Perspectives of Patients With Schizophrenia and Psychiatrists Regarding Ethically Important Aspects of Research Participation
Abstract
OBJECTIVE: Significant controversy surrounds the ethics of psychiatric research. Nevertheless, few data have been gathered to improve our understanding of how individuals with serious mental illness and psychiatrists view ethically important aspects of biomedical research participation. METHOD: The authors assessed views of clinically diagnosed patients with schizophrenia from three sites by means of structured interviews and views of psychiatrists at two sites with written surveys regarding attitudes affecting motivation to participate in biomedical research, attitudes related to autonomy and influences on participation decisions, and attitudes toward the inclusion of vulnerable populations in research. The schizophrenia patients were asked to indicate their personal views; the psychiatrists were asked to provide their personal views and to predict schizophrenia patients’ views. Responses were compared by using repeated measures multivariate analysis of variance. RESULTS: Sixty-three patients with schizophrenia and 73 psychiatry faculty and residents participated. Overall, responses to 23 rated attitudes revealed remarkably similar rank orders and several areas of agreement between patients and psychiatrists. Both groups strongly supported schizophrenia research and autonomous decision making by participants. They saw helping others and helping science as important reasons for protocol participation. Patients endorsed the feeling of hope associated with research involvement, a perspective underestimated by psychiatrists. Psychiatrists also underestimated the patients’ acceptance of physician, investigator, and family influences on participation decisions. Psychiatrists agreed more strongly than patients that vulnerable populations should be included in research. CONCLUSIONS: This study helps to characterize previously neglected attitudes of psychiatric patients and clinicians toward ethically important aspects of biomedical research participation. Schizophrenia patients offered highly discerning views, and interesting similarities and differences emerged in comparing responses of patients and psychiatrists.
Under what circumstances is it ethical for individuals suffering from schizophrenia and other serious mental illnesses to participate in clinical research? This single question has recently occasioned tremendous controversy (1–7). Opponents of psychiatric research argue that current safeguards, such as institutional review and informed consent, are not sufficient to protect mentally ill participants from scientific exploitation (8, 9). Proponents, however, speak of the great need to improve our understanding of mental illnesses, the immediate and future benefits of protocol involvement, and the injustice and disrespect associated with scientific neglect of vulnerable populations (1–6).
Although the bioethics and biomedical research literatures are extensive, little empirical work on the ethics of psychiatric research has been conducted (3, 7, 10–14). What data exist relate primarily to informed consent—the philosophical and legal doctrine upon which ethical experimentation is based (3, 5, 7, 10, 15–19). The doctrine of informed consent derives from the fundamental concept of respect for persons, and it requires that all individuals truly understand and freely make choices about intrusions on their bodies and minds, including acceptance of medical treatments or personal involvement in biomedical experimentation (17, 20, 21). Informed consent studies comparing individuals diagnosed with mental illnesses (e.g., schizophrenia and depressive disorders) to individuals with medical illnesses (e.g., cardiac disease) have demonstrated that those with psychiatric disorders have greater difficulty with consent decision making both in clinical care and research (3, 7, 15). Moreover, the severity of symptoms and cognitive deficits associated specifically with schizophrenia and with Alzheimer’s disease has been shown to adversely affect capacity for informed consent (3). There is reason for optimism, however, because preliminary data clearly suggest that efforts to treat psychiatric patients’ symptoms and to enhance information disclosure procedures do lead to significant improvements in clinical care and research consent (3, 7). Findings also suggest that attributes of the research context, involvement of alternative decision makers, underlying values of patients, and the nature and quality of the investigator-participant relationship may greatly influence informed consent decisions of individuals with mental illness (3, 11, 12, 18). Many other important ethical factors in psychiatric research remain unstudied and poorly understood (1, 3, 7, 11, 18).
Investigators, federal policymakers, bioethicists, and scientific leaders have contributed thoughtfully and passionately to the national discussion of challenging ethics problems in psychiatric research (1, 5, 6, 22, 23). Curiously absent from these deliberations, however, have been the views of schizophrenia patients whose lives may be greatly affected by involvement in experimental protocols. Similarly, we found no data regarding the views of psychiatrists who care for individuals with mental illness and are instrumental in referring their patients to protocols. To address this gap, we examined the perspectives of schizophrenia patients and psychiatrists regarding ethically important aspects of clinical research participation in key domains. We hypothesized that schizophrenia patients would express measurable and meaningful views with respect to 1) attitudes affecting motivation to participate in biomedical research, 2) acceptability of clinicians’ and family members’ influence on patients’ protocol enrollment decisions, and 3) the inclusion of vulnerable groups in biomedical research. We further hypothesized that psychiatrists’ personal views, their predictions regarding schizophrenia patients’ views, and schizophrenia patients’ actual views would differ in a discernible pattern.
METHOD
Eighty-four patients with a clinical diagnosis of schizophrenia (i.e., chronically mentally ill individuals who were candidates for psychiatric protocol referral) were invited to participate in a structured interview for our study. Patients were recruited from the University of New Mexico Mental Health Center, the Albuquerque Veterans Administration (VA) Medical Center, and Johns Hopkins University. Patients were classified as “protocol” if they had ever participated in a biomedical research study (on the basis of patient report and chart verification), and they were classified as “nonprotocol” if there was no history of past participation. By design, we recruited half protocol and half nonprotocol patients for our study. All 88 attending psychiatric faculty and resident psychiatrists at the University of New Mexico and the Albuquerque VA Medical Center were asked to complete a written survey.
Study instruments were developed in part on the basis of the Structured Interview Study from the President’s Commission investigation of the human radiation experiments (18) and the clinical case vignettes of Sachs et al. (24). The interview instrument for patients was extensively pilot-tested and assessed background information, attitudes about biomedical research, and reactions to vignettes describing biomedical research studies. Protocol patients responded to questions about their personal research experiences, and nonprotocol patients were asked parallel questions to predict how they would respond to features of a biomedical protocol. Response formats included categorical, 5-point Likert ratings, and narratives. Patients were shown large, clearly labeled rating scales as a visual aid. An 11-page written survey asked psychiatrists to indicate their personal attitudes on the same questions asked of patients, and they were also asked to predict responses of “schizophrenia patients in general.”
The institutional review board at each site approved the study. All inpatients and outpatients diagnosed with schizophrenia were eligible for participation, but only those assessed by their psychiatrists as capable of decision making and sufficiently stable to tolerate a “minimal risk” 1–2 hour interview were identified as potential participants. They were then invited to participate by a trained interviewer (25), who explained the purpose and procedure of the study and obtained verbal and written informed consent. Recruitment occurred continuously until the established group size was obtained. Patient participants were given $25 for their effort. Psychiatrist participants received written and verbal information regarding the study. All psychiatrists and psychiatric trainees at the University of New Mexico and the Albuquerque VA Medical Center were sent the written survey with no compensation. Three mailings were performed to achieve a high response rate.
Data presented here are responses to 23 Likert-scaled attitude items and participant background information. The views of patients were compared to personal views of psychiatrists and to psychiatrists’ predictions of patient views with two sets of orthogonal repeated measures multivariate analyses of variance (MANOVAs). Attitude items were a repeated measures factor, and rater (patient personal view, psychiatrist personal view, or psychiatrist prediction of patients) was a between-subjects factor. Initial models tested a variety of other factors, such as protocol status, site, status of psychiatrist as attending or resident, and various patient and physician characteristics, for effects, but all were excluded from reported analyses to increase statistical power because they produced nonsignificant results.
Conceptual understanding and exploratory factor analyses organized the 23 attitudes into three sets: 1) attitudes affecting motivation to participate in biomedical research (eight items), 2) attitudes related to the influences on research participation decisions (seven items), and 3) attitudes related to the participation of vulnerable populations in research (eight items). Five of the eight attitudes affecting motivation to participate in research (items 1, 3, 20, 21, 23), all of the eight attitudes related to influence on decisions, and all of the seven attitudes related to vulnerable populations each produced coherent factors with respective alpha coefficients of 0.58, 0.74, and 0.73 as estimates of reliability. Individual items for each set were subjected separately as repeated measures to two MANOVA models that compared patients’ views to psychiatrists’ views or patients’ views to psychiatrists’ predictions. Means were contrasted with Fisher’s least significant difference (p<0.05) only if the overall F test for an effect was significant for a given model.
RESULTS
Characteristics of Respondents
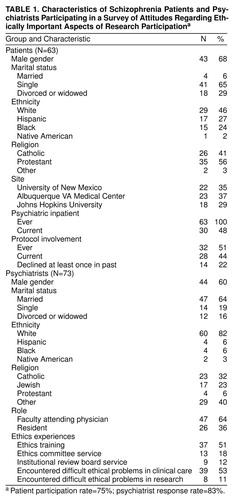
Patient participants (N=63, participation rate=75%) (table 1) averaged almost 43 years of age (range=21–64), and most (68%) were men. On average, they had participated in 2.4 protocols. Roughly 44% (N=28) were currently involved in a clinical protocol, and an additional 7% had been in the past. Of those who had ever participated, 16% had declined participation at least once. Of those who, before our study, had never participated, 28% had refused participation.
All had been psychiatric inpatients (median admissions=6), and 48% were hospitalized during this study. All were currently prescribed a psychotropic medication (92%, an antipsychotic; 44%, side effect medication; 37%, a benzodiazepine; 26%, an antidepressant; and 12%, another mood stabilizer). Their most recent General Adaptive Functioning scores ranged from 20 to 70 (mean=37, SD=12). In rating their own problems with their mental health in the past 3 months, roughly one-fifth rated each point on a 5-point scale (1=none, 5=a lot; mean=3.20, SD=1.44). Most (67%) reported hearing voices in the past 3 months (5-point scale: 1=never, 5=all the time; mean=2.78, SD=1.57), and most (79%) also indicated feeling frightened in the past 3 months (same scale; mean=2.90, SD=1.40). Only 67% reported believing that they actually had schizophrenia, although 95% said that they had been told this diagnosis by a doctor. No significant differences were found on these variables in comparing the protocol and nonprotocol groups. Although patient reports of hearing voices, experiencing fear, and experiencing problems with mental health over the past 3 months were intercorrelated (mean r=0.36, N=63, p<0.005), none of these symptom reports or other clinical characteristics consistently predicted the attitudes reported here.
Seventy-three physician participants (83% response rate) completed written surveys (table 1). All had received M.D. degrees, and two also possessed a Ph.D. Most were faculty attending physicians (N=47, 85% response rate), and others were in the psychiatry residency program (N=26, 74% response rate). Men represented 60% of respondents. They averaged 41 years of age and 10 years of clinical work experience. They typically spent 75% (median) of their time in clinical activities, with 46% overall (67% of faculty) spending at least 10% of their time on research. Over half reported formal ethics training (51%), but few had served on patient care ethics committees (18%) or an institutional review board (12%). Most (53%) reported encountering difficult ethical problems in clinical work, although relatively few (11%) reported such problems in research.
Attitudes Affecting Motivation to Participate in Biomedical Research
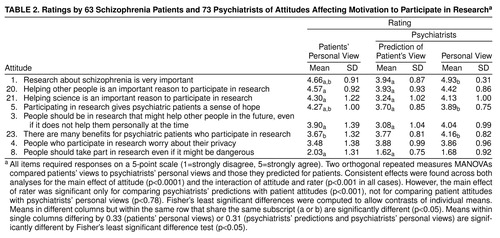
The eight attitudes (table 2) related to motivation to participate in research were rated differently (attitude main effects in both MANOVAs: F>67, df=7, 119, p<0.0001). Patients strongly supported the importance of research about schizophrenia, helping other people as a reason to participate, helping science as a reason to participate, and gaining a sense of hope through participation. Moderate support was shown for helping other people in the future, for many benefits gained from participation, and for worrying about privacy when participating. Finally, most patients expressed opposition to the statement that people should take part in research even it might be dangerous.
A repeated measures MANOVA compared patient attitudes to those predicted for them by psychiatrists. The main effect for rater (F=20.35, df=1, 125, p<0.0001) showed that on average, across attitudes, patients supported research participation more than psychiatrists predicted. Both main effects were qualified by the interaction of attitude by rater (F=8.99, df=7, 119, p<0.0001), revealing that psychiatrists underestimated patients’ support for the five most highly rated attitudes and the lowest rated item. There was a large effect for the average underestimation (Cohen’s d=0.70) (26).
A second repeated measures MANOVA compared patient attitudes to personal views of psychiatrists. The absence of a main effect for rater (F=0.08, df=1, 127, p<0.78) showed that patients and psychiatrists offered similar responses, on average, to attitude items. The interaction of rater by attitude (F=4.97, df=7, 121, p<0.001) revealed that psychiatrists expressed greater support for the statements that research about schizophrenia is very important and that there are many benefits for psychiatric patients who participate in research. Psychiatrists expressed less support than did the patients for believing that participating in research gives psychiatric patients a sense of hope (mean d=0.38, a moderate effect).
Attitudes Related to Influences on Decisions to Participate in Research
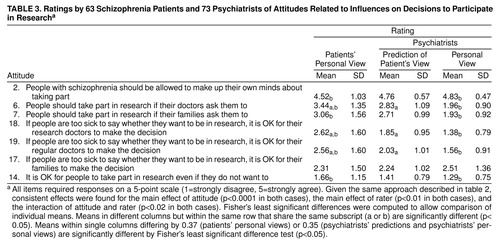
These seven attitudes (table 3) were also rated differently (attitude main effects in both MANOVAs: F>114, df=7, 119, p<0.0001). Patients strongly supported the statement that people with schizophrenia should be allowed to make up their own minds about taking part in research. Slight support was shown for the statement that people should take part in research if their doctors ask them to. The statement that people should take part in research if their families ask them to was rated neutrally. Patients expressed weak opposition to the statements that it is all right for research doctors to make the decision if people are too sick to decide whether to be in research, that it is all right for patients’ regular doctors to make the decision if people are too sick to decide, and that it is all right for patients’ families to make the decision if people are too sick to decide. Finally, patients strongly opposed the statement that it is all right for people to take part in research even if they do not want to.
A repeated measures MANOVA compared patient attitudes to those predicted for them by psychiatrists. The main effect for rater (F=6.88, df=1, 126, p=0.01) revealed that on average, patients agreed more with the attitudes than was predicted by psychiatrists. Main effects were qualified by the interaction of attitude by rater (F=2.81, df=6, 121, p<0.02), which indicated that psychiatrists inaccurately predicted patients’ attitudes to doctors’ influences on patients’ decisions but were accurate in assessing patients’ views about personal and family influence in participation decisions (mean d=0.62).
A second repeated measures MANOVA compared patient attitudes to the personal views of psychiatrists. The main effect for rater (F=29.96, df=1, 127, p<0.0001) indicated that patients’ and psychiatrists’ personal attitudes, on average, differed significantly. The interaction of rater by attitude (F=4.97, df=7, 121, p<0.001) revealed that psychiatrists and patients did agree on the statement that it is all right for families to decide about patients’ research participation if they are too sick to decide. Psychiatrists were more supportive of schizophrenia patients being allowed to make up their own minds about research participation than were the patients themselves. Psychiatrists were also more opposed than patients to people taking part in research against their wishes (mean d=0.30). Psychiatrists were more opposed than patients to the influence of others on patients’ decisions (mean d=0.99, a very large effect).
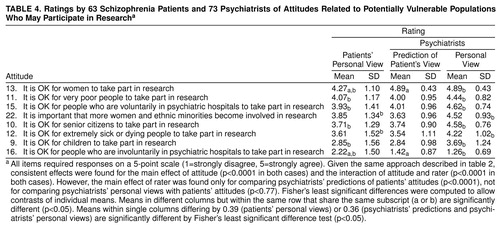
Attitudes Related to the Participation of Potentially Vulnerable Populations in Research
Patients rated these eight attitudes (table 3) very differently (attitude main effects in both MANOVAs: F>84, df=7, 120, p<0.0001). The most strongly supported statements were that it is all right for women and for very poor people to take part in research. Patients showed more moderate support for the statements that is all right for voluntary psychiatric hospital patients, senior citizens, and extremely sick or dying people to take part in research and that it is important that more women and ethnic minorities become involved in research. Patients expressed moderate opposition to the statement that it is all right for children to take part in research and greatest opposition to the statement about involuntary psychiatric hospital patients taking part in research.
A repeated measures MANOVA compared patient attitudes concerning vulnerable populations to attitudes predicted for them by psychiatrists. The absence of a main effect for rater (F=0.09, df=1, 126, p<0.77) revealed that on average, psychiatrists accurately predicted patients’ attitudes. An interaction of attitude by rater (F=5.79, df=7, 120, p<0.0001) was found, however, indicating that psychiatrists were not accurate in predicting certain attitudes (e.g., patients’ support for women taking part in research and patients’ opposition to involuntary psychiatric hospital patients taking part [mean d=0.71]) but were very accurate in predicting the other attitudes.
A second repeated measures MANOVA compared patient attitudes to the personal views of psychiatrists. A main effect for rater (F=17.45, df=1, 129, p<0.0001) revealed that on average, psychiatrists offered greater agreement than did patients for the statements about vulnerable populations taking part in research. Yet, the interaction of rater by attitude (F=9.55, df=7, 123, p<0.0001) showed that psychiatrists opposed more strongly (d=0.87) than patients the statement that it is all right for involuntary psychiatric patients to participate in research. For the other attitudes, psychiatrists were much more supportive than patients (mean d=0.61).
DISCUSSION
This study documents the previously neglected perspectives of schizophrenia patients and of psychiatrists regarding several ethically important aspects of biomedical research participation. Overall, the patients and the clinicians in our study strongly endorsed schizophrenia research. They clearly indicated that helping science and helping others were compelling reasons for research participation. Individuals with schizophrenia, furthermore, saw research participation as offering psychiatric patients a sense of hope—a perspective that was not fully predicted by psychiatrists. The schizophrenia patients and the clinicians both valued autonomous decision making in research participation, but the patients were much more accepting than were psychiatrists regarding the influence of physician-investigators, personal physicians, and family members on such decisions. Both patients and clinicians opposed participation in dangerous research, and they also did not support participation by those who were unwilling or who were undergoing involuntary treatment. Finally, psychiatric patients and clinicians agreed that it was important for more women and minorities to become involved in research. Overall, though, psychiatrists showed much stronger support than did our participants with schizophrenia regarding the inclusion of what have been defined historically as potentially vulnerable groups. In this section, we briefly explore the interpretations, implications, and limitations of our findings.
Schizophrenia Patients as Discerning Individuals
The feasibility of this study is itself a critical finding. When asked, schizophrenia patients were able to offer substantive and discerning perspectives on important issues that bear on the ethical conduct of biomedical research. These attitudes were measurable, and they were often surprisingly similar to the views expressed by psychiatrists. In addition, a fifth of our study group reported having previously refused research participation, and a fourth of those approached for this study declined, indicating both the ability and willingness of people with schizophrenia to exercise personal autonomy related to research enrollment. It is noteworthy that these findings are not attributable to selection of only the healthiest of schizophrenia patients—although all of our participants were judged by their clinicians to be capable of decision making, nearly half of the participants in our study had symptoms severe enough to be voluntary inpatients at the time of our interview, and all required psychotropic medication. Indeed, all had been psychiatric inpatients at some point in the past, typically with multiple hospitalizations. In sum, the people with schizophrenia in this study exhibited considerable strengths and sophistication despite their having a serious mental illness and despite the complexity of the issues presented.
Although this study is preliminary in nature, the feasibility of this work has two implications for psychiatric research. First, since our study’s patient participants were able to express clear views on biomedical research, it is possible that other psychiatric patients’ abilities to appreciate subtle aspects of research participation decisions may be greater than is often recognized. If this is true, assertions of the absolute vulnerability of people with mental illness and negative assessments of capacity for informed consent should not be based solely on the presence of a diagnosed mental disorder (1, 3). This conclusion is consistent with preliminary work showing, for instance, that efforts to address symptom severity and additional efforts related to the dialogue, procedures, relationships, and context of the informed consent process may help to reverse decision-making limitations of some individuals (1–4, 7, 12, 14, 24). Although this may, on the surface, appear to suggest the need for a more burdensome consent process, one potential benefit may be the ability to include, in an ethically sound manner, some individuals who might otherwise be presumed incapable of providing informed consent for relatively elaborate or risky research protocols. Second, the fact that we know little about the perspectives of psychiatric research participants is apparently not due to their inability or unwillingness to express themselves. Further inquiry to explore the experiences, preferences, and concerns of people with mental illness is imperative so that they may have greater voice in the conduct of psychiatric research in the future; this would fulfill the first ethical principle governing human experimentation—respect for persons (17).
Values of Schizophrenia Patients and Psychiatrists
Specific values underlying biomedical research were endorsed by both schizophrenia patients and psychiatrists (17, 20, 21, 27). A clear commitment to science (item 1) and an affirmation of autonomy (items 2, 6, 7, 9, and 14–19) were found in participant responses. Similarly, altruism was seen as an important motivation for research involvement by both patients and clinicians (items 3, 20, and 21). An orientation toward beneficence, the duty to “do good” and help patient well-being, was seen in psychiatrists’ endorsement of the benefits of protocol involvement (item 23) and in the patients’ belief that research participation provides “a sense of hope” to psychiatric patients (item 5). Nonmaleficence, the duty to “do no harm,” and paternalism, a principle linked to beneficence, nonmaleficence, and autonomy, were seen in concern about dangerous research, the inclusion of more highly vulnerable populations, substitute decision making, and involvement of the extremely sick or dying in research (items 8–13, 15–19, and 22). Concern for justice was reflected in support for the inclusion of understudied groups in research and other related attitudes (items 10, 11, 13, and 22). Participants’ concern about privacy was essentially neutral (item 4). With respect to fidelity, defined as faithfulness to the well-being and interests of the patient, respondents were also relatively neutral on the items exploring the desired level of influence of doctors on research enrollment decisions (items 18 and 19). The absence of a difference in responses regarding the personal doctor and the research doctor as a substitute decision maker may reveal trust in mental health professionals generally, but it may also indicate a limited understanding of the potential role conflicts of the clinical investigator (28).
That schizophrenia patients and psychiatrists share many common values is an important constructive finding of this study. The confluence of values in support of science, autonomy, and altruism, however, may also create a heretofore unrecognized motivational “vulnerability” predisposing psychiatric patients, as with other special populations (3, 7, 29, 30, 31), to enroll in research that is not in their best interests. This is a challenging issue requiring careful study.
Comparing Perspectives of Schizophrenia Patients and Psychiatrists
Like the psychiatrists we surveyed, we predicted that the perspectives of psychiatric patients and clinicians would generally differ. And, also like the psychiatrists we studied, we were only partly correct. Indeed, striking agreement existed in many of the responses of schizophrenia patients and psychiatrists, and the overall rank orders assigned to items were nearly identical, as reflected in table 2, table 3, and table 4. Nevertheless, three different patterns did emerge in comparative analyses of the three attitude domains we examined. First, with respect to attitudes toward research participation, the personal views of psychiatrists quite closely resembled those of schizophrenia patients, and yet psychiatrists often were inaccurate in their predictions of patients’ motivations to enroll in clinical research. In the second domain, fairly dramatic differences existed between the personal views of schizophrenia patients and psychiatrists on most items concerning influences on research participation decisions. Although they often appreciated that differences existed, psychiatrists often underestimated the degree to which they disagreed with schizophrenia patients’ views. Finally, when schizophrenia patients and psychiatrists were asked about participation of various vulnerable groups in clinical research, their attitudes differed in degree on every item. Yet, the psychiatrists’ assessments of patients’ views were remarkably accurate on most items in this domain.
The presence of these different patterns points to the necessity of exploring, not presuming, the perspectives held by individual patients with respect to clinical research. To consider just one example, psychiatrists’ personal views and their predictions of patients’ views were strongly weighted toward personal autonomy. Schizophrenia patients did, in fact, endorse autonomy, but they expressed greater acceptance of physicians’ and families’ influence on personal decision making. This difference merits careful consideration. On a practical level, physicians may not think to include family members in research participation decisions. Perhaps more important, however, physicians may not fully appreciate how influential their recommendations may be to prospective protocol participants. Fortunately, our findings also suggest that there may be more common ground between mental health professionals and patients than is presumed.
Limitations
This study provides empirical evidence to help clarify issues that have triggered great controversy in both society and science. However, it measured self-reported attitudes, not actual decisions or behaviors, with a new instrument in a new area of inquiry. Our selection and recruitment processes were designed to mirror those of biomedical research protocols; we sought to capture perspectives of potential recruits for schizophrenia studies on the basis of a clinically documented diagnosis but did not include a formal diagnostic interview or a standard measure of symptom severity. In addition, we excluded individuals of questionable decisional capacity whose perspectives it would be valuable to understand. Similarly, we do not know how the patients who refused to participate in our study would have responded to the attitudinal items. Psychiatrists in our group were from a university setting and had limited research involvement overall. Other, more community- or research-oriented psychiatrists might have responded differently. Site differences were not found in our results, however, supporting generalizability of our data. Finally, psychiatrists’ predictions related to people with schizophrenia generally and do not necessarily indicate accuracy or inaccuracy in assessments of individual patients. For these reasons, we offer our findings as a modest initial effort in an underdeveloped area of research.
CONCLUSIONS
The discussion of biomedical research ethics may be enriched by a greater understanding of the personal perspectives of the people who are most intimately affected by human experimentation: individuals whose illnesses and suffering make biomedical research a societal imperative. In this study, the schizophrenia patients we interviewed revealed meaningful views of biomedical research participation that have important ethical implications, and they were able to articulate these views when asked. Further, a number of core values were recognized and shared by schizophrenia patients and psychiatrists—most notably, belief in the importance of science, in the desire to help others, and in autonomous decision making. Nevertheless, differences in perspectives also exist between patients and clinicians. The process of identifying these similarities and differences promises to help inform clinical research practices of investigators and to enhance the experiences of study participants who give so generously of themselves in the course of science.
Received Jan. 13, 1999; revision received June 2, 1999; accepted June 8, 1999. From the Psychiatric Empirical Ethics Group, Department of Psychiatry, University of New Mexico School of Medicine. Address reprint requests to Dr. Roberts, Psychiatric Empirical Ethics Group, Department of Psychiatry, University of New Mexico School of Medicine, 2400 Tucker N.E., Albuquerque, NM 87131. Supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression and by career development grant IKO 2 AI0173801 from NIMH (Dr. Roberts). The authors thank Graham Redgrave, M.D., Constantine Lyketsos, M.D., Jose Canive, M.D., Fernando Torres, M.D., Brian Roberts, M.D., David Graeber, M.D., Kate Canfield, M.D., Tom DiMatteo, M.D., Russell Horwitz, B.A., Samuel Keith, M.D., Joel Yager, M.D., John Lauriello, M.D., Melinda Rogers, B.S., Melissa Carruth, B.A., and Elma Landgraf, R.N., for their contributions.
 |
 |
 |
 |
1. Expert Panel Report to the National Institutes of Health: Research Involving Individuals With Questionable Capacity to Consent: Ethical Issues and Practical Considerations for Institutional Review Boards (IRBs). Bethesda, Md, NIH, Feb 1998Google Scholar
2. Dresser R: Mentally disabled research subjects: the enduring policy issues. JAMA 1996; 276:67–72Crossref, Medline, Google Scholar
3. Roberts LW: Ethics of psychiatric research: conceptual issues and empirical findings. Compr Psychiatry 1998; 39:99–110Crossref, Medline, Google Scholar
4. Bonnie RJ: Research with cognitively impaired subjects. Arch Gen Psychiatry 1997; 54:105–111Crossref, Medline, Google Scholar
5. Shore D: Ethical principles and informed consent: an NIMH perspective. Psychopharmacol Bull 1996; 32:7–10Medline, Google Scholar
6. Appelbaum PS: Rethinking the conduct of psychiatric research. Arch Gen Psychiatry 1997; 54:117–120Crossref, Medline, Google Scholar
7. Roberts LW, Roberts BR: Psychiatric research ethics: an overview of evolving guidelines and current ethical dilemmas in the study of mental illness. Biol Psychiatry 1999; 46:1025–1038Google Scholar
8. Shamoo AE (ed): Ethics in Neurobiological Research With Human Subjects: The Baltimore Conference in Ethics. New York, Gordon & Breach, 1997Google Scholar
9. Hilts PJ: Psychiatric researchers under fire. New York Times, May 14, 1998, p F1Google Scholar
10. Roth LH, Appelbaum PS, Lidz CW, Benson P, Winslade WJ: Informed consent in psychiatric research. Rutgers Law Rev 1987; 39:425–441Medline, Google Scholar
11. Meisel A, Roth LH: What we do and do not know about informed consent. JAMA 1981; 246:2473–2477Google Scholar
12. Lidz CW, Meisel A, Zerubavel E, Carter M, Sestak RM, Roth LH: Informed Consent: A Study of Decision Making in Psychiatry. New York, Guilford Press, 1984Google Scholar
13. Stanley B, Sieber JE, Melton GB: Empirical studies of ethical issues in research: a research agenda. Am Psychol 1987, 42:735–741Google Scholar
14. Roberts LW, Solomon Z, Roberts BB, Keith SJ: Ethics in psychiatric research: resources for faculty development and resident education. Academic Psychiatry 1998; 22:1–20Crossref, Medline, Google Scholar
15. Appelbaum PS, Grisso T: The MacArthur Treatment Competence Study I, II, III. Law Hum Behav 1995; 19:105–174Crossref, Medline, Google Scholar
16. Cassileth BR, Zupkis RV, Sutton-Smith K, March V: Informed consent: why are its goals imperfectly realized? N Engl J Med 1980; 302:896–900Google Scholar
17. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research: The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Washington, DC, US Government Printing Office, 1979Google Scholar
18. The Human Radiation Experiments: Final Report of the President’s Advisory Committee. New York, Oxford University Press, 1996Google Scholar
19. Levine RJ: Ethics and Regulation of Clinical Research, 2nd ed. Baltimore, Urban & Schwartzenberg, 1986Google Scholar
20. American Medical Association: Code of Medical Ethics Current Opinions With Annotations. Chicago, AMA, 1994Google Scholar
21. Beauchamp T, Childress J: Principles of Biomedical Ethics, 4th ed. New York, Oxford University Press, 1994Google Scholar
22. Maechling C, Pellegrino ED, Shimm DS, Spece RG (Institute of Medicine): Integrity in scientific research, in Emerging Policies for Biomedical Research. Edited by Kelley WN, Osterweis M, Rubin ER. Washington, DC, Association for Academic Health Centers, 1993, pp 127–142Google Scholar
23. Research Involving Persons With Mental Disorders That May Affect Decision Making Capacity, vol 1: Report and Recommendations of the National Bioethics Advisory Commission. Washington, DC, National Bioethics Advisory Commission, Dec 1998Google Scholar
24. Sachs GA, Stocking CB, Stern R, Cox DM, Hougham G, Sachs RS: Ethical aspects of dementia research: informed consent and proxy consent. Clin Res 1994; 42:403–412Medline, Google Scholar
25. Gordon R. Basic Interviewing Skills. Itasca, Ill, FE Peacock, 1992Google Scholar
26. Cohen J: Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, Lawrence Erlbaum Associates, 1987Google Scholar
27. Kahn JP, Mastroianni AC, Sugarman J: Beyond Consent: Seeking Justice in Research. New York, Oxford University Press, 1998Google Scholar
28. Appelbaum PS, Roth LH, Lidz CW: The therapeutic misconception: informed consent in psychiatric research. Int J Law Psychiatry 1982; 5:319–329Crossref, Medline, Google Scholar
29. Daugherty C, Ratain MJ, Grochowski E, Stocking C, Kodish E, Mick R, Siegler M: Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol 1995; 13:1062–1072Google Scholar
30. Harth SC, Thong YH: Parental perceptions and attitudes about informed consent in clinical research involving children. Soc Sci Med 1995; 40:1573–1577Google Scholar
31. Brody J, Gluck JP, Aragon F: Participants’ understanding of the process of psychological research: informed consent. Ethics and Behavior 1997; 7:285–298Crossref, Medline, Google Scholar