2002; 55:249–257Crossref, Medline, Google Scholar
6. Rosenfeld B, Turkheimer E: Multidimensional representation of decision-making in chronic schizophrenics. Multivariate Behavioral Res 1995; 30:199–211Crossref, Google Scholar
7. Rosenfeld B, Turkheimer E, Gardner W: Decision making in a schizophrenic population. Law Hum Behav 1992; 16:651–662Crossref, Google Scholar
8. Weinstein ND, Klein WM: Resistance of personal risk perceptions to debiasing interventions. Health Psychol 1995; 14:132–140Crossref, Medline, Google Scholar
9. Redelmeier DA, Rozin P, Kahneman D: Understanding patients’ decisions: cognitive and emotional perspectives. JAMA 1993; 270:72–76Crossref, Medline, Google Scholar
10. Slovic P, Fischhoff B, Lichtenstein S: Facts versus fears: understanding perceived risk, in Judgement Under Uncertainty: Heuristics and Biases. Edited by Kahneman D, Slovic P, Tversky A. Cambridge, UK, Cambridge University Press, 1982, pp 463–489Google Scholar
11. Breakwell GM: Risk communication: factors affecting impact. Br Med Bull 2000; 56:110–120Crossref, Medline, Google Scholar
12. Lloyd AJ: The extent of patients’ understanding of the risk of treatments. Qual Health Care 2001; 10(suppl 1):i14-i18Google Scholar
13. Rutter DR, Quine L, Albery IP: Perceptions of risk in motorcyclists: unrealistic optimism, relative realism and predictions of behaviour. Br J Psychol 1998; 89:681–696Crossref, Medline, Google Scholar
14. Weinstein ND: Unrealistic optimism about future life events. J Pers Soc Psychol 1980; 39:806–820Crossref, Google Scholar
15. Weinstein ND: Unrealistic optimism about susceptibility to health problems. J Behav Med 1982; 5:441–460Crossref, Medline, Google Scholar
16. Weinstein ND: Perceptions of personal susceptibility to harm, in Primary Prevention of AIDS: Psychological Approaches, vol 13. Edited by Mays VM, Albee GW, Schneider SF. Newbury Park, Calif, Sage Publications, 1989, pp 142–167Google Scholar
17. Hoorens V: Unrealistic optimism in health and safety risks, in Social Psychology and Health: European Perspectives. Edited by Rutter DR, Quine L. Aldershot, UK, Avebury, 1994, pp 153–174Google Scholar
18. van der Pligt J: Risk appraisal and health behavior. Ibid, pp 131–151Google Scholar
19. Weinstein ND: Why it won’t happen to me: perceptions of risk factors and susceptibility. Health Psychol 1984; 3:431–457Crossref, Medline, Google Scholar
20. Zakay D: The relativity of unrealistic optimism. Acta Psychol (Amst) 1996; 93:121–131Crossref, Medline, Google Scholar
21. Rothman AJ, Klein WM, Weinstein ND: Absolute and relative biases in estimations of personal risk. J Appl Soc Psychol 1996; 26:1213–1236Crossref, Google Scholar
22. Greening L, Chandler CC: Why it can’t happen to me: the base rate matters, but overestimating skill leads to underestimating risk. J Appl Soc Psychol 1997; 27:760–780Crossref, Google Scholar
23. National Bioethics Advisory Commission: Research Involving Persons With Mental Disorders That May Affect Decisionmaking Capacity: Report and Recommendations of the National Bioethics Advisory Commission, vol 1. Rockville, Md, NBAC, 1998Google Scholar
24. Michels R: Are research ethics bad for our mental health? N Engl J Med 1999; 340:1427–1430Crossref, Medline, Google Scholar
25. Rosenstein DL, Miller FG: Ethical considerations in psychopharmacological research involving decisionally impaired subjects. Psychopharmacology (Berl) 2003; 171:92–97Crossref, Medline, Google Scholar
26. Appelbaum PS, Grisso T: The MacArthur Treatment Competence Study, I: mental illness and competence to consent to treatment. Law Hum Behav 1995; 19:105–126Crossref, Medline, Google Scholar
27. DeRenzo EG, Conley RR, Love R: Assessment of capacity to give consent to research participation: state-of-the-art and beyond. J Health Care Law Policy 1998; 1:66–87Medline, Google Scholar
28. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
29. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
30. Ackermann R, DeRubeis RJ: Is depressive realism real? Clin Psychol Rev 1991; 11:565–584Crossref, Google Scholar
31. Grisso T, Appelbaum PS: The MacArthur Treatment Competence Study, III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav 1995; 19:149–174Crossref, Medline, Google Scholar
32. Moser DJ, Schultz SK, Arndt S, Benjamin ML, Fleming FW, Brems CS, Paulsen JS, Appelbaum PS, Andreasen NC: Capacity to provide informed consent for participation in schizophrenia and HIV research. Am J Psychiatry 2002; 159:1201–1207Link, Google Scholar
33. Roberts LW, Warner TD, Brody JL: Perspectives of patients with schizophrenia and psychiatrists regarding ethically important aspects of research participation. Am J Psychiatry 2000; 157:67–74Link, Google Scholar
34. Roberts LW: Evidence-based ethics and informed consent in mental illness research. Arch Gen Psychiatry 2000; 57:540–542Crossref, Medline, Google Scholar



