Striatal Dopamine Transporters and Cognitive Functioning in Healthy Men and Women
Abstract
OBJECTIVE: There is growing interest in distinguishing the biological bases of sex differences in behavior from environmental influences. Sex hormone levels seem to be related to some cognitive abilities, particularly memory, and the dopaminergic system participates in the mediation of memory. The dopamine transporter is the primary indicator of dopaminergic tone. This study investigated the relationship between cognition and dopamine transporter availability in healthy men and women. METHOD: Dopamine transporter levels were measured with a technetium-99m radiolabeled analog of cocaine, TRODAT-1, in 66 healthy volunteers (30 men and 36 women). A neuropsychological battery designed to target functions associated with dopaminergic activity was administered during the uptake interval between the radiopharmaceutical injection and image acquisition. RESULTS: Women and younger participants had higher dopamine availability in the caudate nucleus, and these groups also performed better on verbal learning tasks. Furthermore, dopamine transporter availability was correlated with learning performance within groups. Relationships between dopamine availability in the caudate and putamen and executive and motor functioning were observed in women, but not in men. CONCLUSIONS: The results provide further evidence for age effects and sex differences in the neuromodulatory influences of dopamine on behavior in humans.
Sex differences in behavior have both cultural (1) and biological (2–4) underpinnings. Some hormonal modulation of behavior has been established (5, 6), and in humans, dopamine neural transmission participates in the mediation of motor and cognitive tasks. Pharmacological or pathophysiological depletion of dopamine is associated with performance decrements, independent of age-associated loss of neural tissue (7, 8). For example, neuropsychiatric and movement disorders, including Parkinson’s disease and Huntington’s disease, are associated with disturbances in dopamine transmission resulting from basal ganglia lesions (9, 10). Estrogen regulates dopamine transmission (11–13), perhaps in an antidopaminergic fashion in women (14). Estrogen receptors are found in cortical and subcortical areas, including the dopamine-rich caudate and putamen (15, 16).
The dopamine transporter serves as a primary regulator of intrasynaptic dopamine levels (17) by means of the reuptake of dopamine and the resultant termination of its neurotransmission (17, 18). The concentration of the dopamine transporter reflects the homeostatic tone of the dopaminergic system (17, 19). Ligand binding and in situ hybridization studies in mice have demonstrated that animals with deficient dopamine transporters have fewer dopamine receptors (D1 and D2) in the basal ganglia (17), structures that are important in cognitive and motor functioning (9).
The basal ganglia, amygdala, and thalamus are subcortical limbic structures (20). The basal ganglia are composed of the striatum, including the caudate and putamen, and the pallidum (9). The striatum receives input from the neocortex, the thalamus, and the amygdala and sends information to the brainstem and, by means of the thalamus and other areas of the basal ganglia, back to the prefrontal and motor frontal lobe regions (9, 21). The anterior cingulate and the orbitofrontal region are two frontal subcortical circuits that involve the basal ganglia (20). More specifically, there are connections between the caudate and the pallidum and orbitofrontal regions, as well as the hippocampus and posterior cingulate (20). Ventral areas of the striatum and pallidum are connected with the anterior cingulate. As supported by studies of patients with basal ganglia lesions, the caudate is thought to be relatively more involved in complex cognitive functioning, whereas the putamen and globus pallidus are largely motor structures (10).
Behavioral studies have demonstrated sex differences in the performance of some tasks associated with dopaminergic neurotransmission. For example, women perform better than men on verbal learning tasks (22, 23). In addition, verbal learning performance deteriorates with age and is adversely affected by menopause (24, 25). Several authors have reported that men outperform women on measures of motor functioning, including fine motor speed (26, 27). Although there have been studies demonstrating the role of the basal ganglia in motor learning (28, 29) and visuomotor coordination and learning (28, 30–32), studies of sex differences are lacking. One exception is an early study (33) reporting male superiority in the acquisition of mirror drawing by means of mental imagery during early learning trials. By the final trial, however, the performance of men and women was comparable.
Taken together, these findings suggest sex differences in the neuromodulatory effects of dopamine on behavior. However, studies of the coupling between dopamine and behavior in humans are lacking because of the scarcity of reliable ligands for measuring dopamine transporter availability.
TRODAT-1, a technetium-99m-labeled complex ([99mTc] [2[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1] oct-2-yl]-methyl](2-mercaptoethyl)amino]ethyl]amino] ethane-thiolato(3-)-N2,N2′,S2,S2′]oxo-[1R-(exo-exo)]), was developed as an effective dopamine transporter imaging agent (34). This study investigated the relationship between dopaminergic function and behavior in healthy volunteers who underwent single photon emission computed tomography (SPECT) with TRODAT-1 and were administered a neuropsychological battery designed to target functions associated with dopaminergic activity.
Method
Respondents to advertisements placed around the university community were screened to rule out a history of any medical, neurologic, or psychiatric conditions or events that could potentially affect brain function or structure.
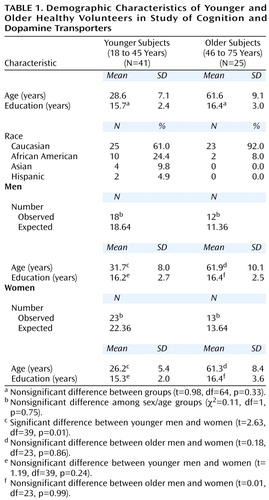
The study group consisted of 66 healthy volunteers (30 men and 36 women; mean age=41.1 years, SD=17.9, range=18–75). Their mean level of education was 16.0 years (SD=2.6, range=12–20). The mean age for the men (43.8 years, SD=17.4) was comparable to the mean age for the women (38.9 years, SD=18.2) (t=1.12, df=64, p=0.27). The mean level of education for the men (16.3 years, SD=2.6) was also comparable to the mean level of education for the women (15.7 years, SD=2.6) (t=0.87, df=64, p=0.39). Most participants were right-hand dominant (right: N=60, left: N=4, ambidextrous: N=2). There were no significant differences in age, race, or education between the men and women overall. The young and older participants did not differ significantly on education. Demographic characteristics are shown in Table 1.
After complete description of the study to the subjects, written informed consent was obtained in accordance with the procedures of the local Committee on Research Involving Human Beings. After the study, the participants received financial compensation for their time.
All studies were performed in the morning. After placement of an intravenous line in an antecubital vein, the participants rested in a supine position for 20 minutes to reduce any stress that might have resulted from the insertion of the catheter. Vital signs were taken every 5 minutes, and ECG rhythm strips were recorded continuously. About 740 MBq (SD=74) (20 mCi) of technetium-99m-labeled TRODAT-1 were then injected as a fast bolus chased with saline through a catheter while planar brain images were acquired for 1 second per frame for 1 minute. The subjects rested for 25 minutes after the injection. The acquisition of dynamic images began 3 hours after the administration of the radioligand. In the interim, the subjects completed the neuropsychological battery and had lunch.
A neuropsychological battery composed of measures of functions related to dopaminergic activity was administered by an investigator (L.H.M.) according to standardized administration procedures. Verbal learning and memory were assessed with the Pennsylvania Verbal Learning Test, a five-trial, 16-item list-learning task modeled after the California Verbal Learning Test (35) and composed of stimuli from the Hopkins Verbal Learning Test (36). The Pennsylvania Verbal Learning Test consists of five learning trials of the original word list, the presentation of a second (distractor) list, immediate free recall of the first list, and cued (categorical) recall of the first list. Long-delay free and cued recall trials were administered after a 20-minute delay. Fine motor speed and coordination were measured with a modification of the Finger Tapping Test (37–39) (three trials per hand), the Grooved Pegboard test (40), and the Thumb-Finger Sequential Touch test (41). Verbal inhibition and executive functioning were assessed by using a computerized version of the color-word trial of the Stroop Test (42–44). The task consisted of congruent trials, in which the color that the word was printed in matched the word content (e.g., “red” printed in red ink), and incongruent trials, in which the text and color were discrepant (e.g., “red” printed in blue ink). Divided attention and working memory were examined with the Auditory Consonant Trigrams task (45, 46). Affective screening included the State-Trait Anxiety Inventory (47) and the Profile of Mood States (48).
Dynamic images and SPECT scans of the brain were acquired on a triple-headed gamma camera equipped with ultra-high-resolution fan beam collimators (Picker 3000, Picker International, Cleveland) at a framing rate of 5 minutes per scan. The acquisition parameters included a continuous mode with 40 projection angles over a 120° arc to obtain data in a 128×128 matrix with a pixel width of 2.11 mm and a slice thickness of 3.56 mm. The center of rotation was always 14.0 cm, regardless of head size. After back-projection, a simple, low pass filter was applied with an order of 4 and a cutoff of 0.351 cm-1. A uniform ellipse was used to estimate attenuation. The images were reinterpolated into 2×2×4-mm voxels on another platform. Representative SPECT images are shown in Figure 1.
A set of standardized templates delineating the basal ganglia and the whole supratentorial brain was placed on a summed image and transposed to each frame (49). To decrease potential problems related to resolution uncertainties, each region of interest was smaller than the actual structure it represented. The regions of interest were placed only on the two contiguous slices with the most intense activity to minimize problems associated with volume averaging in the axial direction. Whole brain boundaries were drawn beginning 12 mm above the uppermost slice containing any basal ganglia activity.
Dynamic scans were used to calculate specific uptake values, representing the ratio of k3:k4 in a conventional three-compartment model at equilibrium. The mean activity per pixel in each region of interest across two slices was calculated (49), yielding specific uptake values for homotopic regions of the left and right hemispheres.
Results
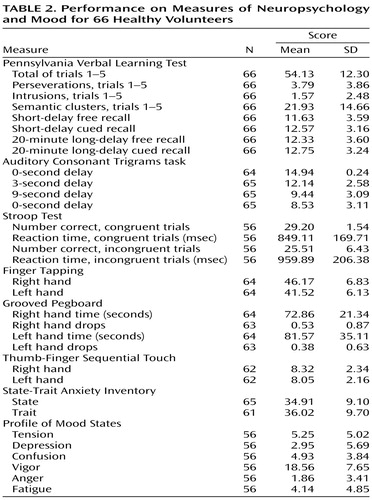
Means and standard deviations for all neuropsychological measures are shown in Table 2. Participants were divided into young (N=41, age 18–45 years, 18 men and 23 women) and older (N=25, age 46–75, 12 men and 13 women) adults, and the memory and dopamine measures were entered as outcome variables in a sex-by-age-group multivariate analysis of variance (MANOVA) design. The memory measure, memory performance z score, was calculated as average z-transformed values (mean=0, SD=1, relative to whole group means) for the following learning and memory measures: 1) total words recalled during acquisition (trials 1–5), 2) number of categorical clusters, an indication of verbal organization (trials 1–5), 3) short-delay free recall, and 4) number of clusters on short-delay free recall. Specific uptake values for the hemispheric caudate and putamen were entered as repeated measures (right versus left) of dopamine transporter availability.
The MANOVA on memory performance z score showed a main effect of sex (F=4.43, df=1, 62, p=0.04), with the women performing better than the men, a main effect of age group (F=5.66, df=1, 62, p=0.02), with the younger participants having higher values, and no interaction between sex and age group (Figure 2, left graph).
The MANOVA on the dopamine measures showed identical effects for the caudate, a main effect of sex (F=4.84, df=1, 61, p=0.01), with the women having higher specific uptake values, and a main effect of age group (F=13.45, df=1, 61, p<0.001), with higher values in the younger participants. There was also an interaction between sex and age (F=3.13, df=1, 61, p=0.05), indicating the sex difference was larger in the younger than in the older group. For the putamen, the main effect of age group was significant (F=11.00, df=1, 60, p<0.001), with higher values in the younger participants (Figure 2, right graph), and there was an interaction between sex and age group (F=3.40, df=1, 60, p=0.04). The interaction reflected higher specific uptake values in the younger women than in the younger men, with no sex differences in the older group. There were no main effects or interactions involving laterality.
A correlational analysis generally supported the group effects in showing robust coupling of the dependent measures within each sex (Figure 3). In the men, the memory performance z score correlated negatively with age (r=–0.51, df=30, p=0.004) and positively with specific uptake value in the caudate (r=0.43, df=30, p=0.02) but not in the putamen. In the women, age and memory performance z score were correlated (r=–0.40, df=36, p=0.02), as were memory performance z score, caudate-specific uptake value (r=0.40, df=36, p=0.02), and putamen-specific uptake value (r=0.40, df=36, p=0.02).
A correlational analysis also revealed relationships between executive and motor functioning in the women, but not in the men. Specifically, in the women, reaction times on congruent trials were correlated with specific uptake value in the caudate (r=–0.64, df=33, p<0.001) and putamen (r=–0.55, df=33, p<0.001). Incongruent trial reaction times were similarly correlated to specific uptake values (r=–0.50, df=33, p<0.01, and r=–0.64, df=33, p<0.001, for the caudate and putamen, respectively). Accuracy, defined as number correct on the incongruent trials, was mildly correlated with specific uptake value in the putamen (r=0.38, df=33, p=0.03). With regard to motor skills, thumb-finger sequencing for the right hand was associated with specific uptake values in the caudate and putamen (r=0.53, df=36, p<0.001, and r=0.67, df=36, p<0.001, respectively). Similar relationships were observed for sequencing in the left hand (r=0.38, df=36, p=0.02, and r=0.52, df=36, p=0.001, for the caudate and putamen, respectively). Time to completion on the Grooved Pegboard test was negatively correlated with striatal specific uptake value in the right hand (r=–0.57, df=36, p<0.001, and r=–0.71, df=36, p=0.0001, for the caudate and putamen, respectively) and in the left hand (r=–0.44, df=36, p<0.01, and r=–0.66, df=36, p=0.0001). Only specific uptake value in the putamen was related to Finger Tapping performance in the left hand (r=0.35, df=36, p<0.05). None of these correlations was significant for the men.
Analysis of gender differences on the components of memory performance z score indicated that the women outperformed the men on clusters for trials 1–5 (t=2.39, df=64, p=0.02) and on short-delay free recall (t=2.18, df=64, p=0.03). The women performed slightly stronger than the men on the short-delay free recall task (t=1.93, df=64, p=0.06). The men and women did not differ in total words recalled on the learning trials. There were no gender differences in Stroop reaction times, Grooved Pegboard speed, or Thumb-Finger Sequential Touch sequences, but the men outperformed the women on Finger Tapping (t=2.89, df=64, p<0.01, and t=2.87, df=64, p<0.01, for the right and left hands, respectively).
The younger participants outperformed the older participants in reaction time on the congruent and incongruent Stroop trials (t=5.53 and t=5.77, df=64, both p<0.0001). The younger subjects were also faster on the Grooved Pegboard (t=5.55 and t=4.98, df=64, both p<0.01, for the right and left hands, respectively) and completed more Thumb-Finger Sequential Touch sequences bilaterally (t=4.00, df=64, p<0.001, and t=2.88, df=64, p<0.01 for the right and left hands). There were no differences in fine motor speed between the age groups.
Planar images of the head were acquired in a subgroup of 10 healthy volunteers to rule out the influence of nonspecific factors on sex differences in TRODAT-1 uptake values. The advantages of using these data included transmission scan-acquired attenuation corrections and absolute quantification of the radioactivity of the whole brain on planar images. There were higher radiation-absorbed doses for the brain in the women (8.8 mrem/mCi injected) than in the men (6.8 mrem/mCi), with virtually identical rates of elimination from the brain (49).
Discussion
The findings of sex differences and age-associated changes in dopaminergic tone seem to provide an explanation at the neuromodulatory level for some effects on cognitive function. This study replicated earlier investigations reporting that younger healthy participants performed significantly better than their older counterparts on list learning and semantic organization (50, 51). With the exception of total recall on the learning trials, we also replicated the findings of stronger performance by the women on verbal list-learning measures (52, 53). Our findings are likewise consistent with reports of age-related declines in D2 receptor availability in the caudate and putamen. Such changes have been associated with performance decrements in tasks involving frontostriatal brain systems, including motor speed, abstraction, mental flexibility, attention, and verbal inhibition (7). Our results demonstrate, for the first time, sex differences in specific uptake values and that better memory is associated with higher specific uptake values, reflecting higher dopaminergic tone, in both the caudate and putamen. Although it has been reported that another specific memory measure was unrelated to the concentration of postsynaptic dopamine receptors, transporter concentrations are probably more sensitive indicators of dopaminergic tone (11, 49).
Better motor coordination, reflected in more completed sequences on the Thumb-Finger Sequential Touch and faster performance on the Grooved Pegboard task, was related to higher striatal specific uptake values in the women, but not in the men. With the exception of a correlation between dopamine availability and left-hand Finger Tapping in the women, there were no associations with fine motor speed. As expected, the men were faster on measures of fine motor speed, but there were no performance differences between the men and women on motor coordination. We also found a relationship between specific uptake value and executive functioning, as measured by reaction time on a computerized verbal inhibition task. Again in the women, but not in the men, faster performance on congruent and incongruent trials was associated with higher dopamine availability in the caudate and putamen. It is unclear why these correlations were present only in the women. There were no gender differences on reaction time. With the exception of Finger Tapping, the younger participants performed better than the older participants on all of these measures as well. The observed dissociation between motor speed and coordination may reflect differential involvement of frontostriatal circuits; however, the absence of the expected superiority of the younger participants on tapping speed suggests that the measure lacks sensitivity, at least as administered here. The pattern of correlations does not support hypotheses linking the caudate to more “intellectual” and the putamen to more “motor” functions. In general, correlations with neurobehavioral measures that are significant for the caudate are also significant, and of comparable magnitude, for the putamen. However, the findings lend further support to the notion that cognitive and motor functioning may be differentially regulated by dopamine in women and men and that these differences are, in part, age dependent.
Evidence for sex differences in the neuromodulatory influences of dopamine on behavior has implications for understanding fundamental brain-behavior relationships. For example, it has been reported that women have lower D2 receptor affinity than men in the left striatum, suggesting “an increased endogenous striatal dopamine concentration in women” (14). Since estrogen regulates dopamine transmission (11–13), sex hormones probably contribute to these sex differences. Changes in the putamen/cerebellum ratio of D2 receptor density across different phases of the menstrual cycle have not been found (13). However, studies examining dopamine transporter availability and neurobehavioral probes across the menstrual cycle, or in women taking hormone-replacement therapy, may help establish the role of estrogen as well as other hormones in these sex differences.
Our failure to find hemispheric asymmetries is disappointing, because others have reported hemispheric laterality differences in right-handed, healthy comparison subjects in the recall of novel and practiced unstructured word lists (54) and in visuospatial abstraction (55) by using [15O]H2O positron emission tomography. Pihlajamäki et al. (56) reported that the left medial temporal lobe was activated during the retrieval of semantically associated words (category fluency) in a functional magnetic resonance imaging study of healthy comparison subjects. Perhaps our “punch biopsy” approach to regional analysis masked any effects of laterality. Alternatively, tonal dopamine availability may not be directly related to regional hemispheric activation during task performance. The association between striatal dopaminergic tone and indices of regional metabolic activity merit specific investigation.
The present study has several limitations. Of most importance, although it shows a coherent coupling of performance with striatal dopaminergic tone, it does not include an experimental manipulation of dopaminergic tone to produce changes in memory performance. Such pharmacologic challenge studies could be carried out in humans and, perhaps more extensively, in animals. The study also focused on a single measure of memory performance in order to control for an experiment-wise (type I) error. A more detailed specification of dopamine involvement in distinct facets of memory, and possibly other aspects of cognitive and emotional processing, can be examined in larger groups. Finally, the single tracer used in this study did not permit the establishment of specificity in the relations observed between neurotransmitter function and memory performance.
However, findings from this study may help elucidate sex differences in memory performance and the effects of healthy aging. They may also have implications for understanding sex differences in brain disorders involving the dopamine system, such as schizophrenia and Parkinson’s disease. Premenopausally, women with schizophrenia have less severe negative symptoms, higher levels of function, and better response to neuroleptic treatment than their male counterparts (57–59). Women also have a lower incidence of Parkinson’s disease than men (60). The results of this study suggest that selectively manipulating hormonal systems could favorably influence the clinical and neurocognitive course of diseases affecting the dopaminergic system.
 |
 |
Received July 18, 2000; revision received April 4, 2001; accepted April 26, 2001. From the Department of Psychiatry and Nuclear Medicine, University of Pennsylvania School of Medicine. Address reprint requests to Dr. Harper Mozley, 1226 N. Broadway St., Indianapolis, IN 46202; [email protected] (e-mail). Funded by grants from the National Institute on Aging (AG-17524), the National Institute on Drug Abuse (DA-09469), NIMH (MH-43880), and the National Institute of Neurological and Communicative Disorders and Stroke (NS-24538) to the Mental Health Clinical Research Center. The authors thank Matthew M. Kurtz, Ph.D., for neuropsychological assessment, Karl Plössl, Ph.D., for radiochemistry, and John Ramsey for study coordination.
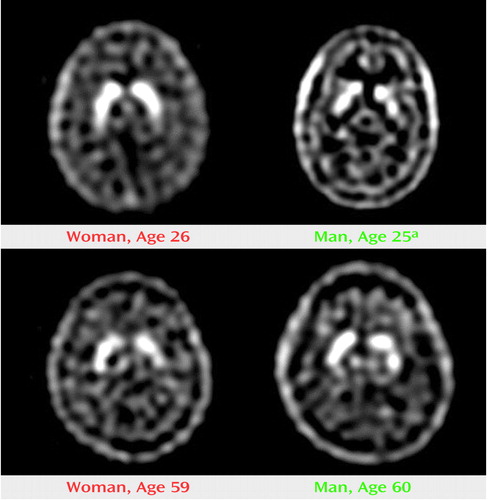
Figure 1. Representative 60-Minute SPECT Brain Scans of Younger and Older Healthy Volunteers
aThe separation between the head of the caudate and putamen is clearly visible.
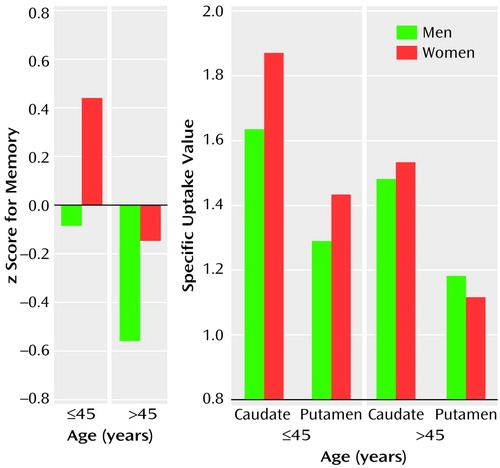
Figure 2. Measures of Memory Performancea and Dopamine Transporter Availabilityb for 41 Younger and 25 Older Healthy Male and Female Volunteers
aComposite of values for words recalled during acquisition, categorical clusters, short-delay free recall, and clusters in short-delay free recall.
bRatio of k3 to k4 in conventional three-compartment model at equilibrium.
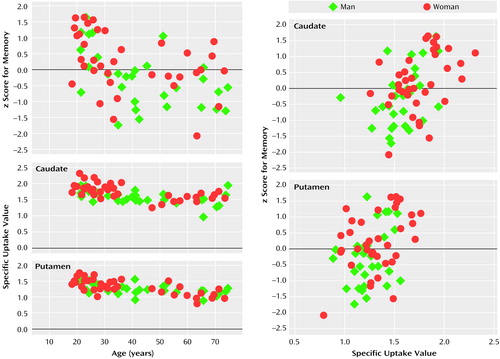
Figure 3. Measures of Memorya and Dopamine Transporter Availabilityb for 30 Male and 36 Female Healthy Volunteers
aComposite of values for words recalled during acquisition, categorical clusters, short-delay free recall, and clusters in short-delay free recall.
bRatio of k3 to k4 in conventional three-compartment model at equilibrium.
1. Frome PM, Eccles JS: Parents’ influence on children’s achievement-related perceptions. J Pers Soc Psychol 1998; 74:435-452Crossref, Medline, Google Scholar
2. Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE: Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci 1999; 19:4065-4072Google Scholar
3. Gur RC, Harper Mozley L, Mozley PD, Resnick SM, Karp JS, Alavi A, Arnold SE, Gur RE: Sex differences in regional cerebral glucose metabolism during a resting state. Science 1995; 267:528-531Crossref, Medline, Google Scholar
4. Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L, Gores JC: Sex differences in the functional organization of the brain for language. Nature 1995; 373:607-609Crossref, Medline, Google Scholar
5. Kimura D: Sex, sexual orientation and sex hormones influence human cognitive function. Curr Opin Neurobiol 1996; 6:259-263Crossref, Medline, Google Scholar
6. Van Goozen SH, Cohen-Kettenis PT, Gooren LJ, Frijda NH, Van de Poll NE: Gender differences in behaviour: activating effects of cross-sex hormones. Psychoneuroendocrinology 1995; 20:343-363Crossref, Medline, Google Scholar
7. Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, Logan J, Moberg PJ, Hitzemann R, Smith G, Pappas N: Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging. Ann Neurol 1998; 44:143-147Crossref, Medline, Google Scholar
8. Volkow ND, Gur RC, Wang G-J, Fowler JS, Moberg PJ, Ding Y-S, Hitzemann R, Smith G, Logan J: Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998; 155:344-349Link, Google Scholar
9. Graybiel AM, Aosaki T, Glaherty AW, Kimura M: The basal ganglia and adaptive motor control. Science 1994; 265:1826-1831Google Scholar
10. Bhatia KP, Marsden CD: The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 1994; 117:859-876Crossref, Medline, Google Scholar
11. Halbreich U: Role of estrogen in postmenopausal depression. Neurology 1997; 48(suppl 7):16-19Google Scholar
12. Lindamer LA, Lohr JB, Harris MJ, Jeste DV: Gender, estrogen, and schizophrenia. Psychopharmacol Bull 1997; 33:221-228Medline, Google Scholar
13. Nordström AL, Olsson H, Halldin C: A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Res 1998; 83:1-6Crossref, Medline, Google Scholar
14. Pohjalainen T, Rinne JO, Någren K, Syvälahti E, Hietala J: Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 1998; 155:768-773Abstract, Google Scholar
15. McEwen BS, Parsons B: Gonadal steroid action on the brain: neurochemistry and neuropharmacology. Ann Rev Pharmacol Toxicol 1982; 22:555-598Crossref, Medline, Google Scholar
16. McEwen BS, Alves SE, Bulloch K, Weiland NG: Ovarian steroids and the brain: implications for cognition and aging. Neurology 1997; 48(suppl 7):8-15Google Scholar
17. Jaber M, Jones S, Giros B, Caron MG: The dopamine transporter: a crucial component regulating dopamine transmission. Mov Disord 1997; 12:629-633Crossref, Medline, Google Scholar
18. Stahl SM: Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, 2nd ed. Cambridge, UK, Cambridge University Press, 2000, pp 135-197Google Scholar
19. Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG: Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA 1998; 95:4029-4034Google Scholar
20. Mega MS, Cummings JL, Salloway S, Malloy P: The limbic system: an anatomic, phylogenetic, and clinical perspective, in The Neuropsychiatry of Limbic and Subcortical Disorders. Edited by Salloway S, Malloy P, Cummings JL. Washington, DC, American Psychiatric Press, 1997, pp 3-18Google Scholar
21. Graybiel AM: The basal ganglia. Curr Biol 2000; 10:R509-R511Google Scholar
22. Kramer JH, Delis DC, Kaplan E, O’Donnell L, Prifitera A: Developmental sex differences in verbal learning. Neuropsychology 1997; 11:577-584Crossref, Medline, Google Scholar
23. Reite M, Cullum CM, Stocker J, Teale P, Kozora E: Neuropsychological test performance and MEG-based brain lateralization: sex differences. Brain Res Bull 1993; 32:325-328Crossref, Medline, Google Scholar
24. Chalfonte BL, Johnson MK: Feature memory and binding in young and older adults. Mem Cognit 1996; 24:403-416Crossref, Medline, Google Scholar
25. Craik FI, McDowd JM: Age differences in recall and recognition. J Exp Psychol 1987; 13:474-479Google Scholar
26. Coleman AR, Moberg PJ, Ragland JD, Gur RC: Comparison of the Halstead-Reitan and infrared Light Beam Finger Tappers. Assessment 1997; 4:277-286Crossref, Medline, Google Scholar
27. Schmidt SL, Oliveira RM, Krahe TE, Filgueiras CC: The effects of hand preference and gender on finger tapping performance asymmetry by the use of an infra-red light measurement device. Neuropsychologia 2000; 38:529-534Crossref, Medline, Google Scholar
28. Jueptner M, Weiller C: A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain 1998; 121:1437-1449Google Scholar
29. Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE: Motor sequence learning: a study with positron emission tomography. J Neurosci 1994; 14:3775-3790Google Scholar
30. Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC: Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. Eur J Neurosci 1996; 8:637-648Crossref, Medline, Google Scholar
31. Doyon J, Gaudreau D, Laforce R, Castonguay M, Bedard PJ, Bouchard JP: Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain Cogn 1997; 34:218-245Crossref, Medline, Google Scholar
32. Doyon J, Laforce R, Bouchard G, Gaudreau D, Roy J, Poirier M, Bédard PJ, Bédard F, Bouchard J-P: Role of the striatum, cerebellum and frontal lobes in the automatization of a repeated visuomotor sequence of movements. Neuropsychologia 1998; 36:625-641Crossref, Medline, Google Scholar
33. Koslow RE: Sex-related differences and visual-spatial mental imagery as factors affecting symbolic motor skill acquisition. Sex Roles 1987; 17:521-527Crossref, Google Scholar
34. Kung MP, Stevenson DA, Plössl K, Meegalla SR, Beckwith A, Essman WD, Mu M, Lucki I, Kung HF: [99mTc]TRODAT-1: a novel technetium-99m complex as a dopamine transporter imaging agent. Eur J Nucl Med 1997; 24:372-380Medline, Google Scholar
35. Delis DC, Kramer JH, Kaplan E, Ober BA: California Verbal Learning Test, Adult Version. San Antonio, Tex, Psychological Corp, 1987Google Scholar
36. Brandt J: The Hopkins Verbal Learning Test: development of a new verbal memory test with six equivalent forms. J Clin Neuropsychol 1991; 5:125-142Crossref, Google Scholar
37. Halstead WC: Brain and Intelligence. Chicago, University of Chicago Press, 1947Google Scholar
38. Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 2nd ed. Tucson, Ariz, Neuropsychology Press, 1993Google Scholar
39. Spreen O, Strauss E: A Compendium of Neuropsychological Tests. New York, Oxford University Press, 1991Google Scholar
40. Matthews CG, Klφve H: Instruction Manual for the Adult Neuropsychology Test Battery. Madison, University of Wisconsin Medical School, 1964Google Scholar
41. Luria AR: Higher Cortical Functions in Man. New York, Basic Books, 1966Google Scholar
42. Stroop JR: Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18:643-662Crossref, Google Scholar
43. Jensen AR, Rohwer WD: The Stroop Color-Word Test: a review. Acta Psychol 1966; 25:36-93Crossref, Medline, Google Scholar
44. Golden CJ: Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Wood Dale, Ill, Stoelting Co, 1978Google Scholar
45. Peterson LR: Short-term memory. Sci Am 1966; 215:90-95Crossref, Medline, Google Scholar
46. Peterson LR, Peterson MJ: Short-term retention of individual verbal items. J Exp Psychol 1959; 58:193-198Crossref, Medline, Google Scholar
47. Spielberger CD: State-Trait Anxiety Inventory (Form Y). Palo Alto, Calif, Consulting Psychologists Press, 1983Google Scholar
48. McNair DM, Lorr M, Droppleman LF: Manual for the Profile of Mood States. San Diego, Educational and Industrial Testing Service, 1981Google Scholar
49. Mozley PD, Acton PD, Barraclough ED, Plössl K, Gur RC, Alavi A, Mathur A, Saffer J, Kung HF: Effects of age on dopamine transporters in healthy humans. J Nucl Med 1999; 40:1812-1817Google Scholar
50. Lezak MD: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995, pp 429-498Google Scholar
51. Pope DM: The California Verbal Learning Test: performance of normal adults aged 55-91 (abstract). J Clin Exp Neuropsychol 1987; 9:50Google Scholar
52. Saykin AJ, Gur RC, Gur RE, Kester DB, Stafiniak P, Harper Mozley L, Robinson LJ, Malamut BL, Watson B, Shtasel DL, Mozley PD: Normative neuropsychological test performance: effects of age, education, gender, and ethnicity. Appl Neuropsychol 1995; 2:79-88Crossref, Medline, Google Scholar
53. Kramer JH, Delis DC, Daniel M: Sex differences in verbal learning. J Clin Psychol 1988; 44:907-915Crossref, Google Scholar
54. Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Recalling word lists reveals “cognitive dysmetria” in schizophrenia: a positron emission tomography study. Am J Psychiatry 1999; 156:386-392Abstract, Google Scholar
55. Wharton CM, Grafman J, Flitman SS, Hansen EK, Brauner J, Marks A, Honda M: Toward neuroanatomical models of analogy: a positron emission tomography study of analogical mapping. Cognit Psychol 2000; 40:173-197Crossref, Medline, Google Scholar
56. Pihlajamäki M, Tanila H, Hänninen T, Könönen M, Laakso M, Partanen K, Soininen H, Aronen HJ: Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol 2000; 47:470-476Crossref, Medline, Google Scholar
57. Gur RE, Petty RG, Turetsky BI, Gur RC: Schizophrenia throughout life: sex differences in severity and profile of symptoms. Schizophr Res 1996; 2:1-12Crossref, Google Scholar
58. Angermeyer MC, Kuhn L, Goldstein JM: Gender and the course of schizophrenia: differences in treated outcomes. Schizophr Bull 1990; 16:293-307Crossref, Medline, Google Scholar
59. Häfner H, Maurer K, Loffler W, Riecher-Rössler A: The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry 1993; 162:80-86Crossref, Medline, Google Scholar
60. Miller DB, Ali SF, O’Callaghan JP, Laws SC: The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann NY Acad Sci 1998; 844:153-165Crossref, Medline, Google Scholar



