Reduced Hippocampal Activation During Encoding and Recognition of Words in Schizophrenia Patients
Abstract
OBJECTIVE: In patients with schizophrenia, impaired hippocampal activation either during encoding or recognition tasks has been observed in a few functional imaging experiments. In this fMRI study, the authors report results of word encoding and recognition in schizophrenia patients and healthy comparison subjects, with a special focus on correcting for behavioral recognition success in order to prevent a bias related to lower task performance in the schizophrenia patients. METHOD: The verbal encoding and recognition tasks were both first analyzed irrespective of recognition success. In a second analysis, recognition success was included in the block-designed encoding task as a covariate of no interest, and incorrectly classified items were rejected from the analysis of the event-related recognition task. RESULTS: Patients performed poorer on the recognition task than the comparison subjects. Bilateral hippocampal activation during encoding and recognition was observed in both groups. Right hippocampal activation in patients during recognition became significant only after exclusion of wrongly classified items. Group comparison revealed greater activation in the healthy comparison subjects in the left anterior hippocampus during encoding and bilaterally during recognition. Greater bilateral hippocampal activation in the healthy subjects and greater activation in the right anterior hippocampus in the schizophrenic patients were revealed after presentation of novel words, which were intermixed with previously encoded words in the recognition task. After exclusion of incorrectly classified items, the differences in the right hippocampus remained significant. CONCLUSIONS: This study provides evidence for disturbed hippocampal function during verbal encoding and recognition in patients with schizophrenia. It extends previous studies by correcting for the possible confound of differences in behavioral task performance. This approach further supports the concept of hippocampal dysfunction in schizophrenia.
Reports from different fields of research have highlighted the role of the hippocampus in the pathology of schizophrenia. Shenton and colleagues (1) summarized 49 magnetic resonance imaging (MRI) studies of medial temporal lobe structures, of which 36 (73%) reported a volume reduction in schizophrenic patients relative to comparison subjects. Magnetic resonance spectroscopy studies have revealed a reduction of hippocampal N-acetylaspartate as an indicator of disturbed neuronal function in schizophrenic patients (2). Positron emission tomography (PET) studies have shown reduced glucose metabolism at rest in the hippocampus of schizophrenic patients (3) and increased activation during auditory hallucinations (4). Studies of hippocampal tissue postmortem have revealed cellular abnormalities (5) and altered expression of various mRNA in subjects with schizophrenia (6).
Despite these findings, the functional relevance of hippocampal damage in schizophrenia is not well understood. While neuropsychological studies have described verbal memory deficits as a core feature of cognitive impairment in patients with schizophrenia (7, 8), and functional imaging has extensively documented the crucial role of the hippocampus in memory tasks in healthy subjects (9), functional imaging studies of memory-related hippocampal dysfunction in schizophrenia are rare.
Zorrilla and colleagues (10) reported a correlation of hippocampal activation during novel picture encoding with recognition success in schizophrenic subjects, while they observed an inverse relationship in healthy comparison subjects. In their article, they cite a previous study that revealed decreased hippocampal activation during novel picture encoding in the same group of patients relative to healthy comparison subjects (11).
In a PET study of verbal recognition, Heckers and colleagues (12) detected less activation in the right hippocampus of schizophrenia patients relative to healthy comparison subjects. The same group reported no difference in hippocampal activation during verbal recognition between patients with deficit and nondeficit schizophrenia (13). In a different PET study, Ragland and colleagues (14) reported less activation in the left hippocampus during recognition of studied words in patients relative to healthy comparison subjects.
A general problem of functional imaging studies in which patients and healthy subjects with different task performance are compared is the interpretation of activation differences. In healthy subjects, hippocampal activation during memory tasks is closely related to behavioral performance (15–17). Therefore, decreased activation in patients with inferior behavioral performance might only represent an effect related to lower task performance rather than being disease specific.
In the study by Heckers and colleagues (12), which reported reduced activation in the right hippocampus during recognition in subjects with schizophrenia, the patients performed worse on the behavioral level than did the comparison subjects. In the study by Ragland and colleagues (14), which reported decreased left mesial temporal activation during recognition in schizophrenia subjects, the patients showed a trend toward worse memory performance. Ragland and colleagues addressed this issue by performing a correlation analysis of task performance and activation. However, they did not observe a relationship between mesial temporal activation and recognition success.
In the present functional MRI (fMRI) study, we approached this issue by performing two analyses of a verbal encoding and recognition task. In the first analysis all items were included. In the second analysis, recognition success was introduced in the block-designed encoding task as a covariate of no interest, and only correctly identified items were included in the analysis of the event-related recognition task. If the hippocampus in schizophrenia patients were intact, there should be no group differences in activation after correcting for recognition success. If activation, however, was decreased after correcting for recognition success, the hypothesis of hippocampal dysfunction in schizophrenia would be strengthened. On the basis of evidence from many fields of research in favor of hippocampal damage in schizophrenia, we expected reduced hippocampal activation even after correcting for recognition success during both encoding and retrieval. Since verbal material was used as study items, we expected the differences to be more pronounced in the left hemisphere.
Method
Subjects
Twelve right-handed, native German-speaking patients with DSM-IV schizophrenia (three women and nine men) were included in the study. None had a history of a neurological or medical illness, head trauma, or substance abuse.
The mean age was 27.4 years (SD=5.6), with a mean age at disease onset of 21.5 years (SD=4.6) and mean disease duration of 5.0 years (SD=5.1). All patients were on a stable regimen of atypical neuroleptic medication. On the day of the fMRI examination, psychopathology was assessed with the Positive and Negative Syndrome Scale (18). The mean scores were 13.0 (SD=3.0) on the positive symptom scale, 14.5 (SD=3.3) on the negative symptom scale, and 30.0 (SD=4.6) on the global scale.
For comparison, a group of 12 healthy right-handed, native German-speaking subjects with no history of psychiatric, neurological, or medical disease, head trauma, or substance abuse was recruited. These subjects were individually matched to the schizophrenic patients with respect to gender (three women and nine men) and age (within 1 year). Accordingly, the mean age of the comparison group was 27.7 years (SD=5.6). Both groups were matched for parental years of education (patients=15.4 years [SD=2.5], comparison group=15.5 years [SD=3.0]) (t=0.15, df=22, p=0.88).
After complete description of the study to the subjects, written informed consent was obtained. The protocol was approved by the local ethical committee.
Experimental Design
The study consisted of an encoding and a recognition task, which were both performed in the MRI scanner. The subjects’ heads were fixed with foam pads to minimize movement during MRI data acquisition. The study material was concrete one-syllable German nouns in white capital letters on a black background, which were projected into the MRI scanner. The behavioral responses, subsequently described, were given via a keypad with the left index finger. The response times were recorded. The encoding and recognition tasks were presented in one session. The time between both tasks did not exceed 3 minutes.
Encoding task
The aim of the first part of the experiment was both to achieve deep encoding of verbal material in a controlled semantic task and to avoid the initiation of variable encoding strategies in different subjects. Therefore, the subjects were not instructed to memorize the presented words but to internally associate a second noun to the presented word (e.g., “station” to the presented word “train”) and to press a response button as soon as the task was completed. They were unaware of the successive recognition task. In total, 50 words were presented in this task. The 50 words were presented in five blocks, 10 items each. A single word was presented for a duration of three seconds. Two strings (XOXO, OXOX), which switched at a rate of 0.3 Hz, were included as a 30-second baseline condition between the blocks with the study items.
Recognition task
For the recognition portion of the experiment, the 50 encoded words were presented intermixed with 50 novel one-syllable nouns. The presentation mode was designed to allow event-related analysis of repeated and novel words. Each item was presented for 2 seconds followed by a fixation cross for 1.5 seconds, yielding an interstimulus interval of 3.5 seconds. Fifty “null events” were included in the design. The term “null event” refers to the omission of an item, which results in a doubled interstimulus interval between two words. This allows the assessment of the entire hemodynamic response and increases the sensitivity of the design with respect to the main effects of the different item classes (19). The 50 novel words, the 50 repeated words, and the 50 null events were ordered in a random sequence. The subjects’ task was to press a left button for previously presented words and a right button for novel words. The novel and repeated words were counterbalanced across subjects.
Behavioral Data Analysis
The number of button presses and the reaction times in the encoding task and the number of correctly identified repeated and novel words and the reaction times of both conditions during the recognition task were compared between the groups with Student’s t test.
MRI Data Acquisition
The study was performed in a 1.5-T system (Philips Gyroscan ACS-NT, Philips Medical Systems, Andover, Mass.) with a single-shot gradient echo planar imaging sequence. Functional volumes were acquired perpendicular to the long axis of the medial temporal lobe to achieve a high in-plane resolution of the hippocampus (Figure 1) (TE=45 msec, TR=3 seconds, flip angle=90°, field of view=256×256, matrix=128×128, slice thickness=4 mm, 16 interleaved slices). High-resolution isotropic three-dimensional T1-weighted gradient echo were acquired to anatomically define the hippocampal formation.
MRI Data Analysis
The analysis of the functional MRI data was performed with SPM 99 (20). The functional volumes of each individual were aligned to the first volume in the time series, done separately for the encoding and recognition tasks. The functional volumes of the recognition task were corrected for timing differences within one volume prior to individual realignment. The realigned volumes were normalized into a standard space by using the echo planar imaging template of SPM 99 as the reference and resliced with a voxel size of 2×2×2 mm. Finally, the data were smoothed with a full width at half maximum filter of 8 mm.
For the analysis of the encoding task, a single-subject linear model was created, which used the boxcar function of SPM 99 convolved with the hemodynamic response as regressor. A bandpass filter of 4–120 seconds was applied. Individual subjects’ contrast images were calculated by contrasting the estimates of encoding versus baseline. These individual contrast images were introduced into a one-sample t test to assess within-group effects. Between-group effects were assessed with a two-sample t test. In the second analysis, individual subjects’ contrast images were entered into a one-factor analysis of variance. The individual numbers of correctly recognized items in the recognition task were entered as a covariate of no interest. Within-group and between-group differences were assessed.
The recognition task was designed as an event-related experiment. The linear model for individual subjects’ hemodynamic response to previously studied and novel words were modeled separately with the canonical hemodynamic response function of SPM 99 and the first-order temporal derivative. A low-pass filter of 4 seconds was applied to eliminate high frequency signals. Single subjects’ contrast images were calculated from the estimates of the hemodynamic response function of encoded and novel words. These were entered into a one-sample t test for within-group analysis and in a two-sample t test to assess between-group effects. For the second analysis, individual subjects’ linear models were created for correctly identified encoded words, correctly identified novel words, false alarms, and misses. The contrast images were calculated from the estimates of the hemodynamic response function of correctly identified encoded and novel words. These individual contrast images were again introduced into one- or two-sample t tests to assess within- and between-group effects. The functional imaging results of false alarms and misses are not presented because the number of items was too low.
The focus of the present study was the encoding- and recognition-related activation within the hippocampus. Therefore, anatomical masks for the left and right hippocampus were created from the normalized and averaged high-resolution anatomical MRI scans of all subjects (Figure 2). The masks were created according to the anatomical landmarks described elsewhere (21). The entire interpretation of the results of this study is restricted to these masks. All statistical maps were transformed into z maps, and the significance threshold was p<0.05 (uncorrected). To reduce the likelihood of false positive results, only clusters of significant activation within these masks and with a size of more than 10 suprathreshold voxels are reported. The number of resolution elements (resels) in this study is 11.5 in the left and the right hippocampus.
Results
Behavioral Data
The number of responses, as a measure of completion of the semantic association task during the encoding condition, was 45.6 (SD=3.7) of 50 in the schizophrenia patients and 47.9 (SD=2.0) in the comparison group (t=1.91, df=22, p=0.07). This difference represents a superior performance trend of the healthy comparison group. The mean reaction time during the encoding task was 1.6 seconds (SD=0.3) in the patient group and 1.4 seconds (SD=0.3) in the healthy group, which was not significantly different (t=1.52, df=22, p=0.15).
In the recognition task, the number of correctly recognized previously studied words was significantly lower in the patient group (mean=38.0, SD=5.2) than in the comparison group (mean=41.8, SD=2.9) (t=2.16, df=22, p<0.05). The number of correctly classified novel words did not differ between the patient group (mean=39.5, SD=5.9) and the healthy group (mean=38.8, SD=5.0) (t=0.30, df=22, p=0.77). The mean reaction time in the recognition task was 1.7 seconds (SD=0.6) in the patient group and 1.5 seconds (SD=0.3) in the comparison group. This difference was not significant (t=0.8, df=22, p=0.41).
fMRI Data
Only right and left hippocampal activation according to the anatomical masks was analyzed, and the results are reported accordingly. Table 1 and Table 2 give detailed description of z scores, cluster sizes, and location of peak maxima of both analyses of the encoding and recognition tasks. Figure 3 depicts the clusters of significant group differences within the hippocampus during word encoding and recognition following correction for recognition success.
Hippocampal activation during encoding
During the encoding task, there was significant activation in the right and left hippocampus in both groups. In direct comparison, there was a region in the left anterior hippocampus with a significantly greater activation in the healthy group compared with the schizophrenia patients. The difference in this region remained significant even after introducing recognition success as a covariate of no interest. There was no region with greater activation in the patient group.
Hippocampal activation during recognition
Both groups had areas of significant activation during the recognition of previously studied words in the right and left hippocampus. In the right hippocampus of the patients, this effect only became significant in the analysis that included only correctly identified items. In direct comparison, healthy subjects showed areas of greater activation in both hippocampi in both analyses. There was no region with greater activation in the group of patients than in the healthy comparison subjects.
Both groups had areas of significant bilateral hippocampal activation during the presentation of novel words. Direct comparison revealed bilateral regions with greater activation in the healthy group. In the analysis of only correctly identified novel words, this difference was only significant in the posterior right hippocampus. In the patient group, there was greater activation in the anterior right hippocampus relative to the healthy comparison group in both analyses.
Discussion
We observed smaller activation in the left hippocampus during verbal encoding and smaller bilateral hippocampal activation during verbal recognition in schizophrenic patients compared with healthy subjects. During the presentation of novel words, patients showed less activation in the posterior part of the right hippocampus and greater activation in the anterior part.
In our study, the patients performed poorer on the memory task than did the comparison subjects. Therefore, the reduced left hippocampal activation during encoding could be related to schizophrenia-specific lesions but also to unspecific poorer memory performance (15, 16). We did, however, still observe reduced activation in the left hippocampus after introducing recognition success as a covariate of no interest. This suggests that left hippocampus engagement is reduced in the patients during successful encoding, which might mirror the functional relevance of the hypothesized hippocampal damage in schizophrenia. Furthermore, this result suggests that impaired hippocampal activation must be in part compensated by other memory processes (e.g., priming) because otherwise a difference in hippocampal activation after correcting for recognition success could not be expected.
Neuronal mechanisms that compensate for schizophrenia-related dysfunction of other brain systems have been suggested for facial affect processing in terms of compensatory activation of the cortical mirror systems (22). The present study, however, is limited in detecting brain activation related to compensatory memory mechanisms. The MRI protocol was designed to optimize the functional imaging of the hippocampus, which requires MRI slices perpendicular to the temporal lobe at the expense of full-brain coverage. Priming, for example, is mediated by frontal and occipital-temporal regions (23), which are not covered in the present study. Therefore, the relationship between successful memory performance, reduced hippocampal activation, and compensatory strategies has to be explored in future studies.
Our results are in agreement with Zorrilla and colleagues (11), who reported smaller hippocampal activation in schizophrenic patients during encoding. In their study with pictures as stimuli, the reduced hippocampal activation was bilateral. The patients performed as well on the recognition task as the healthy subjects, which also argues for compensatory hippocampus-independent memory systems. However, the fact that both groups performed equally well implies a simple memory task. If ceiling effects occur (i.e., perfect task completion because of low task demands), the results might be biased because of differences in effort between both groups. In our study, none of the participants achieved perfect task completion, which reduces this potential bias. Ragland and colleagues (14) did not observe differences in hippocampal activation between patients and healthy subjects during encoding.
The reduced hippocampal activation during encoding in the present study might be one basis for the memory deficit in schizophrenia (7, 8), which is particularly characterized by encoding deficits (24).
The present study confirms earlier reports of decreased hippocampal activation during recognition in subjects with schizophrenia (12–14). It extends these results by detecting decreased activation even after only including correctly identified items in the analysis. This is of importance because in parallel to encoding, hippocampal activation during recognition is related to recognition success in healthy subjects (17). Similar to encoding, the decreased hippocampal activation during the correct identification of previously encoded items supports the concept of hippocampal impairment in schizophrenia. The results also suggest that reduced hippocampal engagement is present during encoding and recognition independently. If it was only relevant during encoding, one would not expect reduced activation during successful recognition of previously encoded words. As during encoding, the results suggest that there are memory mechanisms that compensate for hippocampal dysfunction.
With respect to hemispheric lateralization during recognition, the results of other studies are controversial. Heckers and colleagues (12) detected decreased activation in the right hippocampus, whereas Ragland and colleagues (14) reported left hippocampal activation decreases. In the present study, bilateral decreases were observed. At present, firm conclusions about a possible lateralization of hippocampal dysfunction in schizophrenia can therefore not be drawn.
With respect to the identification of novel words, we observed one area of smaller and one area of greater activation in the right hippocampus of schizophrenic patients. At present, there are no other functional imaging studies that investigated novelty-related activation in the hippocampus of schizophrenic patients. The results of the present study suggest that the identification of novel words might be differently organized within the hippocampus in subjects with schizophrenia but might not be disturbed in general. This is supported by the similar number of correctly identified novel words by both groups.
The present study is limited by the following points. First, as mentioned in the introduction, structural MRI studies have revealed smaller hippocampi in schizophrenic patients in comparison with healthy subjects. Smaller hippocampi in the patients could have contributed to the group difference in the present study. However, the effect of atrophy on cerebral activation in fMRI is not well understood. Johnson and colleagues (25) reported a correlation of increasing activation with increasing atrophy in Alzheimer’s disease patients. In opposition, Prvulovic and colleagues (26) recently observed decreased activation in an area of cortical atrophy in Alzheimer’s disease patients. A different potential confound results from comparing normalized brains instead of regions of interest from individual unnormalized brains. In the case of normalized brains, warping might affect the signal intensity at some voxels or might lead to the comparison of areas with and without brain tissue. If this would be a substantial confound, however, one would expect difference in identical brain areas in all contrasts irrespective of the cognitive task. This was not the case in the present study. Because any conclusion drawn from a potential interaction of hippocampal structure and activation in the context of the present study would be very speculative, volumetric measures have not been included in the study design.
Second, the study used a liberal statistical threshold. This, however, is justified in the view of the authors by several points. First, the study was designed with a clear anatomical and functional hypothesis. Second, the statistical analysis is based on a random effect model, which collapses the individual subjects’ scans to one data point, thus allowing us to generalize the results at the expense of statistical power. Third, hippocampal activation is very subtle, which is documented by numerous imaging studies that failed to detect activation in this area. This point is of particular relevance when analyzing differences of hippocampal activation between groups and when including covariates, as in the present study. Finally, the reported clusters include more than 10 adjacent suprathreshold voxels, which makes a false positive result unlikely.
 |
 |
Received June 17, 2002; revision received Dec. 4, 2002; accepted Dec. 13, 2002. From the Department of Psychiatry and the Department of Radiology, University of Bonn. Address reprint requests to Dr. Jessen, Department of Psychiatry, University of Bonn, Sigmund-Freud-Str. 25, 53105 Bonn, Germany; [email protected] (e-mail).
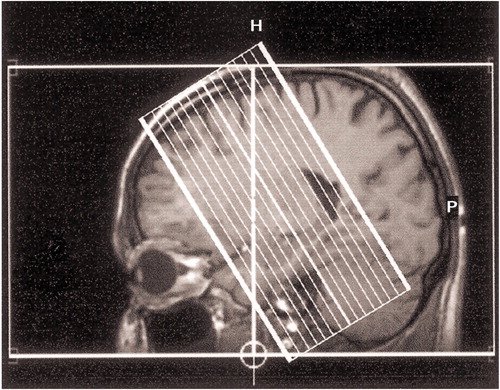
Figure 1. Slice Position of Functional Echo Planar Imaging Scans Obtained From Patients With Schizophrenia (N=12) and Healthy Comparison Subjects (N=12)a
aScans were acquired perpendicular to the long axis of the medial temporal lobe to achieve a high in-plane resolution of the hippocampus.
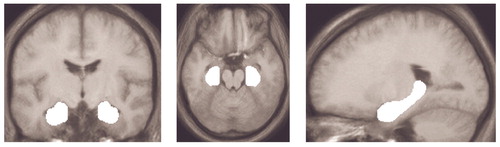
Figure 2. Anatomical Masks of the Left and Right Hippocampus Used in Measuring Activation During Word Encoding and Recognition Among Patients With Schizophrenia (N=12) and Healthy Comparison Subjects (N=12)a
aMasks were created from the normalized and averaged high-resolution T1-weighted MRI scan of all participating subjects. Because the entire study was designed to investigate hippocampal function, only activation within these masks is reported and discussed.
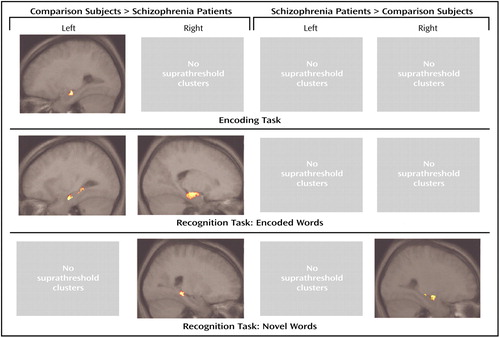
Figure 3. Differences in Hippocampal Activation During Word Encoding and Recognition Between Patients With Schizophrenia (N=12) and Healthy Comparison Subjects (N=12) After Correction for Recognition Successa
aThe clusters are projected on the sagittal slice, which contains the peak voxel of the largest cluster of each contrast. The statistical threshold is p<0.05 uncorrected with a cluster size of >10 voxels. Exact locations, cluster sizes, and stereotactic coordinates are listed in Table 2. Note that only activation located within the anatomical mask is shown, since this study focused on hippocampal activation.
1. Shenton ME, Dickey CC, Frumin M, McCarley RW: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1-52Crossref, Medline, Google Scholar
2. Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CTW, Frank JA, Tedeschi G, Weinberger DR: Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry 1996; 153:1554-1563Link, Google Scholar
3. Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT: Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522-530Crossref, Medline, Google Scholar
4. Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, et al: A functional neuroanatomy of hallucinations in schizophrenia. Nature 1995; 378:176-179Crossref, Medline, Google Scholar
5. Harrison PJ, Eastwood SL: Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus 2001; 11:508-519Crossref, Medline, Google Scholar
6. Weinberger DR: Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry 1999; 45:395-402Crossref, Medline, Google Scholar
7. Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE: Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. Am J Psychiatry 1999; 156:1336-1341Abstract, Google Scholar
8. Kuperberg G, Heckers S: Schizophrenia and cognitive function. Curr Opin Neurobiol 2000; 10:205-210Crossref, Medline, Google Scholar
9. Schacter DL, Wagner AD: Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 1999; 9:7-24Crossref, Medline, Google Scholar
10. Zorrilla LT, Jeste DV, Brown GG: Functional MRI and novel picture-learning among older patients with chronic schizophrenia: abnormal correlations between recognition memory and medial temporal brain response. Am J Geriatr Psychiatry 2002; 10:52-61Crossref, Medline, Google Scholar
11. Zorrilla LT, Jeste DV, Paulus M, Brown GG: Functional abnormalities of medial temporal cortex during novel picture learning among patients with chronic schizophrenia. Schizophr Res 2003; 59:187-198Crossref, Medline, Google Scholar
12. Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM: Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1998; 1:318-323Crossref, Medline, Google Scholar
13. Heckers S, Goff D, Schacter DL, Savage CR, Fischman AJ, Alpert NM, Rauch SL: Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Arch Gen Psychiatry 1999; 56:1117-1123Crossref, Medline, Google Scholar
14. Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE: Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry 2001; 158:1114-1125Link, Google Scholar
15. Fernandez G, Weyerts H, Schrader-Bolsche M, Tendolkar I, Smid HG, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR, Heinze HJ: Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J Neurosci 1998; 18:1841-1847Crossref, Medline, Google Scholar
16. Fernandez G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dumpelmann M, Van Roost D, Elger CE: Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science 1999; 285:1582-1585Crossref, Medline, Google Scholar
17. Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA: Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci 2000; 3:1149-1152Crossref, Medline, Google Scholar
18. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261-276Crossref, Medline, Google Scholar
19. Josephs O, Henson RN: Event-related functional magnetic resonance imaging: modelling, inference and optimization. Philos Trans R Soc Lond B Biol Sci 1999; 354:1215-1228Crossref, Medline, Google Scholar
20. Friston KJ: Statistical parametric mapping and other analyses of functional imaging data, in Brain Mapping: The Methods. Edited by Toga AW, Mazziotta JC. New York, Academic Press, 1996, pp 363-386Google Scholar
21. Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC: Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry 2000; 57:769-775Crossref, Medline, Google Scholar
22. Quintana J, Davidson T, Kovalik E, Marder SR, Mazziotta JC: A compensatory mirror cortical mechanism for facial affect processing in schizophrenia. Neuropsychopharmacology 2001; 25:915-924Crossref, Medline, Google Scholar
23. Schacter DL, Buckner RL: Priming and the brain. Neuron 1998; 20:185-195Crossref, Medline, Google Scholar
24. Harvey PD, Earle-Boyer EA, Weilgus MS, Levinson JC: Encoding, memory, and thought disorder in schizophrenia and mania. Schizophr Bull 1986; 12:252-261Crossref, Medline, Google Scholar
25. Johnson SC, Saykin AJ, Baxter LC, Flashman LA, Santulli RB, McAllister TW, Mamourian AC: The relationship between fMRI activation and cerebral atrophy: comparison of normal aging and Alzheimer disease. Neuroimage 2000; 11:179-187Crossref, Medline, Google Scholar
26. Prvulovic D, Hubl D, Sack AT, Melillo L, Maurer K, Frolich L, Lanfermann H, Zanella FE, Goebel R, Linden DE, Dierks T: Functional imaging of visuospatial processing in Alzheimer’s disease. Neuroimage 2002; 17:1403-1414Crossref, Medline, Google Scholar



