Predictive Values of Neurocognition and Negative Symptoms on Functional Outcome in Schizophrenia: A Longitudinal First-Episode Study With 7-Year Follow-Up
Abstract
OBJECTIVE: The relationship between cognition and outcome in people with schizophrenia has been established in studies that, for the most part, examined chronic patients and were cross-sectional in design. The purpose of this study was to analyze the relationships between neurocognitive variables assessed at illness onset and functional outcome in a longitudinal design. An additional area of interest was whether the severity of negative symptoms would predict outcome independently from neurocognitive variables or whether there would be an overlap in their predictive power. METHOD: The authors administered a comprehensive cognitive battery and clinical assessments to 99 subjects who were in their first episode of illness and analyzed the relationship of cognition and symptom severity at intake with community outcome after an average follow-up period of 7 years. RESULTS: Verbal memory, processing speed and attention, and the severity of negative symptoms at intake were related to subsequent outcome. Global psychosocial functioning was predicted by negative symptoms and attention. Verbal memory was the significant predictor of the degree of impairment in recreational activities. Impairment in relationships was predicted by negative symptoms and memory, whereas attention and negative symptoms were predictive of work performance. There was an overlap in the variance in outcome explained by cognitive variables and negative symptoms. CONCLUSIONS: Verbal memory and processing speed and attention are potential targets for psychosocial interventions to improve outcome. Results from cross-sectional or chronic patient studies do not necessarily correspond to the findings of this prospective first-episode study in which cognition appears to explain less of the variance in outcome.
The past 10 years have seen a surge in interest in the relationship between neurocognitive factors and outcome in schizophrenia. The possibility of predicting long-term outcome from performance on neurocognitive tests would allow clinicians to plan the appropriate psychosocial and possibly cognitive interventions that would target areas of cognitive function most related to outcome that may represent neurocognitive rate-limiting factors restricting the functioning of patients. The functional consequences of neurocognitive deficits in schizophrenia have been reviewed by Green et al. (1, 2) who showed that despite wide variations in neurocognitive and outcome measures, several domains of performance were consistently and reproducibly related to outcome. Community functioning was correlated with secondary verbal memory, card sorting/executive function, and verbal fluency, whereas social problem-solving skills were predicted by vigilance and secondary verbal memory. In addition to neurocognition, the severity of negative symptoms at the onset of illness has been associated with worse outcome in a number of studies (3–5). Studies that have analyzed both cognition and symptoms as predictors of outcome have yielded conflicting results: either that symptoms and neurocognition are independently predictive or that there is an overlap in their prediction of outcome (6–9).
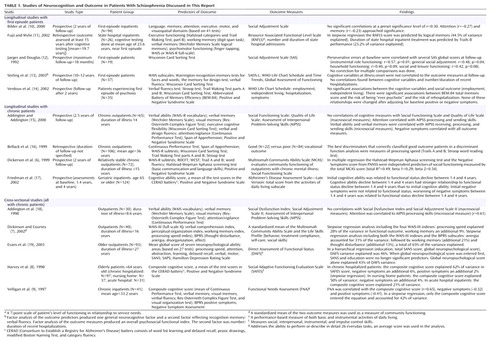
In spite of the fact that the relationships between cognition and outcome are best studied using a longitudinal design with cohorts of first-episode patients, there have been very few studies that have met these criteria (Table 1). Bilder et al. (10) examined the correlations of several cognitive domains with social adjustment; none of these were significant, but memory and attention approached significance. Fujii and Wylie (11) found that verbal memory explained nearly half of the variance in a community outcome measure. They commented on the limitations of their small sample size (N=26) and the archival nature of their neuropsychological testing data. An older study by Jaeger and Douglas (12) used the Wisconsin Card Sorting Test as the only predictor of outcome and found that perseverative errors predicted social adjustment. Stirling and colleagues (13) reported that none of the initial cognitive tests in their battery correlated significantly with psychosocial outcome after 10 years. Verdoux and colleagues (14) found no significant associations between social outcome and tests of memory, executive function, attention, or verbal fluency at intake; none of these relationships were changed after adjusting for baseline positive or negative symptoms. This review of the few longitudinal recent-onset studies indicates a predominance of negative findings, which suggests that the relationships between neurocognition and outcome may differ from the findings of cross-sectional studies or those with chronic patients.
It was therefore of interest to analyze prospectively a cohort of patients from the time of their first hospitalization and to examine the following questions:
| 1. | Are there any significant relationships between neurocognition assessed at the time of first psychiatric hospitalization and subsequent social functioning/quality of life? | ||||
| 2. | Are the relationships between neurocognitive domains and outcome measures specific? | ||||
| 3. | What are the relative contributions of neurocognition and negative symptoms in predicting each measure of outcome? Are cognitive variables and negative symptoms independent predictors of outcome, or is there an overlap in the variance they explain? | ||||
In this study we administered a comprehensive cognitive battery to 99 participants in their first episode of schizophrenia. We grouped the cognitive tests into five domains and analyzed the relationships of these domains, as well as the severity of symptoms at intake, with four measures of community social outcome after an average follow-up period of 7 years.
Method
Study Participants and Clinical Assessment
The 99 first-episode subjects (69 men and 30 women) in this study were among those enrolled in a prospective longitudinal study of recent-onset psychosis described previously (20). They were selected on the basis of having a DSM-IV diagnosis of schizophrenia, schizophreniform disorder, or schizoaffective disorder. Subjects were excluded from the study if 1) their age at intake was more than 40 years, 2) they had a history of neurological disorders such as seizure disorder, stroke, head injury, brain surgery, mental retardation, or severe recurrent headaches, or 3) their diagnosis at a 2-year follow-up assessment was no longer in the schizophrenia spectrum. All the subjects were in their first episode of illness (first hospitalization) at the time of intake. Their average age was 24 years (SD=5, range=16–39). Mean level of education was 12.7 years (SD=2); mean level of parental education was 13.8 years (SD=2.4). Fifty-two subjects were neuroleptic naive. The remaining 47 subjects had minimal neuroleptic exposure at the time of intake, with a median duration of lifetime antipsychotic treatment of 1 month and an interquartile range (25th to 75th) of 3 months.
At intake into this longitudinal study, the subjects underwent an extensive evaluation that included a clinical assessment and the administration of a neuropsychological battery. Two structured interview instruments—the Comprehensive Assessment of Symptoms and History (21) and the baseline version of the Psychiatric Status You Currently Have instrument (22)—were used in the clinical assessment. The Scale for the Assessment of Negative Symptoms (SANS) (23) and the Scale for the Assessment of Positive Symptoms (SAPS) (24) form part of the Comprehensive Assessment of Symptoms and History and the baseline version of the Psychiatric Status You Currently Have instruments. The interrater and test-retest reliability as well as the validity of the Comprehensive Assessment of Symptoms and History, as determined by intraclass correlations, have been previously reported (21). Symptom severity at the time of intake was assessed along three dimensions: psychotic, negative, and disorganized (25). The psychotic dimension was defined as the sum of the SAPS global ratings for hallucinations and delusions. The disorganized dimension was the sum of the SAPS global ratings for positive formal thought disorder, bizarre/disorganized behavior, and inappropriate affect. The negative dimension was defined as the sum of the SANS global ratings for affective flattening, alogia, avolition/apathy, and anhedonia/asociality. The maximum possible scores for the psychotic, disorganized, and negative dimensions are 10, 15, and 20, respectively, and higher scores indicate more severe symptoms.
At the time of testing, there were no significant effects of gender or medication status (naive versus treated with antipsychotics) on the severity of symptoms (multivariate analysis of variance: F≤0.64, df=3, 95, p≥0.60). Current age was related to severity of symptoms (multiple regression: F=4.29, df=3, 95, p=0.007). Only psychotic symptoms were correlated with age (r=0.24, df=97, p=0.01); older patients had more severe psychotic symptoms at intake.
All research protocols were approved by the University of Iowa Institutional Review Board, and all participants gave written informed consent.
Neuropsychological Test Battery
All study subjects were administered a comprehensive neuropsychological assessment by psychometrists trained in standardized assessment and scoring procedures. The only neuropsychological assessment used in this study was the one at the onset of illness, which was the first assessment made at the time of intake into the study. Testing took place in a quiet room at times when the patients were most cooperative and alert and after staff determined that the severity of their symptoms would not interfere with testing. Patients with severe psychotic or disorganized symptoms were tested after being stabilized with antipsychotic medication. Testing generally took 4 hours to complete and, when necessary, occurred over several sessions. Subjects who were neuroleptic naive were tested before the administration of psychiatric medications. Those who were already receiving medication at the time of intake were tested while taking their medication at the prescribed dose.
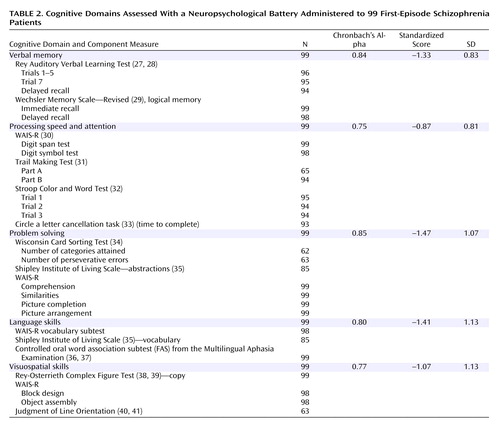
In order to efficiently analyze the cognitive functioning of study subjects, 27 neuropsychological test variables were grouped into five cognitive domains on the basis of a priori theoretical considerations (26, 27). These domains were: verbal memory, processing speed and attention, language skills, visuospatial skills, and problem solving. By summarizing our neuropsychological battery into cognitive domains, we limited capitalizing on chance associations in subsequent statistical analyses. These theoretical groupings were then tested for internal reliability using Cronbach’s alpha analyses. The neuropsychological tests within each of the five cognitive domains have good internal consistency (Cronbach’s alpha ≥0.75).
Before deriving domain scores for the 99 schizophrenia subjects in this study, the raw test scores for each of the 27 neuropsychological test variables were converted to standardized scores on the basis of norms established by use of 275 healthy comparison subjects. By definition, the healthy group had z scores with means of zero and standard deviations set to one. To provide a consistent and uniform basis for establishing the norms, these subjects were recruited through newspaper advertisements from the same geographical area from which the schizophrenia subjects were ascertained. These 275 healthy comparison subjects were selected from our larger overall sample of 550 healthy subjects so as to match the patient group in terms of age, gender, and parental education. Healthy comparison subjects were initially screened by telephone and were further evaluated using an abbreviated version of the Comprehensive Assessment of Symptoms and History to exclude subjects with current or past medical, neurological, or psychiatric illnesses (including alcohol or drug abuse/dependence) and subjects who had a first-degree relative with a schizophrenia spectrum disorder. The same psychometrists who administered the neuropsychological battery to our patients also tested these healthy comparison subjects. Scores were reversed where necessary so that a larger negative score would indicate poorer performance below the mean. Each domain score is the summed average of its component neuropsychological test variable standardized scores. Not all patients completed all tests, and the domain scores are the means of all nonmissing contributing variables. The five domains, their component tests, and descriptive statistics are shown in Table 2.
The standardized scores on the five cognitive domains were not related to the medication status at time of testing (F=0.59, df=5, 93, p=0.71). There were no significant gender differences in performance (F=2.1, df=5, 93, p=0.08), and age did not have a significant effect (F=0.38, df=5, 93, p=0.86).
Outcome Measures
Following the intake assessment, the subjects were evaluated at 6-month intervals with follow-up versions of the Comprehensive Assessment of Symptoms and History and the Psychiatric Status You Currently Have instruments. We analyzed four outcome measures: global psychosocial functioning, relationship impairment, participation and enjoyment of recreational activities, and work impairment. These measures were derived from the most recently administered follow-up Psychiatric Status You Currently Have instrument and reflect the subject’s functioning in the 6 months preceding the most recent follow-up interview. A score is given for each of the 6 months, and the measures are the mean of the six monthly scores. The information for the outcome variables was collected by experienced research assistants who knew our subjects well and were trained for reliability in the assessment instruments. The sources of information for the timeline in the past 6 months were patient interviews, information from an informant (most often the subject’s mother) who knew the patient well, and information from medical records of outpatient or inpatient treatments and from other providers during the follow-up period. All these sources were integrated to produce consensus ratings that were entered into the follow-up version of the Psychiatric Status You Currently Have instrument and used in this study.
The measure of global psychosocial functioning was the rater’s assessment on a scale of 1 to 5 of the global social adjustment of the subject, taking into account the level of functioning in the areas of work, satisfaction, interpersonal relations, and sex, as well as whether the level of functioning was consistent with what would be expected from the subject’s education and social background. Relationship impairment was the average score of the variable for quality of relationship with mother, father, relatives, and friends, each rated from 1 to 5. Enjoyment of recreation was rated from 1 to 5, and work performance was rated from 1 to 6. Lower scores indicated less impairment and better functioning. There were no significant relationships between the outcome measures and the subjects’ age or gender (F≤1.9, df=4, 94, p≥0.12).
Duration of Follow-Up and Antipsychotic Treatment
The mean follow-up duration after intake and cognitive testing in this study was 7.0 years (SD=3.8). The minimum follow-up duration was 2 years. During the follow-up period our subjects received clinical care as usual in the community and were treated with antipsychotics for most of the follow-up period (mean=80.1%, SD=29.2), with 38 patients receiving antipsychotic treatment throughout the entire follow-up. Most patients received the newer atypical antipsychotic medications (i.e., risperidone, olanzapine, quetiapine, or ziprasidone); the mean percentage of time patients were treated with atypical antipsychotics was 57.4% (SD=40.5). Thirty-one patients were treated solely with these newer atypical antipsychotics. Nineteen patients had required clozapine treatment during the follow-up period. The median antipsychotic dose was 470.5 mg/day in chlorpromazine equivalents (quartile range=362.6).
Statistical Analysis
Before analysis data were examined for normality and univariate outliers. The distributions of the visuospatial skills and problem-solving domains were skewed to the right (toward less impairment). Reflected log transformations improved the distributions and were used in further analysis. The distribution of the global psychosocial functioning variable was skewed to the right (toward more severe impairment), and a reflected log transformation was performed on this variable. The distribution of the work performance variable was also skewed to the right, but this distribution could not be improved by transformations. There were no problems related to inhomogeneity of variance, multivariate outliers, or multicollinearity, as assessed with standard methods of regression diagnostics (42).
The analyses were conducted in stages to reduce type I error. We first tested whether there was an overall effect of each of the five cognitive domains on all four outcome variables in five joint omnibus multivariate regression tests in which the four dependent variables were global psychosocial functioning, relationship impairment, participation and enjoyment of recreational activities, and work impairment. As a second step, for cognitive domains in which the omnibus test was significant at the p=0.05 level, follow-up regression analyses were performed with the cognitive domain as an independent variable and each individual outcome measure as a dependent variable. Stepwise regression analyses were used to determine the relative contributions of cognitive domains and symptom severity variables to explaining the variance in outcome measures.
Results
The standardized scores for the cognitive domains in this patient group reflect the standard deviations below the means of the healthy comparison group and indicate a generalized neuropsychological deficit (Table 2). The symptom levels at intake and the scores of the outcome measures at the follow-up assessment are shown in Table 3. Whereas the mean scores for global functioning, relationships, and recreation are consistent with moderate impairment and fair performance at the time of follow-up, the median score for work indicates severe impairment (47 out of the 99 participants were unemployed due to psychopathology).
Cognitive Domains and Outcome Measures
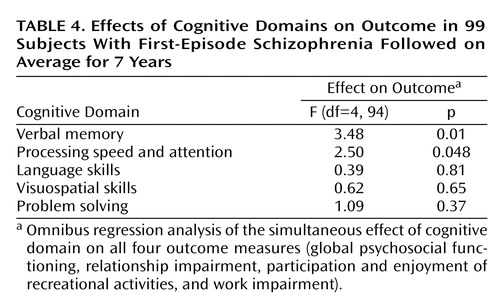
Joint omnibus tests for effects of each of the cognitive domains on all four outcome measures simultaneously were performed (Table 4). The verbal memory and processing speed and attention domains had significant relationships with outcome. The domains of problem solving, language skills, and visuospatial abilities did not have significant relationships with the outcome measures.
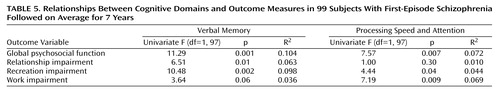
In the next step we examined the relationships between verbal memory and processing speed and attention and the individual outcome variables using regression analysis. The results of these univariate analyses are shown on Table 5. Global psychosocial function and recreation impairment were predicted by both the verbal memory and processing speed and attention domains, with verbal memory exhibiting a higher individual R2. On the other hand, the processing speed and attention domain, but not verbal memory, significantly predicted work impairment. For relationship impairment, the reverse was observed, i.e., verbal memory was a significant predictor while the processing speed and attention domain was not. In all cases where cognitive domains and outcome measures were significantly correlated, worse performance on cognitive tests predicted poorer outcome.
Relationship of Symptom Severity With Cognition and Outcome
In an overall multivariate regression test of the effect of the severity of negative symptoms at intake on the four follow-up outcome measures, the omnibus test was significant (F=3.22, df=4, 94, p=0.02). In univariate regressions the negative symptom dimension was significantly correlated with each of the outcome measures, with more severe negative symptoms predicting worse outcome (Table 6). There were also significant relationships between the severity of negative symptoms and performance on cognitive tests (F≥8.3, df=1, 97, p≤0.005). More severe negative symptoms were related to more impairment in each of the cognitive domains. There were no significant relationships between the severity of psychotic symptoms and either the outcome measures (F=1.37, df=4, 93, p=0.25) or the cognitive domain scores (F=0.87, df=5, 92, p=0.50) (age was used as covariate). Similarly, the severity of disorganized symptoms was not significantly related to the outcome (F=1.25, df=4, 94, p=0.30) or to the cognitive variables (F=1.35, df=5, 93, p=0.25).
Contributions of Negative Symptoms, Memory, and Attention to Prediction of Global Psychosocial Functioning
The relative contributions of cognition and negative symptom severity to the prediction of outcome were examined with stepwise regression analyses where the predictor variables were the significant domain scores and negative symptoms, and the dependent variables were the individual outcome measures. In our exploratory stepwise regression analyses the significance level for entry was set liberally to 0.15 to see whether some of the variables with weaker relationships to outcome measures would contribute unique variance.
For global psychosocial functioning, in a simultaneous entry regression analysis with memory, attention, and negative symptoms as predictors, the overall model was significant (F=5.65, df=3, 95, p=0.001; R2=0.15). In a stepwise regression model with all three predictors, the negative symptom variable entered the equation first and explained 11% of the variance; processing speed and attention entered second and contributed an additional 3.2%. Verbal memory did not enter the equation, although its univariate contribution to the variance of global psychosocial functioning was 10.4%. As shown in Table 7, there is a partial overlap between the variance explained by memory and attention. Moreover, both memory and attention explained variance in addition to negative symptoms, but there was a considerable overlap of each of these cognitive domains with negative symptoms. After both memory and attention are accounted for, negative symptoms continued to make a smaller (3.4% versus 11%) contribution to explaining the variance in global functioning.
Impairment in Recreational Activities
In univariate analyses, the degree of impairment in participation and enjoyment of recreational activities was significantly correlated with verbal memory (9.8% of the variance explained), processing speed and attention (4.4%), and severity of negative symptoms (5.5%). When the three predictors were entered simultaneously in a regression equation, the model was significant (F=3.76, df=3, 95, p=0.01; R2=0.11). Memory was the strongest predictor, and after memory entered the stepwise regression equations first, neither attention nor negative symptom severity made significant contributions to explaining the variance, which indicates an overlap between these predictor variables. When attention was forced into the regression with three predictors, verbal memory entered the equation and negative symptoms did not, but the percent of variance explained only increased from 9.8% to 10%, indicating an almost complete overlap. After the negative symptom variable was forced into the regression equation, memory entered the equation and processing speed and attention did not, resulting in R2=0.10, indicating again that negative symptoms explain very little nonshared variance with memory.
Impairment in Relationships
Negative symptom severity and verbal memory were the significant individual predictors of the degree of impairment in relationships. The simultaneous entry model with these two predictors was significant (F=4.32, df=2, 96, p=0.02; R2=0.083). Individually, memory explained 6.3% of the variance, and severity of negative symptoms explained 6.5%. In a stepwise regression negative symptoms entered first and the contribution of memory was not significant after negative symptoms were accounted for. When memory was forced into the regression, the negative symptom variable did not enter the equation at a significance level of 0.15. This indicates that the two predictor variables explain shared variance in the outcome measure of relationships.
Work Performance
The degree of impairment of work performance at the follow-up assessment was predicted in univariate analyses by the processing speed and attention variable (6.9% of variance) and by the negative symptom variable (5.9%). A simultaneous entry regression analysis was significant (F=5.28, df=2, 96, p=0.007; R2=0.099). In a stepwise regression analysis, processing speed and attention entered first (6.9% of variance explained) and negative symptoms entered second, contributing an additional 3% to the explanation of variance. Therefore, in the prediction of work performance, processing speed and attention and negative symptoms make shared but not entirely overlapping contributions to variance.
Discussion
The findings of this longitudinal follow-up study of a first-episode schizophrenia cohort confirmed our hypothesis that there is a relationship between cognitive function and outcome. In view of the negative findings of three of the five other first-episode longitudinal studies (10, 13, 14) (Table 1), it has been hypothesized (14) that the link between cognitive performance and social outcome may be stronger in populations selected for chronicity or severity of illness. It is also possible that with longer durations of follow-up, the predictive value of cognition or other initial variables on subsequent outcome might decrease because of the effects of factors that act longitudinally, such as treatment with antipsychotic agents (43), availability of comprehensive psychosocial rehabilitation programs (44), and the accumulation during the course of illness of environmental factors, such as stressful life events, lack of social and family support systems, changes in personal reactions to illness, and self-attitudes (45). The role of such factors may explain why in this study cognitive performance and negative symptom severity explain at best 14.2% of the variance in outcome, whereas in other cross-sectional studies of chronic patients cognitive scores predicted between 42% and 62% of the variance of outcome (8, 9, 19) (Table 1). In our study initial negative symptoms explained 11% of the variance in outcome, while the negative symptom score assessed at follow-up concurrently with outcome explained 47.4% of the variance (F=87.5, df=1, 97, p<0.0001). This is similar to the results of Stirling et al. (13) who found no correlations between initial cognitive scores and functional outcome after 10–12 years, but they did find significant relationships when cognition and outcome were assessed concurrently (performance on the Wisconsin Card Sorting Test, WAIS object assembly, picture arrangement and completion, block design, and the general neurocognitive factor were significantly correlated with the overall functional outcome index [r=–0.32 to –0.40]). Similarly, Friedman and colleagues (17) reported that in a path analysis of change in cognitive, functional, and symptom variables between assessment points, the highest standardized regression coefficient (beta=0.61) was for the relationship of the cognitive decline from the second assessment (1.4 years) to the third (4 years) with the functional decline between the second and third assessments. The relationship between initial cognitive status and functional decline between the second and third assessment was weaker (beta=0.36). Initial negative symptoms were not related to functional status, but worsening of negative symptoms between 1.4 and 4 years was related to decline in functional status (beta=0.39) between 1.4 and 4 years.
Our second hypothesis, that the relationships between neurocognitive variables and outcome are specific, was also confirmed. The only cognitive domains that were related to outcome were verbal memory and processing speed and attention. The domains of problem solving, language skills, and visuospatial skills were not related to the outcome measures. There was also specificity in the sense that different domains of outcome differed in their predictors. Global psychosocial function and recreation impairment were predicted by both the verbal memory and processing speed and attention domains, whereas only processing speed and attention predicted the degree of work impairment, and only memory was predictive of the relationship variable. Our results confirm in a first-episode longitudinal study the importance of verbal memory in the prediction of outcome but differ from previous studies that have identified card sorting/executive function and verbal fluency as additional domains predicting community outcome. In our study, attention played a role in predicting community outcome, whereas in most previous studies sustained attention (vigilance) was predictive of social problem-solving skills but not community outcome (1, 2). In this study, the role of processing speed and attention in the prediction of employment and the lack of significant contributions from the other cognitive domains to the variance in employment differ from the findings of most previous research in which memory and Wisconsin Card Sorting Test performance have been found to be important. Our findings, however, are quite consistent with those of Bellack et al. (16), who found that the best discriminators that correctly classified good vocational outcome patients from those with poor outcome were measures of processing speed (such as parts A and B of the Trail Making Test and the Stroop test). Similarly, Dickinson and Coursey (7) found that the processing speed index of the WAIS-III (digit symbol and symbol search tasks) contributed the most (28%) to the variance in a community outcome measure. Our negative results for executive functioning are consistent with the findings of Fujii and Wylie (11) in their first-episode study with a follow-up period of 19.7 years. These authors suggested that the predictive value of executive function does not extend past a few years, since all previous studies were either cross-sectional or had short follow-up periods. In their study verbal memory explained a considerably larger proportion of the variance of outcome (44.5%) than in our study (10.4%). Although a direct comparison is not possible, it appears that their subjects had a worse outcome than in our study. Subjects in their study had a mean score on the Resource Associated Functional Level Scale—a measure of a patient’s level of functioning in relationship to service needs—of 3.19 (a score of 3 indicates lack of activities of daily living/personal skills), whereas subjects in our study exhibited moderate global impairment. Also, the average duration of hospital stay was 2.1 months per year of follow-up in their study versus 0.76 months per year in our study. It is conceivable that in individuals with more severe illness, the relationship between cognition and outcome may be stronger.
Differences between our findings and previous studies may also result from the outcome measures used. The outcome measures chosen in this study (global psychosocial functioning, relationship impairment, participation and enjoyment of recreational activities, and work impairment) belong to the category of “macrosocial” outcome measures, whereas tests of interactional and social problem-solving skills such as a role play test or the Assessment of Interpersonal Problem-Solving Skills are considered to be “microsocial” measures (46). Social problem-solving measures could be conceived as social information processing tasks and are thus more similar to neurocognitive tasks than the macrosocial measures of community functioning. Therefore, associations between neurocognitive functioning and the macrosocial domain would be less likely than associations between neurocognitive functioning and the microsocial domain (15). In our study verbal memory was a stronger predictor of global functioning than processing speed and attention, whereas in studies by Addington et al. (15, 18) that utilized a microsocial measure of outcome, attention exhibited the highest correlation to outcome (r=0.61), and none of the neurocognitive domains was related to macrosocial measures of outcome. If our study had included microsocial measures, it is possible that the magnitude of correlations might have been higher and different predictor domains might have emerged.
Our results indicate that negative symptoms and cognitive variables are not independent predictors of outcome. In all cases there was an overlap in explained variance, although the extent of this overlap varied. This overlap was due in part to the fact that the severity of negative symptoms was correlated with poor performance in all five cognitive domains. This is in agreement with the findings of Bilder et al. (10), who reported significant correlations between the SANS negative symptom scores and memory, attention, executive, and visuospatial domains. Our group (47) has previously reported correlations with verbal memory and processing speed and attention tasks.
In the prediction of global psychosocial functioning, negative symptom severity was the most important factor, followed by attention, and verbal memory was no longer a significant predictor after we accounted for negative symptoms and attention. Memory and attention were not independent of each other (Table 7); severity of negative symptoms shared variance in prediction with both memory and attention, and there was a larger overlap between the severity of negative symptoms and memory (73%) than between negative symptoms and attention (66%) in explaining the variance in global psychosocial functioning. This is consistent with the fact that negative symptoms were more highly correlated with verbal memory (r=–0.55, df=97, p<0.0001) than with processing speed and attention (r=–0.29, df=97, p=0.003). A similar pattern emerged in the prediction of recreational impairment, where memory was the most significant predictor and the overlap between the three predictors appeared to be more extensive. The severity of negative symptoms took precedence over verbal memory in explaining impairment in relationships, and again there was an overlap in the variance explained. Attention was most important in the prediction of work performance, with an additional contribution from negative symptoms; the overlap between the two in explained variance was 51%.
Our results are consistent with the findings of some of the other studies that examined the effects of both cognitive function and symptoms on outcome measures. Velligan et al. (9) conducted a stepwise regression analysis with cognitive score and negative and positive symptom scores, and only the cognitive score entered the equation and explained 42% of the variance in outcome. The negative symptom score was no longer significant, which points to an overlap between symptoms and cognition (Table 1). The authors concluded that negative symptoms may be the behavioral consequences of cognitive deficits. Harvey et al. (8) found that even though both cognition and negative symptoms were highly correlated with outcome, in a stepwise regression the cognitive score predicted 62% of the variance in functional outcome, and negative symptoms contributed only a further 6%, indicating a considerable overlap in explained variance.
On the other hand, Dickerson and colleagues (6) found that community social functioning was predicted independently by a negative symptom variable and a cognitive variable. Dickinson and Coursey (7) asked the question of how symptoms combine with cognitive variables in predicting functional status and also found that they work in an independent and additive fashion. In their stepwise regression analysis the anergia score from the Brief Psychiatric Rating Scale (corresponding to negative symptom severity) entered first and accounted for 31% of the variance, followed by working memory (an additional 21%) and thought disturbance (an additional 13%) for a total of 65% of the variance explained. The authors comment that the strength of the association between symptoms and functional outcome variables in the data probably was due, in part, to areas of overlap between the measurement scales, and that narrower definitions of symptoms or functioning likely would have reduced this relationship to a degree. Similar comments about the conceptual overlap between negative symptom scales and outcome measures have been made by others (1, 3).
These previous studies differ in the tools used for assessment of symptoms and in the outcome measures. In our analysis of negative symptoms, this variable was defined as the sum of the SANS ratings for affective flattening, alogia, avolition/apathy, and anhedonia/asociality. Being well aware of the conceptual overlap between this measure and measures of outcome, we used this definition (25) because it is based on extensive factor-analytic studies and has been traditionally used in research. It was, however, of interest to examine whether a narrower measure of negative symptoms that does not include functioning criteria would still be correlated with cognition and outcome, and whether there would still be a considerable overlap of negative symptoms with verbal memory and attention in the prediction of outcome. In a post hoc analysis that defined severity of negative symptoms as the sum of only the scores for affective flattening and alogia, the correlation with verbal memory was decreased but remained significant (r=–0.47, p<0.0001), whereas the correlation with attention remained practically unchanged (r=–0.31, p=0.002). The severity of negative symptoms defined in this more narrow way predicted less of the variance in global psychosocial functioning (6.3% versus 11%) as well as in the other outcome measures. In the stepwise regression for global psychosocial functioning verbal memory now entered first, and the negative symptom and attention variables were no longer significant. In the prediction of relationship impairment, memory was now the most important predictor; in stepwise regression memory was the first and only significant predictor of recreation, and attention was the only significant predictor of work performance. This shows that with this narrow definition of negative symptoms their predictive value decreases, and there continues to be a considerable overlap with the cognitive domains in the explained variance of outcome. The shared variance between negative symptoms and verbal memory, and, less so, processing speed and attention would indicate that to some extent they may have a common, probably neurobiological, underlying construct (Addington, cited in reference 48).
The relationship of verbal memory and processing speed and attention with outcome identify these cognitive domains as areas of attention for programs directed at improving outcome. The results of the few trials of cognitive remediation of specific areas, including memory and attention, have so far been inconclusive, and a recent meta-analysis (49) did not find any appreciable effects of cognitive remediation. Even when cognitive remediation on a specific task can be achieved, research has not yet addressed the long-term durability of improved performance on specific tasks and to what extent remediation on specific cognitive tasks generalizes to more complex psychosocial functions, such as community outcome (50). It seems that compensatory strategies to bypass the cognitive deficits in schizophrenia that focus on improving functional skills rather than enhancing cognitive capacities may be more feasible (16, 51). From the results of this study it is uncertain whether neurocognitive variables can actually reliably predict future outcome, since the variance in outcome explained by neurocognition is considerably less than in previous cross-sectional studies with chronic patients. For the purposes of predicting global psychosocial outcome, the severity of negative symptoms at intake would provide almost the same information as the results from comprehensive cognitive testing, since there is a considerable overlap in explained variance. The tests for processing speed and attention may be more informative about outcome than other cognitive tests because there is less shared variance with negative symptoms.
In conclusion, this large prospective first-episode study utilized a comprehensive cognitive battery and detailed clinical assessments and confirmed the relationship between verbal memory and outcome, pointed to a relationship between processing speed and attention and community outcome, particularly in the area of work performance, and identified an overlap between cognition and negative symptoms in the prediction of outcome.
 |
 |
 |
 |
 |
 |
 |
Received Sept. 17, 2003; revision received Feb. 27, 2004; accepted May 25, 2004. From the Department of Psychiatry, University of Iowa Health Care, Iowa City; the Mental Health Clinical Research Center, Roy J. and Lucille A. Carver College of Medicine, and the College of Public Health, University of Iowa, Iowa City; the Department of Psychiatry, University of New Mexico, Albuquerque; and the MIND Institute, Albuquerque, N.M. Address correspondence and reprint requests to Dr. Milev, Department of Psychiatry, University of Minnesota Medical School, F282/2A West–B 8393, 2450 Riverside Ave., Minneapolis, MN 55454; [email protected] (e-mail). Supported by NIMH grants MH-31593, MH-40856, and MH-43271 and by a grant from the Nellie Ball Trust Research Fund (Iowa State Bank and Trust Company, Trustee).
1. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Link, Google Scholar
2. Green MF, Kern RS, Braff DL, Mintz J: Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000; 26:119–136Crossref, Medline, Google Scholar
3. Breier A, Schreiber JL, Dyer J, Pickar D: National Institute of Mental Health longitudinal study of chronic schizophrenia: prognosis and predictors of outcome. Arch Gen Psychiatry 1991; 48:239–246; correction: 48:642Crossref, Medline, Google Scholar
4. Ho B-C, Nopoulos P, Flaum M, Arndt S, Andreasen NC: Two-year outcome in first-episode schizophrenia: predictive value of symptoms for quality of life. Am J Psychiatry 1998; 155:1196–1201Link, Google Scholar
5. Wieselgren IM, Lindstrom E, Lindstrom LH: Symptoms at index admission as predictor for 1–5 year outcome in schizophrenia. Acta Psychiatr Scand 1996; 94:311–319Crossref, Medline, Google Scholar
6. Dickerson F, Boronow JJ, Ringel N, Parente F: Social functioning and neurocognitive deficits in outpatients with schizophrenia: a 2-year follow-up. Schizophr Res 1999; 37:13–20Crossref, Medline, Google Scholar
7. Dickinson D, Coursey RD: Independence and overlap among neurocognitive correlates of community functioning in schizophrenia. Schizophr Res 2002; 56:161–170Crossref, Medline, Google Scholar
8. Harvey PD, Howanitz E, Parrella M, White L, Davidson M, Mohs RC, Hoblyn J, Davis KL: Symptoms, cognitive functioning, and adaptive skills in geriatric patients with lifelong schizophrenia: a comparison across treatment sites. Am J Psychiatry 1998; 155:1080–1086Link, Google Scholar
9. Velligan DI, Mahurin RK, Diamond PL, Hazleton BC, Eckert SL, Miller AL: The functional significance of symptomatology and cognitive function in schizophrenia. Schizophr Res 1997; 25:21–31Crossref, Medline, Google Scholar
10. Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JMJ, Woerner MG, Geisler S, Kane JM, Lieberman JA: Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry 2000; 157:549–559Link, Google Scholar
11. Fujii DE, Wylie AM: Neurocognition and community outcome in schizophrenia: long-term predictive validity. Schizophr Res 2003; 59:219–223Crossref, Medline, Google Scholar
12. Jaeger J, Douglas E: Neuropsychiatric rehabilitation for persistent mental illness. Psychiatr Q 1992; 63:71–94Crossref, Medline, Google Scholar
13. Stirling J, White C, Lewis S, Hopkins R, Tantam D, Huddy A, Montague L: Neurocognitive function and outcome in first-episode schizophrenia: a 10-year follow-up of an epidemiological cohort. Schizophr Res 2003; 65:75–86Crossref, Medline, Google Scholar
14. Verdoux H, Liraud F, Assens F, Abalan F, van Os J: Social and clinical consequences of cognitive deficits in early psychosis: a two-year follow-up study of first-admitted patients. Schizophr Res 2002; 56:149–159Crossref, Medline, Google Scholar
15. Addington J, Addington D: Neurocognitive and social functioning in schizophrenia: a 2.5 year follow-up study. Schizophr Res 2000; 44:47–56Crossref, Medline, Google Scholar
16. Bellack AS, Gold JM, Buchanan RW: Cognitive rehabilitation for schizophrenia: problems, prospects, and strategies. Schizophr Bull 1999; 25:257–274Crossref, Medline, Google Scholar
17. Friedman JI, Harvey PD, McGurk SR, White L, Parrella M, Raykov T, Coleman T, Adler DN, Davis KL: Correlates of change in functional status of institutionalized geriatric schizophrenic patients: focus on medical comorbidity. Am J Psychiatry 2002; 159:1388–1394Link, Google Scholar
18. Addington J, McCleary L, Munroe-Blum H: Relationship between cognitive and social dysfunction in schizophrenia. Schizophr Res 1998; 34:59–66Crossref, Medline, Google Scholar
19. Evans JD, Heaton RK, Paulsen JS, Palmer BW, Patterson T, Jeste DV: The relationship of neuropsychological abilities to specific domains of functional capacity in older schizophrenia patients. Biol Psychiatry 2003; 53:422–430Crossref, Medline, Google Scholar
20. Flaum MA, Andreasen NC, Arndt S: The Iowa prospective longitudinal study of recent-onset psychoses. Schizophr Bull 1992; 18:481–490Crossref, Medline, Google Scholar
21. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615–623Crossref, Medline, Google Scholar
22. Andreasen NC: PSYCH-BASE. Iowa City, University of Iowa, 1989Google Scholar
23. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
24. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
25. Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M: Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch Gen Psychiatry 1995; 52:341–351Crossref, Medline, Google Scholar
26. Heinrichs RW, Zakzanis KK: Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998; 12:426–445Crossref, Medline, Google Scholar
27. Lezak MD: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
28. Rey A: L’examen clinique en psychologie. Paris, Presse Universitaire de France, 1958Google Scholar
29. Wechsler D: The Wechsler Memory Scale—Revised. San Antonio, Tex, Psychological Corp, 1987Google Scholar
30. Wechsler D: The Wechsler Adult Intelligence Scale—Revised (WAIS-R) Manual. Cleveland, Psychological Corp, 1981Google Scholar
31. Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, Ariz, Neuropsychology Press, 1985Google Scholar
32. Golden CJ: Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Wood Dale, Ill, Stoelting Co, 1978Google Scholar
33. Talland GA, Schwab RS: Performance with multiple sets in Parkinson’s disease. Neuropsychologia 1964; 2:45–53Crossref, Google Scholar
34. Heaton RK: The Wisconsin Card Sorting Test Manual. Odessa, Fla, Psychological Assessment Resources, 1981Google Scholar
35. Shipley WC: Institute of Living Scale. Los Angeles, Western Psychological Services, 1946Google Scholar
36. Benton AL, Hamsher KD: Multilingual Aphasia Examination. Iowa City, University of Iowa, 1976Google Scholar
37. Benton AL, Hamsher KD, Rey GJ, Sivan AB: Multilingual Aphasia Examination. Iowa City, AJA Associates, 1994Google Scholar
38. Rey A: L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie 1941; 28:286–340Google Scholar
39. Osterrieth PA: Le test de copie d’une figure complexe: contribution a l’étude de la perception et de la mémoire. Archives de Psychologie 1944; 30:206–356Google Scholar
40. Benton AL, Hannay HJ, Varney NR: Visual perception of line direction in patients with unilateral brain disease. Neurology 1975; 25:907–910Crossref, Medline, Google Scholar
41. Benton AL, Sivan AB, Hamsher KD, Varney NR, Spreen O: Contributions to Neuropsychological Assessment: A Clinical Manual. New York, Oxford University Press, 1994Google Scholar
42. Tabachnick BG, Fidell LS: Using Multivariate Statistics, 4th ed. Boston, Allyn & Bacon, 2001Google Scholar
43. Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA: Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56:241–247Crossref, Medline, Google Scholar
44. DeSisto MJ, Harding CM, McCormick RV, Ashikaga T, Brooks GW: The Maine and Vermont three-decade studies of serious mental illness, I: matched comparison of cross-sectional outcome. Br J Psychiatry 1995; 167:331–338Crossref, Medline, Google Scholar
45. Wing JK: Comments on the long-term outcome of schizophrenia. Schizophr Bull 1988; 14:669–673Crossref, Medline, Google Scholar
46. Penn DL, Mueser KT, Spaulding W, Hope DA, Reed D: Information processing and social competence in chronic schizophrenia. Schizophr Bull 1995; 21:269–281Crossref, Medline, Google Scholar
47. O’Leary DS, Flaum M, Kesler ML, Flashman LA, Arndt S, Andreasen NC: Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. J Neuropsychiatry Clin Neurosci 2000; 12:4–15Crossref, Medline, Google Scholar
48. Sharma T, Harvey PD: Cognition in Schizophrenia: Impairments, Importance and Treatment Strategies. New York, Oxford University Press, 2000Google Scholar
49. Pilling S, Bebbington P, Kuipers E, Garety P, Geddes J, Martindale B, Orbach G, Morgan C: Psychological treatments in schizophrenia, II: meta-analyses of randomized controlled trials of social skills training and cognitive remediation. Psychol Med 2002; 32:783–791Medline, Google Scholar
50. Kurtz MM, Moberg PJ, Gur RC, Gur RE: Approaches to cognitive remediation of neuropsychological deficits in schizophrenia: a review and meta-analysis. Neuropsychol Rev 2001; 11:197–210Crossref, Medline, Google Scholar
51. Benedict RH, Harris AE, Markow T, McCormick JA, Nuechterlein KH, Asarnow RF: Effects of attention training on information processing in schizophrenia. Schizophr Bull 1994; 20:537–546Crossref, Medline, Google Scholar



