Diagnostic Specificity and Neuroanatomical Validity of Neurological Abnormalities in First-Episode Psychoses
Abstract
OBJECTIVE: Neurological abnormalities are frequently seen in patients with first-episode psychotic disorders but are generally considered to be diagnostically nonspecific, neurologically nonlocalizing, and, hence, “soft.” This study examined the neuroanatomical correlates and diagnostic specificity of abnormal findings on the neurological examination in first-episode schizophrenia and other psychotic disorders. METHOD: Neuroleptic-naive patients with schizophrenia (N=90) and with nonschizophrenia psychoses (N=39) and carefully matched healthy subjects (N=93) were compared on total and factor scores for a reliable subset of Neurological Evaluation Scale items. The relationship between neurological examination abnormalities and alterations in the relevant brain structures as assessed by magnetic resonance imaging was examined in a subset of subjects. RESULTS: Factor scores for repetitive motor task abnormalities were higher in both patient groups, relative to the healthy group, and did not distinguish between the patient groups. Factor scores for abnormalities in cognitively demanding and perceptual tasks were markedly higher in the schizophrenia group, relative to both comparison groups, and were not different between the nonschizophrenia psychoses group and the healthy comparison group. Higher scores for the cognitive/perceptual abnormalities factor were correlated with smaller volumes of the left heteromodal association cortex. CONCLUSIONS: Neurological signs may serve as expedient bedside measures that are potentially useful in the assessment of idiopathic psychoses, and cognitive/perceptual neurological signs may have a measure of diagnostic specificity. These findings provide neurobiological validation of abnormal findings on the neurological examination. These abnormalities may reflect discrete neuroanatomical alterations in schizophrenia and may have a localizing value.
Bedside neurological evaluations have been employed for several decades in studies addressing the question of schizophrenia’s “organic” basis (reviewed in references 1–3). Neurological examination abnormalities have been generally called “soft” neurological signs, in keeping with their reputed lack of specificity, validity, or localizing value (3, 4). Several studies have shown that patients with schizophrenia have higher scores on measures of neurological abnormalities, compared with healthy persons. Many previous studies were limited by possible confounding factors, including prior use of neuroleptic medications and illness chronicity. Studies of first-episode, never-treated schizophrenia patients can address these confounds. Observations of generalized neuropsychological impairment in first-episode schizophrenia (5–8) have suggested a higher rate of global neurological examination abnormalities in such patients than in healthy comparison subjects. However, only a few studies (9–11) have explored neurological examination abnormalities in first-episode patients.
The diagnostic specificity of neurological examination abnormalities for schizophrenia remains unclear. As with many other aspects of schizophrenia neurobiology, comparisons of neurological examination abnormalities between schizophrenia patients and other psychiatric groups have yielded less consistent results than have comparisons of schizophrenia patients and healthy subjects. Comparisons of neurological examination abnormalities and overlapping neuropsychological deficits in schizophrenia and nonpsychotic psychiatric disorders have yielded mixed results, although a tendency for greater impairment in schizophrenia has been reported (12–15). Boks et al. (16), reviewing this literature, found that only tactile extinction and four conventionally cerebellar signs were significantly more prevalent in schizophrenia than in mood disorders; insufficient data were available to assess the diagnostic specificity of motor sequencing tasks. Prior studies of diagnostic specificity (2, 3) have not accounted for the possible effects of chronicity and prior treatments on neurological performance.
Comparisons between schizophrenia patients and patients with other psychotic disorders in particular domains of neurological performance can shed light on the specific functional deficits of schizophrenia and increase the clinical relevance of the neurological examination in the diagnosis and treatment of psychoses. Psychotic patients are well served by accurate pretreatment diagnosis, particularly because accurate diagnosis influences the likelihood of appropriate treatment (17, 18). Although clinicians sometimes avoid a definitive diagnosis until they have observed the early course of the patient’s illness, the early course is itself influenced by the initial treatment. However, problems with communication and cooperation during initial evaluation of a psychotic person can make it difficult for a clinician to make a diagnosis by using standard phenomenology-based criteria. Supporting data can thus be valuable in the clinical assessment of psychoses, particularly at the patient’s first presentation.
The validity of neurological examination abnormalities in schizophrenia also remains uncertain. Structural brain abnormalities in schizophrenia, as measured by magnetic resonance imaging (MRI), include smaller volumes in the heteromodal association cortex (19), basal ganglia (20–22), and cerebellum (23). Although MRI has been used to examine relations between structure and cognitive deficits (e.g., references 24–26), few studies have examined relationships between neurological abnormalities and brain structure. Given the increasingly held view that schizophrenia is associated with structural changes in a widely distributed network of association cortical structures (19, 27, 28), it is likely to be more fruitful to examine correlates of composite brain structures such as the heteromodal association cortex rather than single structures in isolation. Our first goal was to examine diagnostically specific differences between patients with first-episode schizophrenia, patients with nonschizophrenia psychoses, and healthy comparison subjects. The three groups were compared on motor and cognitive neurological examination abnormalities by using subscales of the Neurological Evaluation Scale (29) derived from principal-components analysis (30). Second, we examined the relationships between neurological examination abnormalities and structural brain abnormalities proposed to underlie the neurological functions that are impaired in schizophrenia. Specifically, we predicted 1) that scores for the more cognitively demanding perceptual tasks included in the neurological examination would be correlated with volumes of the association cortex and 2) that scores for the motor tasks would be correlated with volumes of the basal ganglia and the cerebellum. To address these questions, we examined a subset of first-episode schizophrenia patients with both MRI data and data on neurological examination abnormalities.
Method
Subjects
Patients were recruited from the inpatient and outpatient services of the Western Psychiatric Institute and Clinic, Pittsburgh, for a longitudinal study of first-episode psychosis (31). Patients with previously untreated psychosis were evaluated by trained clinicians using the Structured Clinical Interview for DSM-IV, Patient Edition (SCID-P) (32) and medical, neurological, and psychiatric assessments. Patients who were judged by the treating psychiatrist to be too ill to understand the study and competently provide informed consent were not approached to participate in the study. Patients were eligible to enter the study if they were aged 15–45 years, had an IQ >75, met the DSM-IV criteria for a psychotic disorder, and had no prior treatment with neuroleptics, no significant medical or neurological illness, no history of head injury with loss of consciousness temporally related to psychosis onset, and no current substance abuse or dependence. DSM-IV diagnoses were derived in consensus diagnostic evaluations by the raters and senior clinicians, including the first author, on the basis of all available clinical data and the SCID-P interview data. Ninety subjects were given a diagnosis of schizophrenia (N=74), schizophreniform disorder (N=3), or schizoaffective disorder (N=13), and 39 were given a diagnosis of another psychotic disorder, including psychotic depression (N=12), bipolar disorder with psychosis (N=11), delusional disorder (N=3), and psychosis not otherwise specified (N=13). All diagnoses were formally confirmed after at least 6 months of follow-up.
Ninety-three healthy comparison subjects were recruited by means of advertisements in local neighborhoods and communities in which the patients resided. After participating in an initial telephone screening, these subjects were interviewed with the Structured Clinical Interview for DSM-IV–Non-Patient Edition (SCID-I/NP) (33) by a psychiatrist (the first author) or a clinical psychologist. The comparison subjects had no current or past axis I disorder, no prior exposure to any psychotropic medication within 6 months of the baseline assessment, no history of neurological disorders or any other chronic medical problems with potential to influence neurological function, no mental retardation (IQ <75), and no reported history of schizophrenia or major mood disorder in first-degree relatives. The healthy comparison subjects and the patients did not differ in age, gender, or parental socioeconomic status as measured by the Hollingshead Four-Factor Index (34). However, significantly more patients with nonschizophrenia psychoses were Caucasian, compared with the patients with schizophrenia and the healthy comparison subjects (Table 1). After receiving a full explanation of the study, all patients and comparison subjects provided written informed consent.
Neurological Evaluation
Neurological evaluations were carried out by using a modified version of the Neurological Evaluation Scale (29). The evaluations were done before patients began taking antipsychotic medication. The Neurological Evaluation Scale consists of 29 items, most of which are rated 0, 1, or 2 (strictly dichotomous measures are scored 0 or 2). The Neurological Evaluation Scale was administered in its entirety according to the original instructions of the instruments’ developers (29) by a trained clinician who was unaware of the consensus diagnoses. The examination was supplemented by testing for the palmomental reflex (35). The clinicians who conducted these assessments had established interrater reliability, as previously described (36), and periodically conducted assessments together to avoid rater drift. The reliability studies involving these examiners had determined that 13 items from the supplemented scale showed adequate frequency of abnormality (>10%) and consistently adequate interrater reliability with several groups of psychotic patients and combinations of raters (36). Only these 13 items were entered into the primary analyses reported here. For items measured bilaterally, only the higher of the two ratings was included in the analysis. Lateral dominance items (handedness, etc.) were not considered tests of performance and were therefore omitted from the analysis. Like a previous study conducted by our group (30), the current study included a heterogeneous group of unmedicated patients with schizophrenia and with nonschizophrenia psychoses, and the 13 Neurological Evaluation Scale variables from all patients were entered into a principal-components analysis with varimax normalized rotation. Scores derived by summing the factor components were the primary dependent variables in the current analysis.
Neuroimaging Studies
MRI and Neurological Evaluation Scale data were available for 17 schizophrenia patients (12 male and five female patients; mean age=29.53 years, SD=8.46), nine patients with nonschizophrenia psychoses (five male and four female patients; mean age=22.44 years, SD=5.43), and 18 healthy comparison subjects (13 male and five female subjects; mean age=23.94 years, SD=5.37). MRI scans were conducted by using a 1.5-T Signa whole- body GE Scanner (General Electric Medical Systems, Milwaukee). Regional brain volumes were measured with three-dimensional spoiled gradient recall acquisition in the steady-state pulse sequence, which obtained 2.6-mm thick contiguous axial images (echo time=20 msec, repetition time=40 msec, acquisition matrix=256×192, field of view=20 cm, flip angle=10°). To facilitate image orientation, axial slices were obtained parallel to the anterior commissure–posterior commissure line. NIH Image software (version 1.55) (37) was used to measure brain anatomy. None of the MRI scans in this data set showed motion and magnetic field inhomogeneity artifacts. All measurements were conducted by a trained and reliable rater who was blind to the clinical data and to the subjects’ groups. Interrater and intrarater reliabilities, measured on 10 scans completed about a month apart, were adequate for all the measured brain structures (intraclass r>0.90).
Our approach to measuring caudate volumes has been described earlier (20). The heteromodal association cortex volume was computed by summing the volumes of the dorsolateral prefrontal cortex, the superior temporal gyrus, and the inferior parietal cortex. The dorsolateral prefrontal cortex and superior temporal gyrus were measured by using methods described previously (38, 39). To measure the inferior parietal cortex, a ring was drawn in the subcortical white matter 2 cm from the cortical surface in axial slices (40). The hemispheres were divided into four regions by using three perpendicular lines drawn across the interhemispheric fissure at the most posterior point of the genu, the most posterior point in the splenium of the corpus callosum, and the midpoint between these two points. The parietal cortex was defined as the third most anterior cortical region, with the uppermost slice showing the lateral ventricles as the upper limit and the lowermost slice showing the corpus callosum as the lower limit. To measure cerebellar volumes, consecutive axial slices showing cerebellar structures were measured. Total volumes were measured for the left and right cerebellar hemispheres, including the brainstem, midbrain, and cerebellar vermes.
Data Analyses
Data were analyzed by using analysis of variance (ANOVA), with diagnosis as the grouping variable and the neurological examination abnormalities measures (the factor scores and the total score for the 13 reliable items) as dependent variables. When the ANOVA yielded significant results, Newman-Keuls post hoc tests were used to examine the specific group differences. Correlational analyses were done by using Spearman correlations and partial correlations. Two-tailed significance tests were used throughout, and alpha was set at p<0.05. Bonferroni corrections were applied to address multiple comparisons.
Results
Factor Analysis
The principal-components analysis revealed four factors with eigenvalues >1 (Table 2). The first consisted of tests requiring repetitive hand movements (fist-ring, fist-edge-palm, alternating fist-palm, and rapid alternating movements). The second factor consisted of tasks that were more complex and more cognitively demanding and that involved sensory processing (verbal memory, audiovisual integration, and face-hand test). The Romberg test and the palmomental reflex test loaded onto a third factor, and the graphesthesia test loaded onto a fourth. Tandem gait, tap reproduction, and right-left orientation did not load strongly or selectively on any factor. Together, the four factors accounted for 54% of the total variance.
Neurological Evaluation
Group comparisons by ANOVA (Table 3) were highly significant for the two primary factors but not statistically significant for the third and fourth factors (p>0.10). Post hoc comparisons of groups (Table 3) showed significant relative impairment of motor tasks (tests for repetitive motor neurological abnormalities) in both patient groups and impairment in the cognitively demanding perceptual tasks in only the schizophrenia group. A repeat of the analysis using average Neurological Evaluation Scale factor scores instead of total scores showed the same findings (Figure 1). Inclusion of tap reproduction and right-left orientation in the motor abnormalities factor, as suggested by a previously described factor solution for data from a larger, heterogeneous group of schizophrenia patients (30), also yielded the same results. The conceptually derived sensory integration subscale (face-hand test, audiovisual integration, right-left orientation, stereognosis test, and graphesthesia test) (34) also showed a significant group effect (F=18.57, df=2, 219, p<0.0001); post hoc comparisons of patient groups showed significant differences, with the schizophrenia patients having more neurological examination abnormalities than the patients with nonschizophrenia psychoses (p=0.003, Newman-Keuls).
Because there were fewer Caucasian subjects among the schizophrenia patients than among the patients with nonschizophrenia psychoses, we compared factor scores for Caucasian, African American, and other ethnic group subjects. The cognitive/perceptual neurological abnormalities factor score was significantly higher in the non-Caucasian patients (F=4.07, df=2, 219, p=0.01). Restricting the comparison of diagnostic groups to Caucasian subjects (the number of non-Caucasian patients was insufficient for diagnostic group comparisons) yielded the same results as described earlier. Excluding the schizoaffective disorder group also yielded the same results. Analysis of covariance, with age as a covariate, also had no effect on group differences in neurological examination abnormalities.
Neuroanatomical Correlates of Neurological Examination Abnormalities
In the schizophrenia group, scores for the repetitive motor neurological examination abnormalities factor correlated significantly with left caudate (rs=–0.54, N=17, p=0.03) and cerebellum (rs=–0.6, N=16, p=0.01) volumes. Cognitive/perceptual neurological abnormalities factor scores correlated with left caudate (rs=–0.61, N=15, p=0.02), cerebellum (rs=–0.67, N=16, p=0.005), and left heteromodal association cortex (rs=–0.67, N=16, p=0.005) volumes. The left heteromodal association cortex and cerebellum volumes were significantly correlated with cognitive/perceptual neurological abnormalities factor scores after Bonferroni correction. None of these correlations was significant in the healthy comparison group or the group with nonschizophrenia psychoses. The correlation of cognitive/perceptual neurological abnormalities factor scores with left heteromodal association cortex volumes persisted after correction for intracranial volume.
We also examined the relationships between the components of heteromodal association cortex volume (dorsolateral prefrontal cortex, superior temporal gyrus, and inferior parietal cortex volumes) and the Neurological Evaluation Scale measures. Repetitive motor neurological abnormalities factor scores did not correlate with the volumes of any of the heteromodal association cortex components. On the other hand, cognitive/perceptual neurological abnormalities factor scores correlated significantly with left dorsolateral prefrontal cortex volumes (rs=–0.51, N=16, p=0.04) and with right superior temporal gyrus volumes (rs=–0.55, N=16, p=0.03) in the schizophrenia group. Neither of these correlations was significant in the nonschizophrenia psychoses group or the comparison group.
Discussion
Our main observations are that repetitive motor task performance was impaired in both schizophrenia patients and patients with nonschizophrenia psychoses and that repetitive motor task performance did not distinguish between the two patient groups. On the other hand, cognitively demanding and perceptual tasks were markedly more impaired in schizophrenia patients than in either the patients with nonschizophrenia psychoses or the healthy comparison subjects. Thus, neurological examination abnormalities may have some measure of diagnostic specificity among the idiopathic psychoses. This observation is of clinical relevance and may help clarify the pathophysiological basis of schizophrenia, as contrasted with the other psychoses.
Our findings of a higher rate of cognitive/perceptual neurological examination abnormalities in schizophrenia and of a higher rate of motor abnormalities in both groups of patients with psychoses are somewhat consistent with previous findings. Schizophrenia patients have been found to perform similarly to patients with psychotic depression on motor (41) and pursuit eye movement tasks (42). Patients with schizophrenia and patients with schizoaffective disorder have been found to be more impaired in “parietal” tasks (those measured by the stereognosis, double-simultaneous stimulation, construction, and logical-grammatical relationships tests) but not in motor tasks, compared to patients with psychotic mood disorders (43–45). Not all studies have found such diagnosis effects (8, 46). Nevertheless, our observations raise the possibility that cognitively demanding perceptual components of the neurological examination have neuropsychological parallels in schizophrenia and may be related to neurobiological impairments in specific brain circuits that mediate these functions. Validating this point, we found that MRI measures had a distinct pattern of relationships to the two predominant neurological examination abnormalities factors.
Few studies have examined the neuroanatomical correlates of neurological examination abnormalities in schizophrenia by looking at specific structural-neurological relationships. Two groups (47, 48) found smaller basal ganglia volumes to be associated with motor deficits. In our data, cognitive/perceptual neurological examination abnormalities were significantly and strongly correlated with smaller volumes in the left heteromodal association cortex and the cerebellum and were less strongly correlated with caudate volumes. On the other hand, motor abnormalities tended to be correlated with smaller right and left caudate and cerebellar volumes but not with heteromodal association cortex volumes. Thus, our MRI findings suggest that specific domains of impaired neurological functions observed in first-episode schizophrenia patients may stem from somewhat distinct neuroanatomical alterations in schizophrenia. This view is consistent with our observations that the cognitive/perceptual neurological abnormalities factor correlated more strongly than the motor abnormalities factor with neuropsychological assessments that tap into executive function and memory (49). On the other hand, the motor abnormalities seen in the patients with nonschizophrenia psychoses may be associated with cerebellar and striatal involvement and a relative preservation of the association cortex. The lack of such correlations in the data for the patients with nonschizophrenia psychoses in our study suggests that the observed neurological-anatomical relations are specific to schizophrenia.
The strengths of this study included the relatively large number of well-characterized, neuroleptic-naive psychotic subjects; the use of only reliable items from the Neurological Evaluation Scale in the evaluations; and the use of factor analyses to reduce the number of neurological tests and the number of pathophysiologically meaningful dimensions derived from the tests. In addition, both a well-matched healthy comparison group and a group of first-episode patients with nonschizophrenia psychoses were examined. However, all patients in the study were well enough to provide informed consent and to tolerate extensive testing and interviews before treatment; thus, the patients in the study may not be representative of patients with never-treated psychoses. As stated earlier, examiners were often aware of subjects’ symptoms and histories and therefore may not have been blind to clinical data. However, they were not aware of the consensus research diagnoses that were confirmed weeks after their evaluations, and the schizophrenia-specific finding was not anticipated. The psychotic mood disorder group was restricted to patients with mood-incongruent psychotic features. This limitation may not have weakened the finding of diagnostic specificity but may have diminished the representativeness of the group with nonschizophrenic psychoses. Finally, the significant intersubject variability in the anatomical landmarks made precise partition of the heteromodal association cortex difficult; the geometrical partitioning approach we used may not be neuroanatomically precise, but it has good reliability.
Although replication of the study findings with larger, more heterogeneous groups of patients is needed, our findings provide new evidence suggesting that the so-called “soft” neurological signs have some measure of diagnostic specificity and neuroanatomical validity within the broad group of idiopathic psychoses. These observations have implications for further etiological and pathophysiological research in schizophrenia, as some evidence suggests that “integrative” neurological examination abnormalities may be useful in defining genetic risk for schizophrenia (50). Because these bedside measures are relatively inexpensive and convenient to use, they may be valuable as part of neuropsychiatric assessments in both developed and developing countries.
 |
 |
 |
Received May 9, 2002; revision received Dec. 27, 2002; accepted Jan. 7, 2003. From the Department of Psychiatry, Western Psychiatric Institute and Clinic and University of Pittsburgh School of Medicine; Dayton Veterans Affairs Medical Center, Dayton, Ohio; the Center for Cognitive Medicine, University of Illinois at Chicago, Chicago; and the Department of Psychiatry Research, Hillside Hospital Division, North Shore–Long Island Jewish Health System, Glen Oaks, N.Y. Address reprint requests to Dr. Keshavan, Western Psychiatric Institute and Clinic, 3811 O’Hara St., Room 984, Pittsburgh, PA 15213; [email protected] (e-mail). Supported in part by NIMH grants MH-45203, MH-01180, and MH-45156 (Dr. Keshavan) and by NIH General Clinical Research Center grant M01 RR-00056. The authors thank Nancy McLaughlin, Gina Perez, and Joseph Pierri for conducting the neurological assessments and Drs. Gretchen L. Haas, Elizabeth D. Radomsky, and Debra M. Montrose for supervising the clinical assessments.
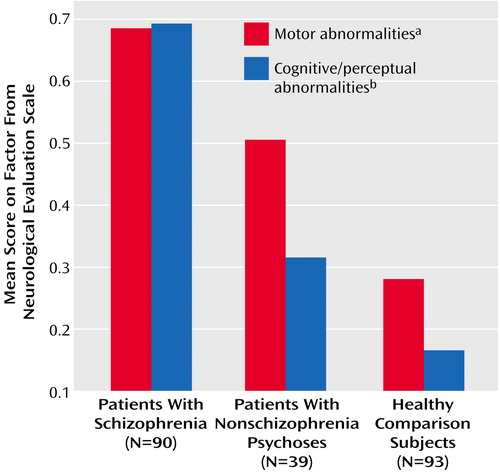
Figure 1. Scores on the Motor and Cognitive/Perceptual Factors of the Neurological Evaluation Scale of First-Episode Patients With Psychoses and Healthy Comparison Subjects
aSignificant differences between patients with schizophrenia and healthy subjects and between patients with nonschizophrenia psychoses and healthy subjects (Newman-Keuls test).
bSignificant differences between patients with schizophrenia and patients with nonschizophrenia psychoses and between patients with schizophrenia and healthy subjects (Newman-Keuls test).
1. Cadet JL, Rickler KC, Weinberger DL: The clinical neurologic examination in schizophrenia, in The Neurology of Schizophrenia. Edited by Nasrallah HA, Weinberger DL. New York, Elsevier, 1986, pp 1-47Google Scholar
2. Heinrichs DW, Buchanan RW: Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry 1988; 145:11-18Link, Google Scholar
3. Sanders RD, Keshavan MS: The neurologic examination in adult psychiatry: from soft signs to hard science. J Neuropsychiatry Clin Neurosci 1998; 10:395-404Crossref, Medline, Google Scholar
4. Keshavan MS, Yeragani VK: Primitive reflexes in psychiatry (letter). Lancet 1987; 1:1264Crossref, Medline, Google Scholar
5. Hoff AL, Riordan H, O’Donnell DW, Morris L, DeLisi LE: Neuropsychological functioning of first-episode schizophreniform patients. Am J Psychiatry 1992; 149:898-903Link, Google Scholar
6. Saykin AJ, Shtasel D, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC: Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry 1994; 51:124-131Crossref, Medline, Google Scholar
7. Rubin P, Holm A, Moller-Madsen S, Videbech P, Hertel C, Povlsen UJ, Hemmingsen R: Neuropsychological deficit in newly diagnosed patients with schizophrenia or schizophreniform disorder. Acta Psychiatr Scand 1995; 92:35-43Crossref, Medline, Google Scholar
8. Albus M, Hubmann W, Wahlheim C, Sobizack N, Franz U, Mohr F: Contrasts in neuropsychological test profile between patients with first-episode schizophrenia and first-episode affective disorders. Acta Psychiatr Scand 1996; 94:87-93Crossref, Medline, Google Scholar
9. Rubin P, Vorstrup S, Hemmingsen R, Andersen HS, Bendsen BB, Stromso N, Larsen JK, Bolwig TG: Neurological abnormalities in patients with schizophrenia or schizophreniform disorder at first admission to hospital: correlations with computerized tomography and regional cerebral blood flow findings. Acta Psychiatr Scand 1994; 90:385-390Crossref, Medline, Google Scholar
10. Gupta S, Andreasen NC, Arndt S, Flaum M, Schultz SK, Hubbard WC, Smith M: Neurological soft signs in neuroleptic-naive and neuroleptic-treated schizophrenic patients and in normal comparison subjects. Am J Psychiatry 1995; 152:191-196Link, Google Scholar
11. Sanders RD, Keshavan MS, Schooler NR: Neurologic examination abnormalities in neuroleptic-naive patients with first-break schizophrenia: preliminary results. Am J Psychiatry 1994; 151:1231-1233Link, Google Scholar
12. Hertzig ME, Birch HG: Neurologic organization in psychiatrically disturbed adolescent girls. Arch Gen Psychiatry 1966; 15:590-598Crossref, Medline, Google Scholar
13. Tucker GJ, Campion EW, Silberfarb PM: Sensorimotor functions and cognitive disturbance in psychiatric patients. Am J Psychiatry 1975; 132:17-21Link, Google Scholar
14. Quitkin F, Rifkin A, Klein DF: Neurologic soft signs in schizophrenia and character disorders: organicity in schizophrenia with premorbid asociality and emotionally unstable character disorders. Arch Gen Psychiatry 1976; 33:845-853Crossref, Medline, Google Scholar
15. Cox SM, Ludwig AM: Neurological soft signs and psychopathology, I: findings in schizophrenia. J Nerv Ment Dis 1979; 167:161-165Crossref, Medline, Google Scholar
16. Boks MP, Russo S, Knegtering R, van den Bosch RJ: The specificity of neurological signs in schizophrenia: a review. Schizophr Res 2000; 43:109-116Crossref, Medline, Google Scholar
17. Gatti F, Bellini L, Gasperini M, Perez J, Zanardi R, Smeraldi E: Fluvoxamine alone in the treatment of delusional depression. Am J Psychiatry 1996; 153:414-416Link, Google Scholar
18. Zanardi R, Franchini L, Gasperini M, Perez J, Smeraldi E: Double-blind controlled trial of sertraline versus paroxetine in the treatment of delusional depression. Am J Psychiatry 1996; 153:1631-1633Link, Google Scholar
19. Pearlson GD, Petty RG, Ross CA, Tien AY: Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology 1996; 14:1-17Crossref, Medline, Google Scholar
20. Keshavan MS, Rosenberg D, Sweeney JA, Pettegrew JW: Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry 1998; 155:774-778Abstract, Google Scholar
21. Shihabuddin L, Buchsbaum M, Hazlett E, Haznedar M, Harvey P, Newman A, Schnur D, Spiegel-Cohen J, Wei T, Machac J, Knesaurek K, Vallabhajousula S, Biren M, Ciaravolo T, Luu-Hsia C: Dorsal striatal size, shape, and metabolic in never-medicated and previously medicated schizophrenics performing a verbal learning task. Arch Gen Psychiatry 1998; 55:235-243Crossref, Medline, Google Scholar
22. Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC: Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry 1999; 156:1200-1204Abstract, Google Scholar
23. Katsetos CD, Hyde TM, Herman MM: Neuropathology of the cerebellum in schizophrenia—an update: 1996 and future directions. Biol Psychiatry 1997; 42:213-224Crossref, Medline, Google Scholar
24. Yates WR, Swayze VW II, Andreasen NC: Neuropsychological effect of global and focal cerebral atrophy in schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol 1990; 3:98-106Google Scholar
25. Flashman LA, Flaum M, Gupta S, Andreasen NC: Soft signs and neuropsychological performance in schizophrenia. Am J Psychiatry 1996; 153:526-532Link, Google Scholar
26. Baare WF, Hulshoff Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS: Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry 1999; 45:1597-1605Crossref, Medline, Google Scholar
27. Andreasen NC, Paradiso S, O’Leary DS: “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:203-218Crossref, Medline, Google Scholar
28. Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, Lonnqvist J, Standerskjold-Nordenstam CG, Narr KL, Khaledy M, Zoumalan CI, Dail R, Kaprio J: Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci USA 2002; 99:3228-3233Crossref, Medline, Google Scholar
29. Buchanan RW, Heinrichs DW: The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res 1989; 27:335-350Crossref, Medline, Google Scholar
30. Sanders RD, Keshavan MS, Forman SD, Pierri JN, McLaughlin N, van Kammen DP, Goldstein G: Factor structure of neurologic examination abnormalities in unmedicated schizophrenia. Psychiatry Res 2000; 95:237-243Crossref, Medline, Google Scholar
31. Keshavan MS, Schooler NR, Sweeney JA, Haas GL, Pettegrew JW: Research and treatment strategies in first-episode psychoses: the Pittsburgh experience. Br J Psychiatry Suppl 1998; 172:60-65Crossref, Medline, Google Scholar
32. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
33. First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (SCID-I/NP), version 2.0. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
34. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
35. Keshavan MS, Kumar YV, Channabasavanna SM: A critical evaluation of infantile reflexes in neuropsychiatric diagnosis. Indian J Psychiatry 1979; 21:267-270Google Scholar
36. Sanders RD, Forman SD, Pierri JN, Baker RW, Kelley ME, van Kammen DP, Keshavan MS: Inter-rater reliability of the neurological examination in schizophrenia. Schizophr Res 1998; 29:287-292Crossref, Medline, Google Scholar
37. Rasband W: NIH Image Manual. Bethesda, Md, National Institutes of Health, 1993Google Scholar
38. Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS: Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry 2001; 158:618-624Link, Google Scholar
39. Keshavan MS, Haas GL, Kahn CE, Aguilar E, Dick EL, Schooler NR, Sweeney JA, Pettegrew JW: Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res 1998; 32:161-167Crossref, Medline, Google Scholar
40. Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD: Decreased regional cortical gray matter volume in schizophrenia. Am J Psychiatry 1994; 151:842-848Link, Google Scholar
41. Jeste DV, Heaton S, Paulsen JS, Ercoli L, Harris MJ, Heaton RK: Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry 1996; 153:490-496Link, Google Scholar
42. Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME: Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol Psychiatry 1999; 46:671-680Crossref, Medline, Google Scholar
43. Schwartz F, Carr A, Munich R, Bartuch E, Lesser B, Rescigno D, Viegener B: Voluntary motor performance in psychotic disorders: a replication study. Psychol Rep 1990; 66(3, part 2):1223-1234Google Scholar
44. Walker E: Attentional and neuromotor functions of schizophrenics, schizoaffectives, and patients with other affective disorders. Arch Gen Psychiatry 1981; 38:1355-1358Crossref, Medline, Google Scholar
45. Mitrushina M, Abara J, Blumenfeld A: A comparison of cognitive profiles in schizophrenia and other psychiatric disorders. J Clin Psychol 1996; 52:177-190Crossref, Medline, Google Scholar
46. Silverstein ML, McDonald C, Meltzer HY: Differential patterns of neuropsychological deficit in psychiatric disorders. J Clin Psychol 1988; 44:412-415Crossref, Medline, Google Scholar
47. Schroder J, Niethammer R, Geider FJ, Reitz C, Binkert M, Jauss M, Sauer H: Neurological soft signs in schizophrenia. Schizophr Res 1991; 6:25-30Crossref, Medline, Google Scholar
48. Dazzan P, Morgan KD, Suckling J, Chitnis X, Orr KGD, Fearon P, Hutchison G, Salvo J, Chapple B, Jones P, Mallett R, Leff J, Murray RM: Neuroanatomical correlates of neurological soft signs: the AESOP first-onset psychosis study (abstract). Schizophr Res 2002; 53(suppl):100Google Scholar
49. Sanders RD, Keshavan MS, Goldstein G, Haas GL, Sweeney JA: Neurological exam abnormalities and neuropsychological performance in neuroleptic-naive psychosis (abstract). Schizophr Res 2001; 49(suppl):119Google Scholar
50. Griffiths TD, Sigmundsson T, Takei N, Rowe D, Murray RM: Neurological abnormalities in familial and sporadic schizophrenia. Brain 1998; 121(part 2):191-203Google Scholar



