Event-Related fMRI of Frontotemporal Activity During Word Encoding and Recognition in Schizophrenia
Abstract
OBJECTIVE: Neuropsychological studies have demonstrated verbal episodic memory deficits in schizophrenia during word encoding and retrieval. This study examined neural substrates of memory in an analysis that controlled for successful retrieval. METHOD: Event-related blood-oxygen-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) was used to measure brain activation during word encoding and recognition in 14 patients with schizophrenia and 15 healthy comparison subjects. An unbiased multiple linear regression procedure was used to model the BOLD response, and task effects were detected by contrasting the signal before and after stimulus onset. RESULTS: Patients attended during encoding and had unimpaired reaction times and normal response biases during recognition, but they had lower recognition discriminability scores, compared with the healthy subjects. Analysis of contrasts was restricted to correct items. Previous findings of a deficit in bilateral prefrontal cortex activation during encoding in patients were reproduced, but patients showed greater parahippocampal activation rather than deficits in temporal lobe activation. During recognition, left dorsolateral prefrontal cortex activation was lower in the patients and right anterior prefrontal cortex activation was preserved, as in the authors’ previous study using positron emission tomography. Successful retrieval was associated with greater right dorsolateral prefrontal cortex activation in the comparison subjects, whereas orbitofrontal, superior frontal, mesial temporal, middle temporal, and inferior parietal regions were more active in the patients during successful retrieval. CONCLUSIONS: The pattern of prefrontal cortex underactivation and parahippocampal overactivation in the patients suggests that functional connectivity of dorsolateral prefrontal and temporal-limbic structures is disrupted by schizophrenia. This disruption may be reflected in the memory strategies of patients with schizophrenia, which include reliance on rote rehearsal rather than associative semantic processing.
Problems in verbal episodic memory in schizophrenia have been well described, and evidence of prominent difficulties in encoding and free recall has been found (1, 2). Long-term storage is relatively spared (3), and less severe impairments are noted on nonrelational recognition tasks (4).
The neural underpinnings of memory can be studied with functional neuroimaging. A review concluded that the prefrontal cortex and hippocampus are most consistently implicated in schizophrenia (5). Hemispheric asymmetry models have emphasized the importance of the left prefrontal cortex to episodic encoding and semantic retrieval and the right prefrontal cortex to episodic retrieval (6). Subsequent studies supported a distinct role for the right anterior prefrontal cortex (Brodmann’s area 10) during episodic retrieval but found less evidence of hemispheric and task specificity for the dorsolateral prefrontal cortex (Brodmann’s area 9, 46) (7, 8). The left dorsolateral prefrontal cortex appears to be involved in semantic organization during encoding (9), and several prefrontal cortex subregions show functional overlap across working, episodic, and semantic memory (10, 11). The importance of the hippocampus to memory was established in early studies of clinical pathology and was supported by functional imaging that attributed hippocampal function to encoding and retrieval, novelty detection, and successful retrieval (12, 13).
Several investigators suggested that the reciprocal relationships between the prefrontal cortex and the mesial temporal lobe in normal cognition (14) may be disrupted or reversed by schizophrenia (15, 16). For example, in a verbal fluency study, investigators reported less prefrontal cortex activation and greater temporal lobe activation in patients than in healthy comparison subjects (17). Other studies reported hippocampal overactivation in patients during baseline assessment and prefrontal cortex overactivation rather than hippocampal recruitment for recognition of words that had undergone deep semantic processing versus words that had undergone shallow perceptual processing (18, 19). A subsequent study of the hippocampus involving word encoding and recognition also found less activation in patients than in healthy comparison subjects during both stages of processing (20).
The purpose of the current study was to examine frontotemporal function in schizophrenia by using a verbal episodic encoding and recognition paradigm that was previously established with positron emission tomography (PET) (8, 21). In previous studies, we found reduced left prefrontal cortex activation during encoding and recognition in patients with schizophrenia. Left superior temporal activity was also reduced during encoding, while activation in the left anterior cingulate, left mesial temporal, and right thalamic regions was reduced during recognition. However, right anterior prefrontal cortex activation during recognition was preserved. Performance correlated with prefrontal cortex activation in healthy participants and with posterior activation in patients. One goal of the present study was to establish the reproducibility of the PET findings with functional magnetic resonance imaging (fMRI). More important, event-related fMRI enables examination of retrieval success. We hypothesized that the left prefrontal cortex regions are disrupted by schizophrenia during both encoding and recognition but that schizophrenia patients would not differ from healthy comparison subjects in activation in the right frontal pole during recognition. Retrieval success was hypothesized to be related to prefrontal cortex activity only in the comparison subjects.
Method
Participants
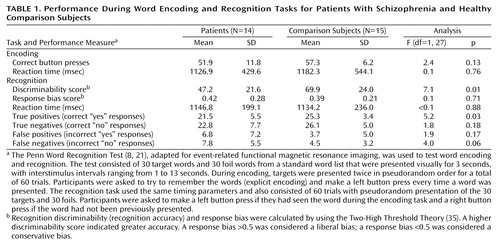
Participants were 14 patients with schizophrenia and 15 healthy comparison subjects from the Schizophrenia Center at the University of Pennsylvania. Both groups included six female subjects. The patient group excluded one subject with excessive movement artifact (i.e., >4 mm in any dimension) and two subjects who could not comply with the research procedure. The comparison subjects were matched with the patients in age (comparison subjects: mean age=28.4 years, SD=7.0; patients: mean age=32.7 years , SD=8.3), parental education (comparison subjects: mean=15.6 years, SD=2.8; patients: mean=13.1 years, SD=2.2), and handedness (two left-handed comparison subjects and one left-handed patient). As expected, the patients had less education than the comparison subjects (patients: mean=12.6 years, SD=2.1; comparison subjects: mean=14.8 years, SD=2.6) (t=2.4, df=27, p<0.05). The patients had a DSM-IV consensus diagnosis (criterion reliability ≥0.85) of schizophrenia established by medical, neurological, and psychiatric evaluations (22). The comparison subjects also underwent standard evaluations (23). The participants had no history of substance abuse or of other medical, psychiatric, or neurological disorders that might affect brain function.
All patients were mildly to moderately ill according to the Scale for the Assessment of Negative Symptoms (SANS) (24) (mean score=24.2, SD=22.4, range=0–86), the Scale for the Assessment of Positive Symptoms (SAPS) (25) (mean score=12.5, SD=12.9, range=0–48), and the Brief Psychiatric Rating Scale (BPRS) (26) (mean score=28.9, SD=6.4, range=19–46). Examination of clinical subtypes (27) revealed that 12 patients met the criteria for the “mild” subcategory, one for the “deficit” subcategory, and one for the “paranoid” subcategory. Excluding the two patients with more severe symptoms did not change the overall pattern of fMRI results (analysis available upon request). All patients were receiving medication; two patients received only typical antipsychotics, nine received only atypical antipsychotics, and three received both typical and atypical antipsychotics. The average dose of typical antipsychotics was 68.7 mg/day in chlorpromazine equivalents (SD=26.6, range=0–250), and the average dose of atypical antipsychotics was 14.6 mg/day in olanzapine equivalents (SD=2.6, range=0–35.8). No patient was receiving anticholinergic medication. The patients’ mean age at illness onset was 20.5 years (SD=4.9), and the mean duration of illness was 12.2 years (SD=7.6). After complete description of the study to the subjects, written informed consent was obtained.
Tasks
Images were acquired during three conditions: word encoding, letter n-back, and word recognition. Letter n-back (28) served as a distracter to prevent rehearsal, and the results for this condition will not be reported. Encoding and recognition utilized the Penn Word Recognition Test (8, 21), adapted for event-related fMRI. The task consists of 30 targets and 30 foils from a standard word list (29) balanced for frequency, concreteness, and imageability. Words were visually presented for 3 seconds, with “jittered” interstimulus intervals ranging from 1 to 13 seconds (Figure 1). During interstimulus intervals, the subjects were instructed to fixate on a crosshair image and told to relax. During encoding, targets were presented twice in pseudorandom order for a total of 60 trials. Participants were asked to try to remember the words (explicit encoding) and make a left button press every time a word was presented. The recognition task used the same timing parameters and also consisted of 60 trials with pseudorandom presentation of the 30 targets and 30 foils. Participants were asked to make a left button press if they had seen the word during the encoding task and a right button press if the word had not been previously presented. The tasks were triggered by the scanner and coupled to image acquisition by using Power Laboratory (30) on a Macintosh laptop computer. Stimuli were rear projected to the center of the visual field by using a PowerLite 7300 video projector (Epson America, Inc., Long Beach, Calif.) and viewed through a mirror mounted on the head coil. Responses were recorded by means of a nonferromagnetic keypad (FORP, Current Design Inc., Philadelphia). Practice sessions were provided, and all participants understood the instructions and use of the keypad.
Image Acquisition
Data were acquired on a 4-T GE Signa Scanner (General Electric Medical Systems, Milwaukee) by using established procedures (28). Structural T1-weighted imaging was used for anatomic overlays of functional data and spatial normalization (31). Blood-oxygen-level-dependent (BOLD) (32, 33) images were obtained with a gradient-echo echoplanar sequence (22 slices, TR=2000, TE=40, voxel size=3.75×3.75×4 mm). Images were corrected for geometric distortion on the basis of a magnetic field map acquired after acquisition of the T1 localizer (34).
Data Analysis
Button presses were recorded to index task engagement during encoding. During recognition, scoring distinguished correct “yes” responses to targets, correct “no” responses to foils, incorrect “no” responses to targets, and incorrect “yes” responses to foils. Recognition discriminability (recognition accuracy) and response bias were calculated from these scores by using the two-high threshold theory (35). A response bias >0.5 was considered a liberal bias; a response bias <0.5 was considered a conservative bias. Median reaction time for correct responses indexed psychomotor speed. Analysis of variance with one grouping factor (diagnosis) was used to test the effect of schizophrenia on performance.
Functional images were preprocessed in MEDx (Sensor Systems, Sterling, Va.) by using standard procedures (28). Images were motion corrected (36), proportionally scaled (37), and band-pass filtered (0.016–0.166 Hz) (38). Talairach (31) transformation occurred in two steps. First, a least-squares surface registration algorithm (39) was used to coregister raw functional images to the structural localizer. Second, a nonlinear transformation into Talairach space was done on the basis of commissural landmarks identified by a trained investigator. The transformed data were spatially smoothed (8 mm full width at half maximum, isotropic spatial resolution).
Statistical analysis was carried out in two stages. First, a multiple linear regression model was used to estimate the hemodynamic response. Predictor variables were a set of lagged indicators of stimulus type (target, foil, crosshair) associated with each scan. Convolution with a priori hemodynamic response functions was not required. Assumptions were 1) that temporally overlapping hemodynamic responses elicited by adjacent stimuli add together linearly and 2) that hemodynamic response to an individual stimulus is negligible after 16 seconds. Both assumptions are supported for interstimulus intervals of ≥2000 msec (40). The resulting regression coefficients (beta parameters) represented unbiased estimators of incremental hemodynamic responses at each time point, beginning 4 seconds before stimulus presentation and continuing for 16 seconds poststimulus.
In the second stage, beta parameters were treated as subject data for a multisubject analysis with SPM 99 (41). To identify significant stimulus-related activity, averaged poststimulus peak responses (beta parameters 2, 4, and 6 seconds after stimulus onset) were contrasted to averaged prestimulus baseline responses (beta parameters 2 and 4 seconds before stimulus onset). Contrasts were performed both within and between groups. Within-group contrasts were performed on the whole brain. For between-group analyses, inclusive maps were used in SPM 99 to restrict contrasts to voxels with above-threshold responses for either group. The resulting SPM{T} maps were transformed to the unit normal distribution SPM{Z}. Significance thresholds were based on spatial extent (k) and peak height (u) (42). We used a height threshold corresponding to an uncorrected p value of 0.005, which required a minimum of eight voxels in any individual cluster. This selection resulted in a corrected probability of p<0.05, based on the theory of Gaussian fields (42, 43).
Results
Performance
Performance data are summarized in Table 1. All participants were attentive during encoding, and there were no group differences in number of button presses or reaction times. When asked about strategies for encoding, eight comparison subjects and one patient reported making word associations. The remaining subjects either could not describe a strategy or reported using rehearsal or visualization. Recognition discriminability was lower for the patients. However, there was no difference in reaction time or response bias, and both groups maintained a conservative bias. Patients had more difficulty correctly identifying targets (correct “yes” responses) than correctly rejecting foils (correct “no” responses). There were no significant correlations between performance and medication dose nor any differences in reaction time (T=0.5, df=9, p=0.60), discriminability (T<0.1, df=9, p=0.96), or response bias (T=0.1, df=9, p=0.88) between patients who received typical versus atypical medications.
Task-Related BOLD Change
Word encoding
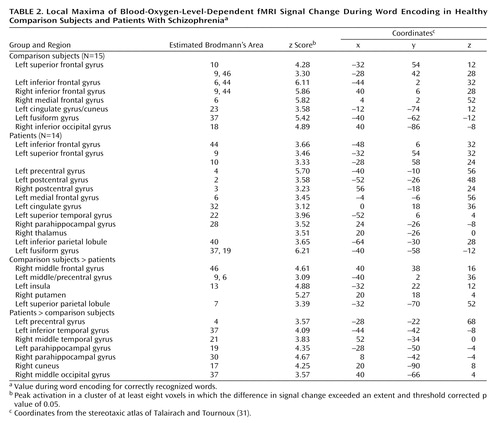
Because of group differences in recognition discriminability, analysis was restricted to correctly recognized words. Table 2 presents local maxima for encoding. As in previous studies (8, 21), the comparison subjects had extensive frontal activation, including activation in the left frontal pole (Brodmann’s area 10), left dorsolateral prefrontal cortex (Brodmann’s area 9, 46), bilateral Broca’s area (Brodmann’s area 6, 9, 44), and right supplementary motor regions (Brodmann’s area 6). The comparison subjects also had activation in the posterior cingulate (Brodmann’s area 23) and the bilateral visual association cortices (Brodmann’s area 18, 37). Activation in the patients was more diffuse. Like the comparison subjects, the patients activated the left Broca’s area (Brodmann’s area 44), left frontal pole (Brodmann’s area 9, 10), and left visual association areas (Brodmann’s area 37, 19). However, the patients did not activate the left dorsolateral prefrontal cortex, and they showed extensive bilateral activation of primary and supplementary sensorimotor areas (Brodmann’s area 2, 3, 4, 6). Unlike the comparison subjects, the patients activated the left anterior cingulate (Brodmann’s area 32), left superior temporal gyrus (Brodmann’s area 22), right parahippocampal gyrus (Brodmann’s area 28), right thalamus, and left parietal cortex (Brodmann’s area 40).
Between-group contrasts (Table 2, Figure 2) revealed that the comparison subjects had greater frontal activation in the left (Brodmann’s area 9, 6) and right dorsolateral prefrontal cortex (Brodmann’s area 46). The comparison subjects also had greater activation in the left insula (Brodmann’s area 13), right putamen, and left superior parietal cortex (Brodmann’s area 7). The patients showed greater temporal-limbic activation, including activation in the left inferior temporal gyrus (Brodmann’s area 37), right middle temporal gyrus (Brodmann’s area 21), and bilateral parahippocampal gyrus (Brodmann’s area 19, 30). Patients also had greater activation in left motor region (Brodmann’s area 4) and right visual association area (Brodmann’s area 17, 37).
Word recognition
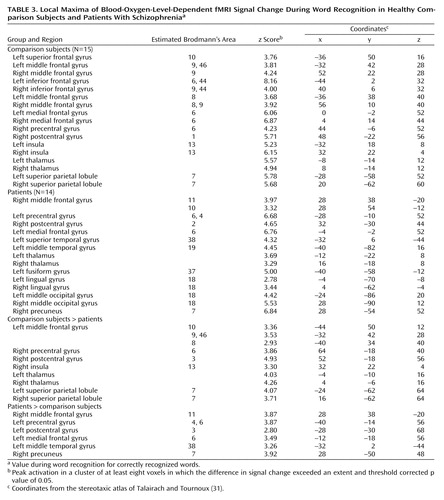
Table 3 presents local maxima for correct word recognition. The comparison subjects again had extensive prefrontal cortex activation, including activation in the left frontal pole (Brodmann’s area 10), bilateral dorsolateral (Brodmann’s area 9, 46), and bilateral Broca’s area (Brodmann’s area 6, 9, 44). The comparison subjects also showed bilateral activation of insula (Brodmann’s area 13), thalamus, superior parietal cortex (Brodmann’s area 7), and primary (Brodmann’s area 1, 6) and supplementary motor areas (Brodmann’s area 6, 8, 9). The patients also had activation in the bilateral sensorimotor (Brodmann’s area 2, 4, 6) and thalamic regions. However, instead of having anterior and dorsolateral prefrontal cortex activation, the patients showed bilateral orbitofrontal activation (Brodmann’s area 10, 11). Patients also activated the left superior (Brodmann’s area 19, 38) and middle temporal (Brodmann’s area 19) regions and the bilateral visual association areas (Brodmann’s area 7, 18, 37).
Between-group contrasts (Table 3, Figure 3) found greater activation in the comparison subjects in the left frontopolar (Brodmann’s area 10) and dorsolateral (Brodmann’s area 9, 46) prefrontal cortex. The comparison subjects also had larger effects in the bilateral sensorimotor areas (Brodmann’s area 3, 6, 8), right insula (Brodmann’s area 13), bilateral thalamus, and bilateral superior parietal regions (Brodmann’s area 7). The analysis of activation in the patients minus activation in the comparison subjects again revealed group differences in the left sensorimotor areas (Brodmann’s area 3, 4, 6). The patients also had more activation in the left orbitofrontal region (Brodmann’s area 11), left middle temporal gyrus (Brodmann’s area 38), and right precuneus (Brodmann’s area 7).
Retrieval success
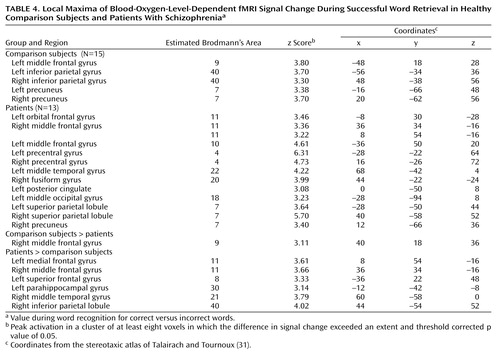
Successful retrieval (Table 4) was investigated by contrasting the BOLD response for the correctly identified targets with that for the correctly identified foils. This analysis did not include one patient who had no correct “no” responses. The comparison subjects had activation in the left dorsolateral prefrontal cortex (Brodmann’s area 9) and bilateral inferior parietal gyrus (Brodmann’s area 40) and precuneus (Brodmann’s area 7). Activation was more diffuse in the patients. In the frontal cortex, patients had activation in the left anterior prefrontal cortex (Brodmann’s area 10) and the bilateral orbitofrontal (Brodmann’s area 11) and motor regions (Brodmann’s area 4). They also activated the left middle temporal gyrus (Brodmann’s area 22), left posterior cingulate, and bilateral visual association areas (Brodmann’s area 7, 18, 20).
Between-group contrasts (Table 4, Figure 4) showed that retrieval success was related to greater right dorsolateral prefrontal cortex activation (Brodmann’s area 9) in the comparison subjects than in the patients. The patients showed greater activation in the bilateral orbitofrontal (Brodmann’s area 11), left superior frontal (Brodmann’s area 8), left parahippocampal (Brodmann’s area 30), right middle temporal (Brodmann’s area 21), and right inferior parietal regions (Brodmann’s area 40).
Discussion
The current study reproduces our earlier PET findings (21) of dorsolateral prefrontal cortex dysfunction in patients with schizophrenia during word encoding and recognition. Whereas dorsolateral prefrontal cortex dysfunction was restricted to the left hemisphere during PET, in the current study there was evidence in the patients of bilateral deficits during encoding, left hemisphere impairments during recognition, and right-sided dysfunction during retrieval success. In accord with earlier correlational results, the patients relied on a more distributed set of temporal-limbic and posterior regions, suggesting possible use of a language-based compensatory network (44). As found previously, there was no group difference in right anterior prefrontal cortex activation during recognition, suggesting that the associated episodic retrieval mechanism (45) is relatively intact in patients with schizophrenia. In contrast to our prior findings of less activation in the superior and mesial temporal cortex in patients with schizophrenia, the current study found that patients had greater temporal-limbic activation than comparison subjects during word encoding and recognition and during successful retrieval.
The dorsolateral prefrontal cortex was the frontal lobe area most clearly disabled by schizophrenia. Reproducing this result supports the importance of this region to episodic verbal memory and to schizophrenia pathophysiology (21, 46). In contrast, there was no group difference in the left Broca’s area during encoding. Unimpaired activity in Broca’s area has been noted in other schizophrenia studies and attributed to intact verbal rehearsal in short-term memory (47) (see reference 48 for exception). Discrepant effects of schizophrenia on the left dorsolateral prefrontal cortex and Broca’s activity may reflect encoding strategies found in patients. On the basis of self-report, the comparison subjects relied more frequently on associative semantic processing, which is linked with left dorsolateral prefrontal cortex function (9). In contrast, the patients may have relied on verbal rehearsal mediated by Broca’s area (49). Previous findings that patients with schizophrenia benefit from semantic encoding in a levels-of-processing paradigm (18, 19, 50) suggest that failure to encode words semantically relates to difficulties in generating organizational strategies (51) rather than to an inability to process words semantically.
Dorsolateral prefrontal cortex dysfunction in patients extended to the right hemisphere during encoding and successful retrieval. This dysfunction was not found in our earlier PET study and should be viewed with caution. Recent event-related fMRI studies of successful retrieval in healthy individuals (52, 53) have suggested that the right dorsolateral prefrontal cortex is important for postretrieval monitoring (e.g., monitoring the relevance of the retrieved information). Rather than engaging in this evaluative processing, the patients may have focused on processing the stimulus characteristics of the words, as they showed greater activation than the comparison subjects in sensorimotor and visual association areas.
Within the prefrontal cortex, the patients also showed several areas of overactivation. Activity in left frontal pole was unimpaired in the patients during encoding. The patients also overactivated the orbitofrontal cortex during recognition and retrieval success. Heckers and colleagues (18, 19) also found frontopolar and orbitofrontal overactivation in schizophrenia. Given patients’ better performance for words that had undergone shallow perceptual versus deep semantic processing, the investigators attributed this overactivation to greater retrieval effort (18). This relationship also seems to exist in the patients in the current study, for whom the use of a less efficient encoding strategy may have led to greater retrieval effort. Regional group differences within the prefrontal cortex clarify the need to examine effects in specific subregions, as has been done in recent studies of working memory and episodic memory (10, 11), and illustrate that not all aspects of prefrontal cortex are equally affected by schizophrenia. The dynamic nature of these abnormalities (both under- and overactivation) in patients suggests a disruption in the modulation of prefrontal cortex function in schizophrenia rather than a focal lesion in which the prefrontal cortex is unable to participate in memory processing.
Evidence of temporal-limbic overactivation in patients should be viewed with caution, as it does not replicate our earlier PET findings. However, there are several methodological reasons why hippocampal activity is not always detected by PET (13), and the low temporal resolution of our PET method may account for this inconsistency. Nevertheless, dorsolateral prefrontal cortex underactivation and parahippocampal overactivation in patients suggest that the normal functional connectivity of prefrontal and temporal-limbic structures is disrupted or reversed by schizophrenia (15, 16). The direction of this reversal has varied, with some studies showing the current pattern (17, 54) and others showing less hippocampal activation and greater prefrontal cortex activation (18, 19). Findings of both prefrontal cortex and hippocampal abnormalities should not be surprising, given the strong reciprocal connections between the hippocampus and the neocortex (55). Neurodevelopmental hippocampal abnormalities in schizophrenia may mimic adult prefrontal cortex lesions since the hippocampus is a gateway for efferent pathways from the prefrontal cortex to the nucleus accumbens (56).
This study had several limitations. Hippocampal activation was not seen in the comparison subjects, as in several previous studies (18, 19). However, unlike prior studies, our study did not test recall and did not manipulate the encoding level. Eldridge and colleagues (57) demonstrated that the hippocampus is engaged during retrieval only when subjects are required to make a conscious recollection. Lack of activation may reflect the use of a familiarity-based strategy. Another potential limitation is that patients were receiving medication. However, in our PET study we did not find any differences between medicated and unmedicated patients (21), and in the current study medication dose did not correlate with performance. Others have also been unable to find medication effects on prefrontal or mesial temporal function in schizophrenia (18, 19, 58) (see reference 59 for exception). Because of the small size of the medication subgroups in our study, we were unable to contrast fMRI results for patients who received typical versus atypical medications. Although performance did not differ between these medication subgroups, this issue deserves further attention, given findings suggesting differences in the effect of typical and atypical agents on cerebral blood flow in schizophrenia (60). Finally, the current paradigm did not constrain encoding and retrieval conditions. We chose this approach because our initial goal was to assess reproducibility of a previously established paradigm. This lack of constraint had the advantage of revealing putative differences in strategic processes. Further studies employing levels-of-processing encoding conditions (61) and source memory (62) tasks will better clarify the role of encoding and retrieval strategies in episodic memory and related frontotemporal function in schizophrenia.
 |
 |
 |
 |
Presented at the International Congress of Schizophrenia Research, Colorado Springs, Colo., March 29–April 2, 2003. Received July 8, 2003; revision received Oct. 14, 2003; accepted Oct. 14, 2003. From the Departments of Psychiatry and Radiology, University of Pennsylvania. Address reprint requests to Dr. Ragland, Department of Psychiatry, University of Pennsylvania, Gates Building/HUP, 10th Floor, Philadelphia, PA 19104; [email protected] (e-mail). Supported by NIMH grants MH-62103 and MH-19112, grant NS-045839 from the National Institute of Neurological and Communicative Disorders and Stroke, and grant RR-0040 from the NIH Division of Research Resources.
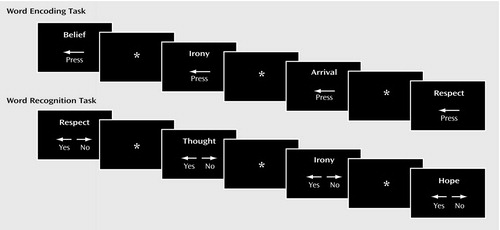
Figure 1. Illustration of Word Presentation During Word Encoding and Word Recognition Tasksa
aEncoding stimuli included a left arrow at the bottom of the page to remind participants to make a left button press when the word was presented. In the word recognition task, arrows reminded participants to make a left button press if they thought they had seen the word before (targets) and a right button press if the word had not been previously presented (foils). In both tasks, words were presented for 3 seconds followed by a visual fixation cross-hair that was presented from 1 to 13 seconds (jittered interstimulus interval).
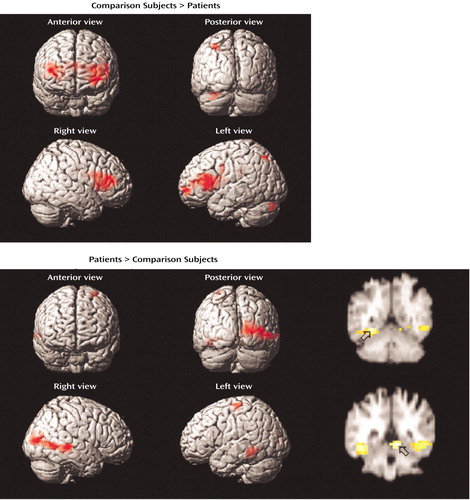
Figure 2. Brain Images Showing Differences Between Comparison Subjects (N=15) and Patients With Schizophrenia (N=14) in Blood-Oxygen-Level-Dependent fMRI Signal Change During Word Encodinga
aStatistical parametric maps are surface-rendered on smoothed brain images. Significant differences in the maxima of activation in which the value for the comparison subjects exceeded that for the patients (upper panel) were found for the right middle frontal gyrus, left middle/precentral gyrus, left insula, left superior parietal lobule, and right putamen. Significant differences in which the value for the patients exceeded that for the comparison subjects (lower panel) were found for the bilateral parahippocampal gyrus, left inferior temporal gyrus, left precentral gyrus, right middle temporal gyrus, right middle occipital gyrus, and right cuneus. The coronal slice images show greater activation in the patients in the left parahippocampal gyrus (upper coronal image; peak activation: y=–50) and right parahippocampal gyrus (lower coronal image; peak activation: y=–42). Colored areas indicate a difference in signal change that exceeds a threshold corresponding to a corrected p value of 0.05.
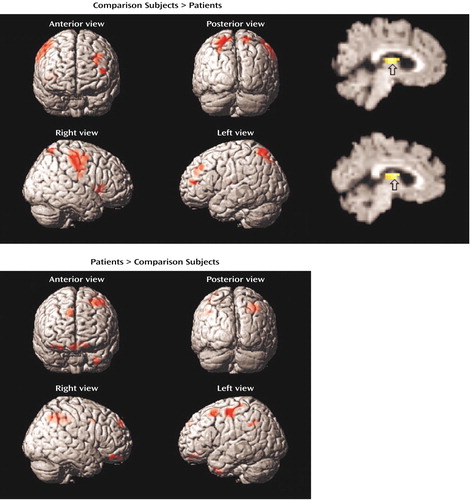
Figure 3. Brain Images Showing Differences Between Comparison Subjects (N=15) and Patients With Schizophrenia (N=14) in Blood-Oxygen-Level-Dependent fMRI Signal Change During Word Recognitiona
aStatistical parametric maps are surface-rendered on smoothed brain images. Significant differences in the maxima of activation in which the value for the comparison subjects exceeded that for the patients (upper panel) were found for the left middle frontal gyrus, right precentral gyrus, right postcentral gyrus, right insula, bilateral thalamic regions, and bilateral superior parietal regions. Significant differences in which the value for the patients exceeded that for the comparison subjects (lower panel) were found for the right middle frontal gyrus, left precentral gyrus, left postcentral gyrus, left medial frontal gyrus, left middle temporal gyrus, and right precuneus. The sagittal slice images show greater activation in the comparison subjects in the left thalamus (upper sagittal image; peak activation: x=–4) and right thalamus (lower sagittal image; peak activation: x=4). Colored areas indicate a difference in signal change that exceeds a threshold corresponding to a corrected p value of 0.05.
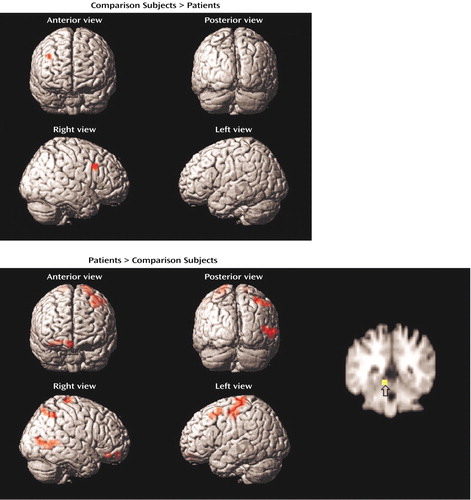
Figure 4. Brain Images Showing Differences Between Comparison Subjects (N=15) and Patients With Schizophrenia (N=13) in Blood-Oxygen-Level-Dependent Signal Change During Successful Word Retrievala
aStatistical parametric maps are surface-rendered on smoothed brain images. Significant differences in the maxima of activation for which the value for the comparison subjects exceeded that for the patients (upper panel) were found for the right middle frontal gyrus. Significant differences in which the value for the patients exceeded that for the comparison subjects (lower panel) were found for the left medial frontal gyrus, right middle frontal gyrus, left superior frontal gyrus, left parahippocampal gyrus, right middle temporal gyrus, and right inferior parietal lobule. The coronal slice image shows greater activation in the patients in the left parahippocampal gyrus (peak activation: y=–42). The colored area indicates a difference in signal change that exceeds a threshold corresponding to a corrected p value of 0.05.
1. Aleman A, Hijman R, de Haan EHF, Kahn RS: Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry 1999; 156:1358–1366Abstract, Google Scholar
2. Saykin AJ, Gur RC, Gur RE, Mozley D, Mozley LH, Resnick SM, Kester B, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
3. Paulsen JS, Heaton RK, Sadek JR, Perry W, Delis DC, Braff D, Kuck J, Zisook S, Jeste DV: The nature of learning and memory impairments in schizophrenia. J Int Neuropsychol Soc 1995; 1:88–99Crossref, Medline, Google Scholar
4. Danion J-M, Rizzo L, Bruant A: Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Arch Gen Psychiatry 1999; 56:639–644Crossref, Medline, Google Scholar
5. Weiss AP, Heckers S: Neuroimaging of declarative memory in schizophrenia. Scand J Psychol 2001; 42:239–250Crossref, Medline, Google Scholar
6. Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S: Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA 1994; 91:2016–2020Crossref, Medline, Google Scholar
7. Buckner RL: Beyond HERA: contributions of specific prefrontal brain areas to long-term memory retrieval. Psychon Bull Rev 1996; 3:149–158Crossref, Medline, Google Scholar
8. Ragland JD, Gur RC, Lazarev MG, Smith RJ, Schroeder L, Raz J, Turetsky BI, Alavi A, Gur RE: Hemispheric activation of anterior and inferior prefrontal cortex during verbal encoding and recognition: a PET study of healthy volunteers. Neuroimage 2000; 11:624–633Crossref, Medline, Google Scholar
9. Nohara S, Suzuki M, Kurach M, Yamashita I, Matsui M, Seto H, Saitoh O: Neural correlates of memory organization deficits in schizophrenia: a single photon emission computed tomography study with 99mTc-ethyl-cysteinate dimer during a verbal learning task. Schizophr Res 2000; 42:209–222Crossref, Medline, Google Scholar
10. Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE: Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage 2001; 14:48–59Crossref, Medline, Google Scholar
11. Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, Ingvar M: Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia 2003; 41:371–377Crossref, Medline, Google Scholar
12. Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C: Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus 1999; 9:83–98Crossref, Medline, Google Scholar
13. Schacter DL, Wagner AD: Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 1998; 9:7–24Crossref, Google Scholar
14. Grasby PM, Frith CD, Friston KJ, Bench C, Frackowiak RSJ, Dolan RJ: Functional mapping of brain areas implicated in auditory verbal memory function. Brain 1993; 116:1–20Crossref, Medline, Google Scholar
15. Weinberger DR, Berman KF, Suddath R, Torrey EF: Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 1992; 149:890–897Link, Google Scholar
16. Friston KJ, Frith CD: Schizophrenia: a disconnection syndrome? Clin Neurosci 1995; 3:89–97Medline, Google Scholar
17. Yurgelun-Todd DA, Waternaux CM, Cohen BM, Gruber SA, English CD, Renshaw PF: Functional magnetic resonance imaging of schizophrenic patients and comparison subjects during word production. Am J Psychiatry 1996; 153:200–205Link, Google Scholar
18. Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM: Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1998; 1:318–323Crossref, Medline, Google Scholar
19. Weiss AP, Schacter DL, Goff DC, Rauch SL, Alpert NM, Fischman AJ, Heckers S: Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry 2003; 53:48–55Crossref, Medline, Google Scholar
20. Jessen F, Scheef L, Germeshausen L, Tawo Y, Kockler M, Kuhn K-U, Maier W, Schild HH, Heun R: Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. Am J Psychiatry 2003; 160:1305–1312Link, Google Scholar
21. Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE: Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry 2001; 158:1114–1125Link, Google Scholar
22. Shtasel DL, Gur RE, Gallacher F, Heimberg C, Cannon T, Gur RC: Phenomenology and functioning in first episode schizophrenia. Schizophr Bull 1992; 18:449–462Crossref, Medline, Google Scholar
23. Shtasel DL, Gur RE, Mozley PD, Richards J, Taleff MM, Heimberg C, Gallacher F, Gur RC: Volunteers for biomedical research: recruitment and screening of normal controls. Arch Gen Psychiatry 1991; 48:1022–1025Crossref, Medline, Google Scholar
24. Andreasen NC: The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
25. Andreasen NC: The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa 1984Google Scholar
26. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. J Operational Psychiatry 1980; 11:46–64Google Scholar
27. Gur RE, Mozley PD, Shtasel DL, Cannon TD, Gallacher F, Turetsky B, Grossman R, Gur RC: Clinical subtypes of schizophrenia: differences in brain and CSF volume. Am J Psychiatry 1994; 151:343–350Link, Google Scholar
28. Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE: Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology 2002; 16:370–379Crossref, Medline, Google Scholar
29. Paivio A, Yuille JC, Madigan SA: Concreteness, imagery, and meaningfulness values for 925 nouns. J Exp Psychol 1968; 76(Jan suppl):1–25Google Scholar
30. Chute DL, Westall RF: Power Laboratory. Devon, Pa, MacLaboratory, 1997Google Scholar
31. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
32. Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS: Time course of EPI of human brain function during task activation. Magn Reson Med 1992; 25:390–397Crossref, Medline, Google Scholar
33. Kwong K, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, Brady TJ, Rosen BR: Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 1992; 89:5675–5679Crossref, Medline, Google Scholar
34. Alsop DC: Correction of ghost artifacts and distortion in echoplanar MR imaging with an iterative reconstruction technique (abstract). Radiology 1995; 197P:388Google Scholar
35. Snodgrass JG, Corwin J: Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 1988; 117:34–50Crossref, Medline, Google Scholar
36. Woods RP, Mazziota JC, Cherry SR: Automated image registration, in Quantification of Brain Function: Tracer Kinetics and Image Analysis in Brain PET. Edited by Uemura K. Amsterdam, Elsevier Science, 1993, pp 391–398Google Scholar
37. Andersson JL: How to estimate global activity independent of changes in local activity. Neuroimage 1997; 6:237–244Crossref, Medline, Google Scholar
38. Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline JB: To smooth or not to smooth? bias and efficiency in fMRI time-series analysis. Neuroimage 2000; 12:196–208Crossref, Medline, Google Scholar
39. Pellizari CA, Chen GTY, Spelbring DR, Weichselbaum Chen CT: Accurate three-dimensional registration of CT, PET and/or MR images of the brain. J Comput Assist Tomogr 1989; 13:20–26Crossref, Medline, Google Scholar
40. Rosen BR, Buckner RL, Dale AM: Event-related functional MRI: past, present, and future. Proc Natl Acad Sci USA 1998; 95:773–780Crossref, Medline, Google Scholar
41. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
42. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33:636–647Crossref, Medline, Google Scholar
43. Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC: Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1994; 1:210–220Crossref, Medline, Google Scholar
44. Curtis VA, Bullmore ET, Morris RG, Brammer MJ, Williams SCR, Simmons A, Sharma T, Murray RM, McGuire PK: Attenuated frontal activation in schizophrenia may be task dependent. Schizophr Res 1999; 37:35–44Crossref, Medline, Google Scholar
45. Desgranges B, Baron J, Eustache F: The functional neuroanatomy of episodic memory: the role of the frontal lobes, the hippocampal formation, and other areas. Neuroimage 1998; 8:198–213Crossref, Medline, Google Scholar
46. Weinberger DR, Berman KF, Zec RF: Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry 1986; 43:114–124Crossref, Medline, Google Scholar
47. Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, Cohen JD: Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry 2001; 58:280–288Crossref, Medline, Google Scholar
48. Stevens AA, Goldman-Rakic PS, Gore JC, Fullbright RK, Wexler BE: Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Arch Gen Psychiatry 1998; 55:1097–1103Crossref, Medline, Google Scholar
49. Paulesu E, Frith CD, Frackowiak RSJ: The neural correlates of the verbal component of working memory. Nature 1993; 362:342–345Crossref, Medline, Google Scholar
50. Ragland JD, Moelter ST, McGrath C, Hill SK, Gur RE, Bilker WB, Siegel SJ, Gur RC: Levels-of-processing effect on word recognition in schizophrenia. Biol Psychiatry 2003; 54:1154–1161Crossref, Medline, Google Scholar
51. Stone M, Gabrieli JDE, Stebbins GT, Sullivan EV: Working and strategic memory deficits in schizophrenia. Neuropsychology 1998; 12:278–288Crossref, Medline, Google Scholar
52. Henson RNA, Rugg MD, Shallice T, Dolan RJ: Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. J Cogn Neurosci 2000; 12:913–923Crossref, Medline, Google Scholar
53. McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL: Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: an event-related fMRI study. J Cogn Neurosci 2000; 12:965–976Crossref, Medline, Google Scholar
54. Warkentin S, Nilson A, Risberg J, Carlson S: Absence of frontal lobe activation in schizophrenia (abstract). J Cereb Blood Flow Metab 1989; 9(suppl 1):S354Google Scholar
55. Squire LR, Knowlton B, Musen G: The structure and organization of memory. Annu Rev Psychol 1993; 44:453–495Crossref, Medline, Google Scholar
56. Grace AA: Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res 2000; 31:330–341Crossref, Google Scholar
57. Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA: Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci 2000; 3:1149–1152Crossref, Medline, Google Scholar
58. Miller DD, Rezai K, Alliger R, Andreasen NC: The effect of antipsychotic medication on relative cerebral blood perfusion in schizophrenia: assessment with technetium-99m hexamethyl-propyleneamine oxime single photon emission computed tomography. Biol Psychiatry 1997; 41:550–559Crossref, Medline, Google Scholar
59. Medoff DR, Holcomb H, Lahti AC, Tamminga CA: Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus 2001; 11:543–550Crossref, Medline, Google Scholar
60. Miller DD, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Comparison of the effects of risperidone and haloperidol on regional cerebral blood flow in schizophrenia. Biol Psychiatry 2001; 49:704–715Crossref, Medline, Google Scholar
61. Craik F, Lockhart R: Levels of processing: a framework for memory research. J Verbal Learning and Verbal Behavior 1972; 11:671–684Crossref, Google Scholar
62. Schacter DL, Harbluk JL, McLachlan DR: Retrieval without recollection: an experimental analysis of source amnesia. J Verbal Learning and Verbal Behavior 1984; 23:593–611Crossref, Google Scholar



