Low Dopamine D2 Receptor Binding in Subregions of the Thalamus in Schizophrenia
Abstract
OBJECTIVE: Several structural and functional brain imaging studies have pointed to a disturbance of thalamic subnuclei in patients with schizophrenia. The dopamine hypothesis of schizophrenia has, however, not been thoroughly examined in terms of this complex structure, which has connections with most brain regions of central interest in schizophrenia research. The aim of the present study was to examine dopamine D2 receptor binding in subregions of the thalamus in patients with schizophrenia. METHOD: The authors used positron emission tomography and the radioligand [11C]FLB457 to examine dopamine D2 receptor binding in thalamic subregions of 10 drug-naive patients with schizophrenia. Binding potential was calculated by the reference tissue method and used as an index for dopamine D2 receptor binding. Comparisons were made with 19 healthy subjects. Subregions of interest were defined on individual magnetic resonance images using a percentage-based operational approach. Clinical symptoms were rated by using the Brief Psychiatric Rating Scale (BPRS). RESULTS: The [11C]FLB457 binding potential was lower in the central medial and posterior subregions of the thalamus in patients with schizophrenia. At a functional level, there was a significant negative correlation between binding potential and BPRS positive symptom scores. CONCLUSIONS: The subregions with low D2 receptor binding comprise primarily the dorsomedial nucleus and pulvinar, two important components in circuitries previously suggested in the pathophysiology of schizophrenia. Aberrant dopaminergic neurotransmission in thalamic subregions might be a mechanism underlying positive symptoms in schizophrenia.
The dopamine D2 receptor has long been of central interest in research on the pathophysiology of schizophrenia. Early positron emission tomography (PET) studies focused on the striatum, a region with a high density of D2 receptors. Improvements in PET methodology now allow the examination of low-density dopamine D2 receptor populations in several limbic and cortical regions in which structural or biochemical abnormalities have been reported in schizophrenia. In a recent analysis of cortical and subcortical regions, we found low radioligand binding to dopamine D2 receptors in the anterior cingulate cortex of patients with schizophrenia and a correlation with positive symptom scores (1).
On the other hand, functional abnormality of schizophrenia has also been discussed in terms of thalamic circuitry (2). Imaging studies have consistently revealed smaller thalamic volumes in patients with schizophrenia as well as altered thalamic perfusion and metabolism (2–13). Neuropathological studies have found a reduction in the number of neurons in the mediodorsal nucleus of the thalamus in schizophrenia brains (14–17). Schizophrenia-associated neuronal loss has also been found in the pulvinar (17). A synapse-related protein study reported that the thalamic abnormalities include synaptic disturbances (18).
The thalamus was not included in early maps showing dopaminergic innervation in the rodent brain (19). However, dopamine D2 receptors have more recently been identified in the human thalamus in vitro (20, 21) and in vivo using PET (22–24). The possibility that dopamine D2 receptors in the thalamus are involved in the therapeutic actions of antipsychotics has been supported by PET studies that demonstrated dopamine D2 receptor occupancy in the thalamus by antipsychotic drugs (25). Each of the major 23 subnuclei of the thalamus (26) has a unique set of efferent and afferent projections with different functional implications. In a previous study, we found a tendency toward low density of dopamine D2 receptors in the thalamus (1). However, in that study we focused on the thalamus as a whole and did not take its detailed complexity into account.
The aim of the present PET study was to examine separately dopamine D2 receptor binding in five major subregions of the thalamus. Ten neuroleptic-naive patients with schizophrenia and 19 healthy comparison subjects were examined with the radioligand [11C]FLB457, a substituted benzamide with a very high affinity for dopamine D2 receptors (27). We then investigated whether there were correlations between subregional binding and psychopathology score as assessed with the Brief Psychiatric Rating Scale (BPRS) (28).
Method
Subjects
This study was approved by the Ethics and Radiation Safety Committee of the National Institute of Radiological Sciences, Chiba, Japan. After complete description of the study, written informed consent was obtained from all subjects. The patients were recruited from the outpatient units of five university-affiliated psychiatric hospitals and the psychiatric divisions of general hospitals in the prefectures of Tokyo and Chiba. Ten drug-naive right-handed male patients with schizophrenia (mean age=29.5 years, SD=7.8) who met DSM-IV criteria for schizophrenia or schizophreniform disorder were included. One patient satisfying the criteria for schizophreniform disorder (duration of illness was 1 month at the time of study entry) met the criteria for schizophrenia at 6-month follow-up. His behavioral ratings were evaluated at the time of study entry. Eight of the 11 patients in a previous study (1) had been examined with magnetic resonance imaging (MRI) and could thereby be included in the present analysis that also included two newly recruited patients. The other three patients had refused to participate in the MRI scan (1), and we could not include them in the present analysis. The duration of illness ranged from 1 month to 7 years, with a median of 2 years. Psychopathology was assessed by the 18-item Oxford version of the BPRS translated into Japanese (item score range=0–6 points) (28). Sum scores for positive and negative symptoms were calculated (1, 29), with the positive symptom subscale including the following eight items: conceptual disorganization, mannerisms and posturing, hostility, grandiosity, suspiciousness, hallucinatory behavior, unusual thought content, and excitement. The negative symptom subscale included these three items: emotional withdrawal, motor retardation, and blunted affect. BPRS total scores ranged from 14 to 42 (mean=29.3, SD=8.9), the mean positive symptom score was 14.6 (SD=4.6), and the mean negative symptom score was 5.5 (SD=4.6). The healthy subjects were recruited through notices on bulletin boards at universities and their affiliated hospitals where the patients were diagnosed. The 19 healthy right-handed male comparison subjects were age-matched (mean=29.6 years, SD=7.5). Parental socioeconomic status was determined on the basis of the Hollingshead-Redlich scale, and no significant differences between patients (mean=2.6, SD=0.7) and comparison subjects (mean=2.3, SD=0.5) were found (t=1.30, df=27, p>0.20). The comparison subjects did not meet criteria for any psychiatric or neurological disorder and had no first-degree relatives with neuropsychiatric disorders.
PET and MRI Procedures
The PET system ECAT EXACT HR+ (CTI-Siemens, Knoxville, Tenn.) was used to measure radioactivity in the brain. The field of view of this system is 15.5 cm. To minimize head movement, a head fixation device (Fixster, Stockholm) was used. A transmission scan for attenuation correction was performed using a 68Ge-68Ga source. Acquisitions were performed in three-dimensional mode with the interplane septa retracted. A bolus of 89.5–249.0 MBq (mean=172.5, SD=40.0) of [11C]FLB457 with high specific radioactivity (64.9–534.9 GBq/μmol) was injected intravenously into a cannula inserted in an antecubital vein. The cannula was then flushed by the rapid injection of 20 ml of saline. Radioactivity in brain was measured in a series of scans for 80 minutes starting immediately after the injection. The emission scans were reconstructed with a Hanning filter cutoff frequency of 0.4 (full width at half maximum=7.5 mm). Images from the reconstructed volume were displayed as 67 horizontal sections. MR images were acquired on a Phillips Gyroscan NT, 1.5 tesla. T1-weighted images of the brain were obtained to allow for differentiation between white and gray matter. The scan parameters for 1-mm-thick, three-dimensional T1 images in the transversal plane were as follows: TR/TE=19/10 msec, flip angle=30°, matrix=256×256, field of view=256×256 mm, number of excitations=1.
Quantitative Analysis of [11C]FLB457 Binding
Quantitative analysis was performed using the three-parameter simplified reference tissue model (30, 31). The cerebellum was used as a reference region because it has been shown to be almost devoid of D2 receptors (23, 31). The model provides an estimation of the binding potential, which is defined by the following equation: BP=k3/k4=f2 Bmax/{Kd [1 + Σi Fi/Kdi]}, where k3 and k4 describe the bidirectional exchange of tracer between the free compartment and the compartment representing specific binding, f2 is the “free fraction” of nonspecifically bound radioligand in brain, Bmax is the receptor density, Kd is the equilibrium dissociation constant for the radioligand (32), and Fi and Kdi are the free concentration and the dissociation constant of competing endogenous dopamine, respectively. The model also provides the parameter R1, which represents the ratio of radioligand delivery in the region of interest to that in the reference region (influx ratio).
Thalamic Subdivisions
Regions of interest for five operationally defined subregions of the thalamus were defined on MR images according to a manual tracing technique that has been described in the literature and applied previously for the study of thalamic volumes in schizophrenia (5, 7). The regions of interest were delineated three-dimensionally on MR images and displayed by a distinct color as described previously (33) (Figure 1).
In the first step, the boundaries of the whole thalamus were identified. The mamillary body was used as the anterior boundary. The internal capsule was the lateral boundary, the third ventricle the medial boundary, and the inferior border of the third ventricle the inferior boundary. The posterior boundary was defined as the location where the hemispheres of the thalamus merged under the crux fornix. The superior boundary was the main body of the lateral ventricle (7). In the second step, the thalamus was subdivided into five distinct regions. The thalamus was first divided into medial and lateral parts. A line drawn parallel to the lateral border of the midbrain, the interhemispheric fissure, and the cerebral aqueduct represented the vertical bisection (coronal view in Figure 1). This line was continued through all thalamic slices to create a plane of bisection parallel to the interhemispheric fissure. The individual number of contiguous coronal slices in which the thalamus appeared was then calculated.
The thalamus was divided into anterior, central, and posterior divisions that were defined as fixed percentages of the total number of coronal slices. The anterior and central divisions each contained 40% of the total number of slices, and the posterior division contained 20%. Using this approach, the thalamus was divided into six subregions. The medial and lateral portions of the posterior thalamus were then combined, since they both corresponded to the pulvinar (axial view in Figure 1). In the final step, the regions of interest were linearly transformed using the parameters obtained from the coregistration of the individual MRI and PET images. This was done using SPM 99 (34), with the default parameter option of mutual information (35). After transformation of the regions of interest from MRI to PET, the regional radioactivity of each region of interest was calculated for each frame, corrected for decay, and plotted against time. The average values for regions of interest in the right and left hemisphere were used to increase the signal-to-noise ratio for the calculations. Subdivision of the thalamus and measurement of binding potential values was performed in duplicate by a single investigator (F.Y.) in 10 of the healthy subjects, and intrarater reliability was assessed. High intraclass correlations (ICCs) for subregions were seen for the two sets of measurements (anterior medial: ICC=0.96, anterior lateral: ICC=0.94, central medial: ICC=0.92, central lateral: ICC=0.90, posterior: ICC=0.82).
Morphological analysis was performed on the volume of the region of interest defined on MRI images. The size of the area was calculated, summed across slices, and multiplied by the slice thickness (1 mm), yielding approximate volumes. Intracranial volume was used as a covariate when comparing volumetric measures between the groups.
Statistical Comparisons
The binding potential and influx ratio (R1) values for the whole thalamus were compared between patients and healthy subjects by Student’s t test. The volumes for the whole thalamus were compared between patients and comparison subjects using one-way analysis of covariance (ANCOVA) with the intracranial volume as covariate. Group differences in the binding potential values of the thalamic subregions were compared by using multivariate analysis of variance (MANOVA). Follow-up serial one-way analyses of variance (ANOVAs) were performed to specify regional differences. To examine the influence of regional differences of blood flow and volumes on those of the binding potential values, serial one-way ANOVAs and ANCOVAs with intracranial volume as covariate were performed to specify regional differences of the influx ratios and volumes, respectively. A p value of 0.05 (two-tailed) was chosen as the significance threshold. The relationship between regional binding potential values and BPRS scores (total score as well as positive and negative symptom subscale scores) was evaluated in the correlation analysis. In consideration of the effect of the duration of illness, we examined the relationship of its variables to regional binding potential values. We also evaluated the relationships of age and parental socioeconomic status to the binding potential values in healthy subjects and patients. In the correlation analysis, we used the Pearson correlation method, and p<0.01 [0.05/5] was considered as significant to avoid type I errors due to the multiplicity of statistical analyses.
Results
With regard to the binding potential values for [11C]FLB457 binding in the whole thalamus, there was no significant difference between the two groups (schizophrenia patients: mean=3.31, SD=0.33; comparison subjects: mean=3.54, SD=0.45) (t=1.45, df=27, p>0.15). However, multivariate analyses of the binding potential values in the thalamic subregions showed significant differences between patients with schizophrenia and comparison subjects. Follow-up ANOVAs revealed that the binding potential values in the central medial region and the posterior region were significantly lower in the patients (Table 1, Figure 2).
The influx ratio (R1) for the whole thalamus (schizophrenia patients: mean=0.84, SD=0.50; comparison subjects: mean=0.87, SD=0.57) did not differ significantly between patients and comparison subjects (t=1.62, df=27, p>0.12) nor did it differ in any subregion (Table 1). The volume of the whole thalamus was not significantly different between patients (mean=8.65, SD=1.16) and healthy subjects (mean=8.65, SD=1.19) (F=0.005, df=1, 26, p>0.94), and no significant difference was found for any of the thalamic subregions (Table 1).
For the central medial region and the posterior region there was a statistically significant negative correlation between binding potential and positive symptom subscores on the BPRS (Table 1, Figure 3). There was no significant correlation between binding potential and the BPRS total scores or negative symptom subscore for any region. Further, no significant relationship was observed between regional binding potential values and the duration of illness, age, or parental socioeconomic status in comparison subjects and patients.
Discussion
In a previous PET study, we reported low dopamine D2 receptor binding in the anterior cingulate in patients with schizophrenia and a statistical trend for low binding in the thalamus (1). In the present study with a partly overlapping patient group, a more detailed analysis of the thalamus indicated low [11C]FLB457 binding potential in the central medial and posterior subregions of the thalamus in patients with schizophrenia. Highly elevated levels of dopamine have been found postmortem in the thalamus of schizophrenia (36), and the reduction in binding could be attributed to an increase in endogenous dopamine. However, our previous study indicated that extrastriatal [11C]FLB457 was not sensitive to endogenous dopamine, as [11C]FLB457 binding in the cortex and thalamus was not significantly affected by a 1-mg/methamphetamine challenge (37). Therefore, the present findings may be attributed to the receptor density. A longitudinal study will be required to settle the issue of whether low density is acquired during the course of disease or whether it represents abnormal brain development (38).
The subregions with low D2 receptor binding comprise primarily the dorsomedial nucleus and pulvinar, two important components in circuitries previously suggested in the pathophysiology of schizophrenia (5, 7). The thalamus has long been suggested to have a gating function that filters the sensory input to the cortex, thereby providing protection against sensory overload and hyperarousal (39). Startle prepulse inhibition, a sensitive measure of sensory gating, has indeed been suggested as being related to dopamine neurotransmission in the thalamus (40). Both animal and human studies have provided evidence that the mediodorsal thalamus has a particular role in the regulation of startle prepulse inhibition (41–43). In experimental studies, it has been shown that prepulse inhibition of startle can be disrupted after microinjection of the dopamine agonist apomorphine into the medial thalamus (43). The dorsomedial nucleus has connections to the prefrontal cortex and anterior cingulate (44). Both these regions have also been included in discussions on the gating function (39). An abnormality of the dorsomedial nucleus in schizophrenia may be attributed to functional disturbances in any of the brain regions discussed in relation to sensory gating.
The other subnuclei with low binding potential are found in the pulvinar. This is one of several structures implicated in attentional processing and salience (45–47). The pulvinar has connections with the visual and auditory cortex as well as the prefrontal cortex and temporal association areas (48). A primary abnormality in the pulvinar may thus induce unusual associations and sensory disturbances in schizophrenia (26). Taken together, these two regions may have a functional role consistent with several of the disturbed functions in schizophrenia and underlie the correlation with BPRS positive symptom score.
The observation of a significant negative correlation between subregional binding potential and positive symptom score is similar to that previously reported for [11C]FLB457 binding in the anterior cingulate. The anterior cingulate has direct connections with the dorsomedial nucleus and the pulvinar (48–50). When we examined the relationship between dopamine D2 receptor binding in the central medial and posterior regions of the thalamus and that of the anterior cingulate using the Pearson correlation method, we found a significant interrelationship between those regions in patients with schizophrenia (p<0.05). The abnormality shown in the thalamic subregions might have a similar background to that in the anterior cingulate. However, the cellular localization of D2 receptors in the thalamic subregions that might allow speculation about the altered regulatory function of interneurons with D2 receptors has not yet been determined, as discussed in the previous study (1).
Reduced regional blood flow has been reported in the thalamus of schizophrenia (51). However, the reduction of binding potential is unlikely to be a result of altered blood flow, since the R1 value did not differ significantly between the healthy subjects and patients. While morphological changes have been reported in the thalamic mediodorsal nucleus and pulvinar in schizophrenia (7, 8), and this can affect binding potential, no significant difference was observed in the volumes of thalamic subdivisions, including the central medial and posterior regions, between our examined patient and comparison groups. The relatively moderate severity and short duration of illness in our patient group may explain the lack of volume change (52, 53). In any case, low dopamine D2 receptor binding can therefore not be attributed to reductions in gross brain anatomy.
There are several confounding factors in this study. First, the number of patients was small, raising the question of adequate statistical power, and thus it cannot be ruled out that a larger study population might reveal that the binding potential values of other thalamic subregions and volumetric measurements will also show significant differences. In addition, [11C]FLB457 has high affinity not only for dopamine D2 receptors but also for dopamine D3 receptors (27). Dopamine D3 receptors are distributed mainly to the ventral striatum and the islands of Calleja in the postmortem human brain, but they have as yet not been distinctly identified in the thalamus (54–56). Thus, there is a possibility that our findings could be partly explained by the reduction of dopamine D3 receptors, but this will have to await the outcome of future studies on the amount of dopamine D3 receptors in the thalamus. Another factor is that psychopathology was assessed by the 18-item BPRS, but this scale mainly measures the affective component of the negative symptoms and does not cover well the additional components that identify cognitive, anergic, and social dimensions (57).
Finally, our measurement of thalamic subdivisions has several limitations. We were unable to delineate and employ an intrathalamic marker as a consistent landmark for our regional subdivisions. Rather, we relied upon approximate percentage-based divisions of the total thalamic area as a means of dividing the thalamus. This automated method reduced some of the subjectivity and systematic bias involved in defining subthalamic areas with limited resolution imaging. However, without manual editing, the assumptions that all thalamic nuclei are consistently represented by these rigid subdivisions cannot be assured, and the volume of each subdivision would not be comparable with data from carefully edited volumetric studies.
 |
Received Jan. 7, 2003; revisions received May 6 and Oct. 7, 2003; accepted Oct. 9, 2003. From the Brain Imaging Project, National Institute of Radiological Sciences, Chiba, Japan; CREST Japan Science and Technology Corporation, Saitama, Japan; Biofunctional Informatics, Graduate School of Allied Health Sciences, Tokyo Medical and Dental University, Tokyo, Japan; the Department of Psychiatry, Jikei University School of Medicine, Tokyo, Japan; and the Psychiatry Section, Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden. Address reprint requests to Dr. Suhara, Brain Imaging Project, National Institute of Radiological Sciences, 4–9–1 Anagawa, Inage-ku, Chiba 263–8555, Japan; [email protected] (e-mail). Supported by the PET project of the National Institute of Radiological Sciences in Chiba, Japan. The authors thank T. Saijo, T. Ando, A. Yamamoto, Y. Asai, S. Ito, and T. Nakayama for their help in data acquisition and N. Kinukawa for discussions and comments about the statistics.
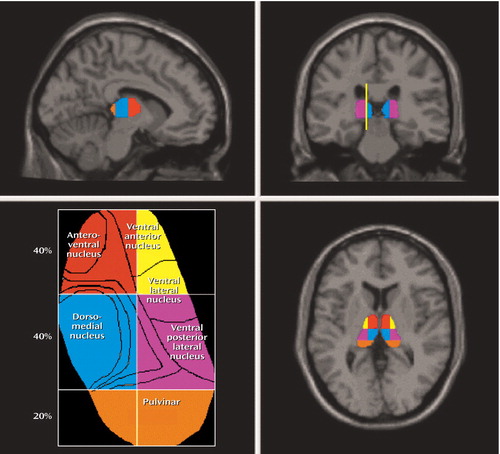
Figure 1. Thalamic Subdivisional Regions of Interesta
aRegions of interest were defined on T1-weighted MR images by a manual tracing technique as described in a previous article examining the thalamic volumes in schizophrenia (7). Approximate regions of specific thalamic nuclei are depicted in the representation of the axial view of the thalamus (lower left) (5, 7). The line drawn in the coronal view of the MRI—which is parallel to the lateral border of the midbrain, interhemispheric fissure, and cerebral aqueduct—represents the line of vertical bisection of each thalamus.
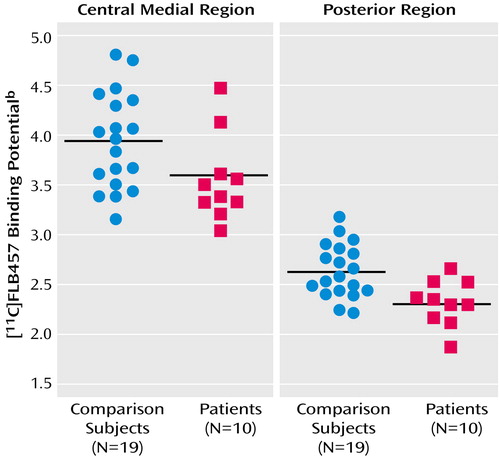
Figure 2. [11C]FLB457 Binding Potential in the Central Medial and Posterior Subregions of the Thalamus in Patients With Schizophrenia (N=10) and Healthy Comparison Subjects (N=19)a
aThe horizontal line represents group mean.
bIndex of dopamine D2 receptor binding.
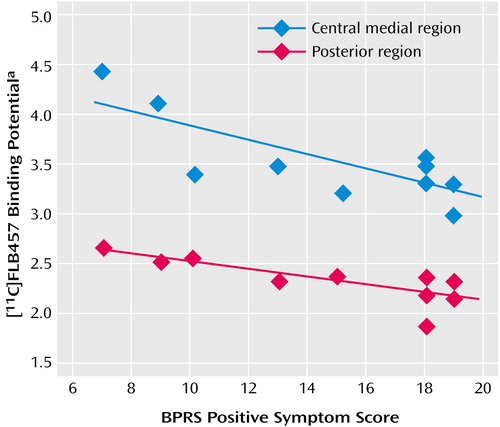
Figure 3. Relationship Between [11C]FLB457 Binding Potential in the Central Medial and Posterior Subregions of the Thalamus and BPRS Positive Symptom Score in Patients With Schizophrenia (N=10)
aIndex of dopamine D2 receptor binding.
1. Suhara T, Okubo Y, Yasuno F, Sudo Y, Inoue M, Ichimiya T, Nakashima Y, Nakayama K, Tanada S, Suzuki K, Halldin C, Farde L: Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch Gen Psychiatry 2002; 59:25–30Crossref, Medline, Google Scholar
2. Andreasen NC, Arndt S, Swayze V II, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WTC: Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 1994; 266:294–298Crossref, Medline, Google Scholar
3. Andreasen NC, Ehrhardt JC, Swayze VW II, Alliger RJ, Yuh WT, Cohen G, Ziebell S: Magnetic resonance imaging of the brain in schizophrenia: the pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry 1990; 47:35–44Crossref, Medline, Google Scholar
4. Pakkenberg B: The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophr Res 1992; 7:95–100Crossref, Medline, Google Scholar
5. Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE Jr: PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 1996; 153:191–199Link, Google Scholar
6. Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC: Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155:1711–1717Link, Google Scholar
7. Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS: Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry 2001; 158:618–624Link, Google Scholar
8. Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitropoulou V, Siever LJ: Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry 2001; 58:133–140Crossref, Medline, Google Scholar
9. Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RSJ, Dolan RJ: Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel-based morphometry. Am J Psychiatry 2002; 159:1497–1505Link, Google Scholar
10. Rodriguez VM, Andree RM, Castejon MJ, Zamora ML, Alvaro PC, Delgado JL, Vila FJ: Fronto-striato-thalamic perfusion and clozapine response in treatment-refractory schizophrenic patients: a 99mTc-HMPAO study. Psychiatry Res 1997; 76:51–61Crossref, Medline, Google Scholar
11. Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA: Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry 1996; 153:41–49Link, Google Scholar
12. Heimberg C, Komoroski RA, Lawson WB, Cardwell D, Karson CN: Regional proton magnetic resonance spectroscopy in schizophrenia and exploration of drug effect. Psychiatry Res 1998; 83:105–115Crossref, Medline, Google Scholar
13. Gunther W: MRI-SPECT and PET-EEG findings on brain dysfunction in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 1992; 16:445–462Crossref, Medline, Google Scholar
14. Pakkenberg B: Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 1990; 47:1023–1028Crossref, Medline, Google Scholar
15. Young KA, Manaye KF, Liang C, Hicks PB, German DC: Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry 2000; 47:944–953Crossref, Medline, Google Scholar
16. Popken GJ, Bunney WE Jr, Potkin SG, Jones EG: Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci USA 2000; 97:9276–9280Crossref, Medline, Google Scholar
17. Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L: Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry 2002; 159:59–65Link, Google Scholar
18. Blennow K, Bogdanovic N, Heilig M, Grenfeldt B, Karlsson I, Davidsson P: Reduction of the synaptic protein rab3a in the thalamus and connecting brain regions in post-mortem schizophrenic brains. J Neural Transm 2000; 107:1085–1097Crossref, Medline, Google Scholar
19. Ungerstedt U: Stereotaxic mapping of monoamine pathways in the rat brain. Acta Physiol Scand 1971; 367:1–48Crossref, Google Scholar
20. Kessler RM, Whetsell WO, Ansari MS, Votaw JR, de Paulis T, Clanton JA, Schmidt DE, Mason NS, Manning RG: Identification of extrastriatal dopamine D2 receptors in post mortem human brain with [125I]epidepride. Brain Res 1993; 609:237–243Crossref, Medline, Google Scholar
21. Langer O, Halldin C, Dolle F, Swahn CG, Olsson H, Karlsson P, Hall H, Sandell J, Lundkvist C, Vaufrey F, Loc’h C, Crouzel C, Maziere B, Farde L: Carbon-11 epidepride: a suitable radioligand for PET investigation of striatal and extrastriatal dopamine D2 receptors. Nucl Med Biol 1999; 26:509–518Crossref, Medline, Google Scholar
22. Sedvall G, Farde L: Chemical brain anatomy in schizophrenia. Lancet 1995; 346:743–749Crossref, Medline, Google Scholar
23. Suhara T, Sudo Y, Okauchi T, Maeda J, Kawabe K, Suzuki K, Okubo Y, Nakashima Y, Ito H, Tanada S, Halldin C, Farde L: Extrastriatal dopamine D2 receptor density and affinity in the human brain measured by 3D PET. Int J Neuropsychopharmacol 1999; 2:73–82Crossref, Medline, Google Scholar
24. Okubo Y, Olsson H, Ito H, Lofti M, Suhara T, Halldin C, Farde L: PET mapping of extrastriatal D2-like dopamine receptors in the human brain using an anatomic standardization technique and [11C]FLB 457. Neuroimage 1999; 10:666–674Crossref, Medline, Google Scholar
25. Farde L, Suhara T, Nyberg S, Karlsson P, Nakashima Y, Hietala J, Halldin C: A PET-study of [11C]FLB 457 binding to extrastriatal D2-dopamine receptors in healthy subjects and antipsychotic drug-treated patients. Psychopharmacology (Berl) 1997; 133:396–404Crossref, Medline, Google Scholar
26. Jones EG: The Thalamus. New York, Plenum, 1985Google Scholar
27. Halldin C, Farde L, Hogberg T, Mohell N, Hall H, Suhara T, Karlsson P, Nakashima Y, Swahn CG: Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med 1995; 36:1275–1281Medline, Google Scholar
28. Kitamura T, Machizawa S, Maruyama S, Nakagawa Y, Morita M, Sato T: Test-retest reliability of Oxford University version of the Brief Psychiatric Rating Scale (BPRS): a preliminary survey of multicenter collaborative study initiated by the National Institute of Mental Health. J Ment Health 1985; 32:1–15Google Scholar
29. Kane J, Honigfeld G, Singer J, Meltzer H: Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
30. Lammertsma AA, Hume S: Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4:153–158Crossref, Medline, Google Scholar
31. Olsson H, Halldin C, Swahn CG, Farde L: Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab 1999; 19:1164–1173Crossref, Medline, Google Scholar
32. Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ: A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol 1984; 15:217–227Crossref, Medline, Google Scholar
33. Yasuno F, Hasnine AH, Suhara T, Ichimiya T, Sudo Y, Inoue M, Takano A, Ou T, Ando T, Toyama H: Template-based method for multiple volumes of interest of human brain PET images. Neuroimage 2002; 16:577–586Crossref, Medline, Google Scholar
34. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
35. Studholme C, Hill DL, Hawkes DJ: Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys 1997; 24:25–35Crossref, Medline, Google Scholar
36. Oke AF, Adams RN: Elevated thalamic dopamine: possible link to sensory dysfunctions in schizophrenia. Schizophr Bull 1987; 13:589–604Crossref, Medline, Google Scholar
37. Okauchi T, Suhara T, Maeda J, Kawabe K, Obayashi S, Suzuki K: Effect of endogenous dopamine on extrastriatal [11C]FLB 457 binding measured by PET. Synapse 2001; 41:87–95Crossref, Medline, Google Scholar
38. Goldsmith SK, Shapiro RM, Joyce JN: Disrupted pattern of D2 dopamine receptors in the temporal lobe in schizophrenia. Arch Gen Psychiatry 1997; 54:649–658Crossref, Medline, Google Scholar
39. Carlsson A, Waters N, Waters S, Carlsson ML: Network interactions in schizophrenia—therapeutic implications. Brain Res Brain Res Rev 2000; 31:342–349Crossref, Medline, Google Scholar
40. Perry W, Feifel D, Minassian A, Bhattacharjie I, Braff DL: Information processing deficits in acutely psychotic schizophrenia patients medicated and unmedicated at the time of admission. Am J Psychiatry 2002; 159:1375–1381Link, Google Scholar
41. Hazlett EA, Buchsbaum MS, Haznedar MM, Singer MB, Germans MK, Schnur DB, Jimenez EA, Buchsbaum BR, Troyer BT: Prefrontal cortex glucose metabolism and startle eyeblink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology 1998; 35:186–198Crossref, Medline, Google Scholar
42. Kodsi MH, Swerdlow NR: Regulation of prepulse inhibition by ventral pallidal projections. Brain Res Bull 1997; 43:219–228Crossref, Medline, Google Scholar
43. Young KA, Randall PK, Wilcox RE: Startle and sensorimotor correlates of ventral thalamic dopamine and GABA in rodents. Neuroreport 1995; 6:2495–2499Crossref, Medline, Google Scholar
44. Jones EG: Cortical development and thalamic pathology in schizophrenia. Schizophr Bull 1997; 23:483–501Crossref, Medline, Google Scholar
45. Robinson DL, Petersen SE: The pulvinar and visual salience. Trends Neurosci 1992; 15:127–132Crossref, Medline, Google Scholar
46. LaBerge D, Buchsbaum MS: Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci 1990; 10:613–619Crossref, Medline, Google Scholar
47. Morris JS, Friston KJ, Dolan RJ: Neural responses to salient visual stimuli. Proc R Soc Lond B Biol Sci 1997; 264:769–775Crossref, Google Scholar
48. Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS: Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol 1997; 379:313–332Crossref, Medline, Google Scholar
49. Vogt BA, Pandya DN, Rosene DL: Cingulate cortex of the rhesus monkey, I: cytoarchitecture and thalamic afferents. J Comp Neurol 1987; 262:256–270Crossref, Medline, Google Scholar
50. Vogt BA, Pandya DN: Cingulate cortex of the rhesus monkey, II: cortical afferents. J Comp Neurol 1987; 262:271–289Crossref, Medline, Google Scholar
51. Vita A, Bressi S, Perani D, Invernizzi G, Giobbio GM, Dieci M, Garbarini M, Del Sole A, Fazio F: High-resolution SPECT study of regional cerebral blood flow in drug-free and drug-naive schizophrenic patients. Am J Psychiatry 1995; 152:876–882Link, Google Scholar
52. Portas CM, Goldstein JM, Shenton ME, Hokama HH, Wible CG, Fischer I, Kikinis R, Donnino R, Jolesz FA, McCarley RW: Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biol Psychiatry 1998; 43:649–659Crossref, Medline, Google Scholar
53. Saijo T, Abe T, Someya Y, Sassa T, Sudo Y, Suhara T, Shuno T, Asai K, Okubo Y: Ten year progressive ventricular enlargement in schizophrenia: an MRI morphometrical study. Psychiatry Clin Neurosci 2001; 55:41–47Crossref, Medline, Google Scholar
54. Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC: Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 1990; 347:146–151Crossref, Medline, Google Scholar
55. Landwehrmeyer B, Mengod G, Palacios JM: Dopamine D3 receptor mRNA and binding sites in human brain. Brain Res Mol Brain Res 1993; 18:187–192Crossref, Medline, Google Scholar
56. Murray AM, Ryoo HL, Gurevich E, Joyce JN: Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA 1994; 91:11271–11275Crossref, Medline, Google Scholar
57. Welham J, Stedman T, Clair A: Choosing negative symptom instruments: issues of representation and redundancy. Psychiatry Res 1999; 87:47–56Crossref, Medline, Google Scholar



