Recalling Word Lists Reveals “Cognitive Dysmetria” in Schizophrenia: A Positron Emission Tomography Study
Abstract
OBJECTIVE: This study explored the neural circuitry used during recall of unstructured verbal material in schizophrenic patients and healthy volunteer subjects. METHOD: The subjects were 13 healthy volunteers and 14 schizophrenic patients. All patients were free of medication, and all subjects were right-handed. Two experimental cognitive conditions were used: recall of novel and practiced word lists (two 15-item lists from the Rey Auditory Verbal Learning Test). Both active recall tasks were compared with an eyes-closed resting baseline condition. A nonparametric randomization test was used to determine within- and between-group differences in regional cerebral blood flow. RESULTS: Performance on both the practiced and novel memory tasks was nonsignificantly different in the patients and control subjects. During the novel memory task, the patients showed decreased flow in the right anterior cingulate, right thalamus, and bilateral cerebellum (left greater than right) relative to the control subjects. When recalling the practiced word lists, the patients showed decreased flow in the left dorsolateral prefrontal cortex, bilateral medial frontal cortex, left supplementary motor area, left thalamus, left cerebellar regions, anterior vermis, and right cuneus. CONCLUSIONS: Patients with schizophrenia fail to activate cortical-cerebellar-thalamic-cortical circuitry during recall of both well-learned and novel word lists. (Am J Psychiatry 1999; 156:386–392)
Historically it has been emphasized that patients with schizophrenia have a cognitive disorder that may represent the characteristic trait of this illness (1, 2). Several studies (3–5) have shown that this deficit is prominent in specific cognitive domains, such as memory, attention, and executive function.
Identification of impaired cognitive functions is only an initial step in the understanding of the pathophysiology of schizophrenia. The natural second step entails study of the intrinsic neural mechanisms that are associated with these impairments (6, 7). One approach, used by numerous investigators, seeks to associate individual schizophrenic symptoms and cognitive dysfunctions with specific brain regions (8–11). More recently, we and others (3, 12–14) have chosen to pursue a strategy that posits the disruption of one fundamental cognitive process that defines the phenomenotype of schizophrenia and affects multiple cognitive domains. If this more parsimonious hypothesis proves to be correct, the heterogeneous array of psychological dysfunctions in schizophrenia may be explained by a disruption in a fundamental cognitive process that can be attributed to a dysfunctional neural circuit (14).
In light of the fundamental nature of memory in human cognition and earlier reports of marked memory impairment in schizophrenia in relation to performance on other cognitive tests (5, 15), we have focused our efforts on developing an understanding of the neural mechanisms of human memory. Frontal, thalamic, and cerebellar regions may constitute a circuit that is the core network used by the human brain to perform a variety of memory tasks (16–20). Structural and functional abnormalities of the frontal lobe, thalamus, and cerebellum have been demonstrated in schizophrenia (7–11, 21–26). The emerging body of studies showing the role of the cerebellum in several cognitive processes have led to a great interest in understanding its role in psychiatric illnesses (16, 17, 27, 28).
We previously reported (7) that during recall of complex narrative material, schizophrenic patients failed to activate the frontal-cerebellar-thalamic circuitry used by healthy individuals to perform the same memory task. However, these findings may be paradigm-specific and not generalizable to other cognitive processes.
In the present study we used positron emission tomography (PET) to examine the neural circuitry used during recall of unstructured verbal material in schizophrenic patients and healthy volunteer subjects. We hypothesized that recalling a different sort of verbal material might elicit abnormalities in cortical-cerebellar-thalamic circuitry in schizophrenic patients as well. However, because of the different mental mechanisms involved in recalling structured versus unstructured verbal information (29), task-specific differences in this circuit were also anticipated.
METHOD
Subjects
The subjects were 13 healthy volunteers (six men, seven women) recruited from the community and 14 patients (10 men, four women) suffering from schizophrenia who were evaluated at the University of Iowa Mental Health Clinical Research Center. This is the same sample examined in our previous verbal memory study (7). Volunteers were screened to rule out current or past history of psychiatric, neurological, or medical illness by using a short version of the Comprehensive Assessment of Symptoms and History (30), medical history, and physical examination. For the schizophrenic patients, the mean duration of illness was 10.6 years (SD=12.2). Clinical symptoms were rated by using the Scale for the Assessment of Negative Symptoms (31) and the Scale for the Assessment of Positive Symptoms (32), and these were used to summarize psychopathology in three dimensions—negative, disorganized, and psychotic; the mean negative symptom dimension score was 2.482 (SD=0.68), the mean psychotic symptom dimension score was 3.357 (SD=0.77), and the mean disorganized symptom dimension score was 1.429 (SD=1.67). The mean age of the volunteers was 28.6 years (SD=7.2), and their mean educational achievement was 14.7 years (SD=1.5). The mean age of the patients was 30.7 years (SD=11.3), and their mean educational achievement was 13.2 years (SD=2.2). The patients either were withdrawn from all medication for a 3-week period prior to study (N=11) or had never been treated (N=3). All patients and volunteers were right-handed. After complete description of the study to the subjects, written informed consent was obtained.
Tasks
Two experimental cognitive conditions were used: recall of novel and practiced word lists (two 15-item lists from the Rey Auditory Verbal Learning Test). For the practiced free recall task, the subjects were asked to learn the group of words during an initial training session that occurred 1 week prior to the imaging study. The words were presented auditorily, at the rate of one per second, and the items recalled by the subjects were spoken aloud. In this session, presentation and recall of the word list were repeated until all 15 words were recalled (irrespective of order). On the day before the PET study the subjects were given a “refresher course,” during which the group of words was presented again to ensure that recall was 100% accurate. For the condition referred to as “novel recall,” the subjects heard the word list 60 seconds before the imaging study and were told that they would be requested to recall as many words as they could during the PET scan. For both conditions the word list was read to the subject immediately prior to the image acquisition, and recall began approximately 10 seconds prior to the arrival of the bolus of [15O]water in the brain, with bolus arrival time determined from an initial low-activity sham study (33). The subjects were asked to recall as much of the list as possible and to continue to try to recall for the duration of image acquisition, repeating words until image acquisition was complete. Both active recall tasks were compared with a reference condition, lying quietly with eyes closed, which is referred to as REST (random episodic silent thought). Four additional conditions were included in the overall PET experiment (16).
PET and MR Data Acquisition
Magnetic resonance (MR) scans, to be used for anatomic localization of functional activity, were obtained with a standard T1-weighted three-dimensional gradient-echo sequence on a 1.5-T GE scanner (TE=5 msec, TR=24 msec, flip angle=40°, number of excitations=2, field of view=26 mm, matrix=256×192, slice thickness=1.5 mm).
PET data were acquired with a bolus injection of 75-mCi [15O]water in 5–7 ml saline by using a GE PC4096-plus 15-slice whole-body scanner. The PET acquisition details have been described elsewhere (7, 33). Cerebral blood flow (CBF) was calculated on a voxel-by-voxel basis by using an autoradiographic method (34).
Image Analysis
The quantitative PET blood flow images and MR images were analyzed by using the locally developed software package BRAINS (35, 36). The PET image of each individual was then fit to that individual’s MR scan by using a surface-fit algorithm (37, 38). The PET image could be localized on coregistered MR and PET images where the MR image represented the “average brain” of the subjects in this study (24). Specific between-group differences in neural activation were examined by a direct statistical comparison of the patients and healthy volunteers using our specific randomization analysis (39).
Two types of randomization analysis were conducted. One type examined the differences between the two groups by using an initial subtraction analysis (practiced recall minus REST in patients versus control subjects and novel recall minus REST in patients versus control subjects). This analysis reflects differences between the two groups in both conditions and removes differences in head shape or size, but it does not reveal whether the differences were due to the memory task or to REST. To address this latter point we also did randomization comparisons of the two groups for each of the individual conditions with no subtractions of REST.
The selection of a significance threshold for PET studies is always arbitrary. Consistent with our previous studies, we used an uncorrected p value of 0.005. This threshold closely approximates the sizes of peaks defined by t=3.61 when the Montreal method is used (39). Every peak has been defined by the number of contiguous significant voxels that constitute the peak and by the standard Talairach atlas coordinates (40). We report only the peaks that contain more than 50 adjacent voxels. The region name given to each peak was based on direct visual inspection of the coregistered MR and PET images.
RESULTS
The patients and control subjects did not differ significantly in task performance either during the practiced condition (mean=12.2 words, SD=4.0, versus mean=14.2 words, SD=2.9) (t=1.40, df=25, p=0.17) or during the novel condition (mean=5.4 words, SD=1.9, and mean=6.6 words, SD=1.5, respectively) (t=1.76, df=25, p=0.09). Therefore, it is unlikely that between-group differences in blood flow were due to differences in task performance.
Results of the randomization analysis comparing the patients and control subjects during the practiced task are shown in table 1 and displayed in figure 1a. The patients showed a relative decrease in flow in the left dorsolateral prefrontal cortex (Brodmann area 46), bilateral medial frontal cortex (Brodmann areas 32 and 43), left supplementary motor area (Brodmann area 6), right premotor cortex, left thalamus, left cerebellar regions, anterior vermis, and the right cuneus. Similar results were obtained by using the randomization method without subtracting the REST condition. The patients showed a relative decrease in flow in the right frontal cortex, anterior and posterior cingulate, and left cerebellar regions.
Results for the novel memory task are shown in table 1 and figure 1b. Brain regions showing a relative decrease in flow in the patients included the left inferior frontal cortex (Brodmann area 44), right anterior cingulate, right thalamus, and bilateral cerebellum (left greater than right). Prefrontal flow decreases were not observed in this analysis when the p<0.005 threshold was used. In an exploratory post hoc analysis we used one-tailed t tests and repeated this analysis using a p<0.01 threshold. At this threshold, a peak of relatively decreased flow was found in the right prefrontal lobe (Brodmann area 47/11) (x=20, y=22, z=–14). Identical results were found when we used between-group comparisons without subtracting the REST condition. The patients showed a relative decrease in flow in the right anterior cingulate, thalamus, and cerebellum.
We also examined the results for both cognitive tasks for each subject group independently, using the standard Montreal (Worsley) method for subtracting two experimental conditions (39). In this analysis we examined practiced recall minus REST and novel recall minus REST in the healthy volunteers and in the patients. Comparison of the activation patterns in these analyses revealed that schizophrenic patients fail to activate brain regions that healthy volunteers utilize during performance of the two memory tasks (figure 2). Thus, convergent statistical analysis using three different methods confirms that patients with schizophrenia have abnormalities in cortical-cerebellar-thalamic-cortical circuitry.
Only one area with a relative increase in blood flow, the left parietal lobe, was found in the patients with schizophrenia during either memory task.
DISCUSSION
The present study has shown that, compared to healthy individuals, patients with schizophrenia who recall a list of words fail to activate frontal-cerebellar-thalamic circuitry. These findings confirm the hypothesis, based on our previous PET studies, that patients suffering from schizophrenia have a relative decrease in CBF in the same interconnected cerebral regions while performing different types of cognitive tasks (7, 41). As anticipated, abnormalities in the cortical-cerebellar-thalamic-cortical circuitry are generalized across a variety of memory tasks and are not task-specific.
Lower flow during the practiced task was observed in the left dorsolateral prefrontal cortex (Brodmann area 46), left supplementary motor area (Brodmann area 6), and right premotor cortex. The prefrontal cortex has a pivotal role in carrying out higher cognitive functions by virtue of its reciprocal connections with other brain regions (42, 43). The results from the present study are consistent with the construct of frontal lobe dysfunction in schizophrenia (11, 44–)47). The dorsolateral prefrontal cortex (Brodmann area 46) has been associated with verbal working memory and with the generation of willed actions in healthy individuals (20, 48). It has also been hypothesized that a defect in working memory function or a disorder of willed actions could be at least one of the crucial cognitive impairments in schizophrenia (3, 49). Taken together, these results allow us to suggest that a basic impairment of the prefrontal cortex may lead to a core cognitive disturbance in schizophrenia that is shown in several cognitive domains (i.e., working memory or generation of willed actions) (14).
The lack of differences between patients and control subjects in prefrontal lobe regional CBF during the novel task performance was an unexpected finding. This may be because one strategy used during the novel recall task is the maintenance of the verbal information in an active state (short-term maintenance of verbal information), which has been shown to activate dorsolateral prefrontal cortical areas (50). This may be why schizophrenic patients display a close-to-normal pattern of prefrontal cortex activation in response to the challenge of the higher prefrontal requirement allocated to task performance. If so, the two groups would not differ in this region when our standard significance threshold is used, although differences might emerge at a lower one. Because our search was hypothesis-driven by our previous work, we examined results obtained by using a lower threshold. With this lower threshold (p<0.01), the schizophrenic patients did show a relative decrease in regional CBF in the right prefrontal cortex.
Another striking finding of our study is the relative decrease in CBF in a large area that encompasses the left lateral and medial rostral supplementary motor area (Brodmann area 6). The rostral supplementary motor area is critically involved in internal representation of time and in internal selection of movement, and it is activated during “complex” tasks requiring selection of response (51, 52). Although little is known about changes in the supplementary motor area in schizophrenia, relatively decreased activation of the supplementary motor area in schizophrenic patients during motor tasks has been reported (53).
Decreased thalamic regional CBF in schizophrenic patients was observed on the right for the novel task and on the left for the practiced task. Neuropathological (23, 54), in vivo anatomical (24, 55), and functional (7, 56) studies have shown thalamic abnormalities in schizophrenia. A defect in information processing capacity and in filtering input stimuli has been proposed as one component of the core underlying deficit in schizophrenia (13, 57). Because of the role of the thalamus in modulating attention and in filtering external information in order to exclude redundant or distracting stimuli, it has been suggested that thalamic dysfunction might play a crucial role in the etiopathological model of schizophrenia (24, 58).
The patients with schizophrenia also showed a relative decrease in flow in the right anterior cingulate during performance of the recall task involving the novel word list. The anterior cingulate has an important role in emotional and attentional mechanisms (59), which are also altered in schizophrenia (4, 60). The healthy individuals showed greater neural activation in the anterior cingulate cortex during performance of the novel task, which has a higher attentional demand than do practiced tasks (17, 61). Low flow or activity in the anterior cingulate has been reported in schizophrenic patients during several cognitive tasks (7, 62, 63). Our results showing a decreased flow in the right anterior cingulate, right prefrontal cortex, and right thalamus only during the novel condition suggest that schizophrenic patients may have an inability to activate a right attentional circuit during memory tasks for which the attentional demands are also high.
Multiple cerebellar regions were found to have a relative decrease in flow in schizophrenic patients during both conditions. Our group (7, 41) has previously demonstrated that schizophrenic patients display relatively lower blood flow in diverse cerebellar regions during the recall of complex narratives and during recognition memory for words. Intriguingly, neuroleptic-naive schizophrenic patients had a relative increase of CBF in the cerebellum when they were examined in a REST condition (46).
It has been proposed that a cerebellar dysfunction in schizophrenia may lead to “poor mental coordination,” resembling abnormalities in motor coordination and sequencing (64). Results from the present study also show that patients with schizophrenia have a relatively lower blood flow during recall of a practiced word list in the anterior vermis. Interestingly, some anatomical studies (22, 65) have shown a smaller anterior vermis in schizophrenia.
Historically, dysmetria has been defined as the inability of the individual to time the control at onset and offset of activity in the appropriate pairs of muscles. Dysfunction in the central timing process has been attributed to the lateral cerebellum, the putamen, and the supplementary motor area (64, 66). It seems possible that these brain regions related to the timing and programming of movement may also be involved in timing and controlling the fluid coordination of mental activity (67–69).
A dysfunctional circuitry linking brain regions involved in timing and sequencing mental functions (i.e., cerebellum and rostral supplementary motor area) to regions involved in high-level cognitive processes (i.e., prefrontal lobe) provide strong support for a “cognitive dysmetria” model of schizophrenia. This model posits that a neural misconnection in cortical-cerebellar-thalamic-cortical circuitry in schizophrenic patients may lead to difficulty in coordinating and sequencing mental processes, such as receiving, processing, retrieving, and expressing information, that are needed to achieve desired cognitive acts. While this circuit is hypothesized to be impaired across a broad range of cognitive tasks, specific differences in regional CBF patterns across different types of cognitive tasks are also expected, since different interconnected brain regions could be recruited according to specific features of the cognitive task. On the basis of these findings it is possible to speculate that the clinical heterogeneity of schizophrenia can be understood in the context of dynamic shifts in this disturbed circuitry, whereas the disturbed circuitry represents a basic neural phenomenon common to patients suffering from schizophrenia.
Presented at the 4th International Conference on Functional Mapping of the Human Brain, Montreal, June 7–12, 1998. Received April 7, 1998; revision received Sept. 8, 1998; accepted Oct. 19, 1998. From the Mental Health Clinical Research Center, Department of Psychiatry, College of Medicine, University of Iowa Hospitals and Clinics; and the Instituto de Salud “Carlos III,” Madrid. Address reprint requests to Dr. Andreasen, Department of Psychiatry, University of Iowa, MHCRC, 2911 JPP, 200 Hawkins Dr., Iowa City, IA 52242-1057. Supported by NIMH grants MH-31593 and MH-40856 and NIMH Clinical Research Center grant MH-43271.
 |
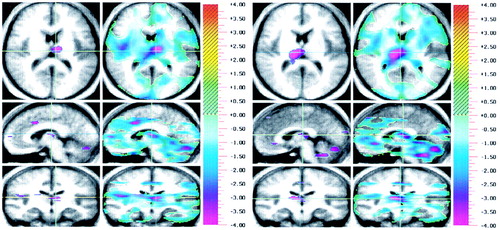
FIGURE 1. Brain Images Showing Differences in Regional Cerebral Blood Flowa Between 13 Healthy Volunteers and 14 Schizophrenic Patients During Recall of (a) Practiced and (b) Novel Word Listsb, c
aThree orthogonal views are shown: axial (top), sagittal (middle), and coronal (bottom). Crosshairs are used to show the location of the slice. Statistical (randomization) maps of the PET data are superimposed on a composite magnetic resonance (MR) image derived by averaging the MR scans from the subjects. Within each condition, the “peak map” (left) provides a descriptive picture of areas where all contiguous voxels exceed the predefined threshold for statistical significance. The “t map” (right) represents the value of t for all voxels in the image and shows the general geography of the activations; the color bar at the right shows the t statistic values. Only regions with a relative decrease in flow for schizophrenic patients are shown.
bFor recall of the practiced word list (part a), regions in blue/purple tones indicate lower flow in patients. The crosshairs point to the left thalamus. The view of the sagittal plane indicates that blood flow in the left medial frontal, left thalamus, and left cerebellum is significantly lower in schizophrenic patients.
cFor recall of the novel word list (part b), regions in blue/purple tones indicate lower flow in patients during the novel recall condition. The crosshairs point to the right thalamus. The view of the sagittal plane indicates that blood flow in the right anterior cingulate, right thalamus, and several regions in the cerebellum is significantly lower in schizophrenic patients.
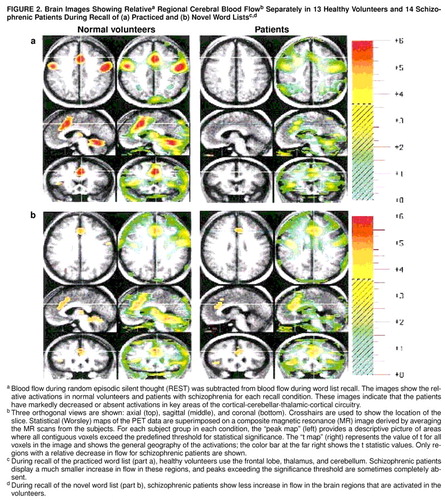
FIGURE 2. Brain Images Showing Relativea Regional Cerebral Blood Flowb Separately in 13 Healthy Volunteers and 14 Schizophrenic Patients During Recall of (a) Practiced and (b) Novel Word Listsc, c
aBlood flow during random episodic silent thought (REST) was subtracted from blood flow during word list recall. The images show the relative activations in normal volunteers and patients with schizophrenia for each recall condition. These images indicate that the patients have markedly decreased or absent activations in key areas of the cortical-cerebellar-thalamic-cortical circuitry.
bThree orthogonal views are shown: axial (top), sagittal (middle), and coronal (bottom). Crosshairs are used to show the location of the slice. Statistical (Worsley) maps of the PET data are superimposed on a composite magnetic resonance (MR) image derived by averaging the MR scans from the subjects. For each subject group in each condition, the “peak map” (left) provides a descriptive picture of areas where all contiguous voxels exceed the predefined threshold for statistical significance. The “t map” (right) represents the value of t for all voxels in the image and shows the general geography of the activations; the color bar at the far right shows the t statistic values. Only regions with a relative decrease in flow for schizophrenic patients are shown.
cDuring recall of the practiced word list (part a), healthy volunteers use the frontal lobe, thalamus, and cerebellum. Schizophrenic patients display a much smaller increase in flow in these regions, and peaks exceeding the significance threshold are sometimes completely absent.
dDuring recall of the novel word list (part b), schizophrenic patients show less increase in flow in the brain regions that are activated in the volunteers.
1. Kraepelin E: Dementia Praecox and Paraphrenia (1919). Translated by Barclay RM; edited by Robertson GM. New York, Robert E Krieger, 1971Google Scholar
2. Bleuler E: Dementia Praecox or the Group of Schizophrenias (1911). Translated by Zinkin J. New York, International Universities Press, 1950Google Scholar
3. Goldman-Rakic PS: Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 1994; 6:348–357Crossref, Medline, Google Scholar
4. Posner MI, Early TS, Reiman E, Pardo JV, Dhawan M: Asymmetries in hemispheric control of attention in schizophrenia. Arch Gen Psychiatry 1988; 45:814–821Crossref, Medline, Google Scholar
5. Saykin AJ, Gur RC, Gur RE, Mozley PD, Resnick SM, Kester DB, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
6. Frith CD, Done DJ: Towards a neuropsychology of schizophrenia. Br J Psychiatry 1988; 153:437–443Crossref, Medline, Google Scholar
7. Andreasen NC, O"Leary DS, Cizadlo T, Arndt S, Rezai K, Boles Ponto LL, Watkins GL, Hichwa RD: Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA 1996; 93:9985–9990Crossref, Medline, Google Scholar
8. Andreasen NC, Rezai K, Alliger R, Swayze VW II, Flaum M, Kirchner P, Cohen G, O"Leary DS: Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with Xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943–958Crossref, Medline, Google Scholar
9. Liddle PF, Friston KJ, Frith C, Hirsch SR, Jones T, Frackowiak RSJ: Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992; 160:179–186Crossref, Medline, Google Scholar
10. Silbersweig DA, Stern E, Frith CD, Cahill C, Holems A, Grootoonik S, Seaward J, McKenna P, Chua SE, Schnorr L, Jones T, Frackowiak RSJ: A functional neuroanatomy of hallucinations in schizophrenia. Nature 1995; 378:176–179Crossref, Medline, Google Scholar
11. Weinberger DR, Berman KF, Zec RF: Psychologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional blood flow evidence. Arch Gen Psychiatry 1986; 43:114–124Crossref, Medline, Google Scholar
12. Frith CD: The Cognitive Neuropsychology of Schizophrenia. Hove, UK, Lawrence Erlbaum, 1992Google Scholar
13. Braff DL: Information processing and attentional dysfunctions in schizophrenia. Schizophr Bull 1993; 19:233–259Crossref, Medline, Google Scholar
14. Andreasen NC: Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science 1997; 275:1586–1593Crossref, Medline, Google Scholar
15. Gold JM, Randolph C, Carpenter CJ, Goldberg TE, Weinberger DR: Forms of memory failure in schizophrenia. J Abnorm Psychol 1992; 101:487–494Crossref, Medline, Google Scholar
16. Andreasen NC, O"Leary DS, Arndt S, Cizadlo T, Rezai K, Watkins GL, Ponto LLB, Hichwa RD: PET studies of memory, I: novel and practiced free recall of complex narratives. Neuroimage 1995; 2:284–295Crossref, Medline, Google Scholar
17. Andreasen NC, O"Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LLB, Hichwa RD: PET studies of memory, II: novel versus practiced free recall of word lists. Neuroimage 1995; 2:296–305Crossref, Medline, Google Scholar
18. Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S: Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA 1994; 91:2016–2020Crossref, Medline, Google Scholar
19. Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ: Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature 1994; 368:633–635Crossref, Medline, Google Scholar
20. Petrides M, Alivisatos B, Meyer E, Evans AC: Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA 1993; 90:878–882Crossref, Medline, Google Scholar
21. Raz S, Raz N: Structural brain abnormalities in the major psychoses: a quantitative review of the evidence from computerized imaging. Psychol Bull 1990; 108:93–108Crossref, Medline, Google Scholar
22. Weinberger DR, Kleinman JE, Luchins DJ, Bigelow LB, Wyatt RJ: Cerebellar pathology in schizophrenia: a controlled postmortem study. Am J Psychiatry 1980; 137:359–361Link, Google Scholar
23. Blennow K, Davidsson P, Gottfries CG, Ekman R, Heilig M: Synaptic degeneration in thalamus in schizophrenia. Lancet 1996; 348:692–693Crossref, Medline, Google Scholar
24. Andreasen NC, Arndt S, Swayze V II, Cizadlo T, Flaum M, O"Leary D, Ehrhardt JC, Yuh WTC: Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 1994; 266:294–298Crossref, Medline, Google Scholar
25. Goldman-Rakic PS, Selemon LD: Functional and anatomical aspects of schizophrenia. Schizophr Bull 1997; 23:437–458Crossref, Medline, Google Scholar
26. Benes FM, Davidson J, Bird ED: Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry 1986; 43:31–35Crossref, Medline, Google Scholar
27. Leiner HC, Leiner AL, Dow RS: Does the cerebellum contribute to mental skills? Behav Neurosci 1986; 100:443–454Google Scholar
28. Schmahmann JD: An emerging concept: the cerebellar contribution to higher function. Arch Neurol 1991; 48:1178–1187Crossref, Medline, Google Scholar
29. Paradiso S, Crespo-Facorro B, Andreasen NC, O’Leary DS, Watkins LG, Boles Ponto L, Hichwa RD: Brain activity assessed with PET during recall of word lists and narratives. Neuroreport 1997; 8:3091–3096Crossref, Medline, Google Scholar
30. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615–623Crossref, Medline, Google Scholar
31. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
32. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1983Google Scholar
33. Hurtig RR, Hichwa RD, O"Leary DS, Boles Ponto LL, Narayana S, Watkins GL, Andreasen NC: Effects of timing and duration of cognitive activation in [15O]water PET studies. J Cereb Blood Flow Metab 1994; 14:423–430Crossref, Medline, Google Scholar
34. Raichle ME, Martin WRW, Herscovitch P, Mintun MA, Markham J: Brain blood flow measured with intravenous [ 15O]H2O, II: implementation and validation. J Nucl Med 1983; 24:790–798Medline, Google Scholar
35. Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW II: Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci 1992; 4:125–133Crossref, Medline, Google Scholar
36. Andreasen NC, Cizadlo T, Harris G, Swayze V II, O’Leary DS, Cohen G, Ehrhardt J, Yuh WT: Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci 1993; 5:121–130Crossref, Medline, Google Scholar
37. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
38. Cizadlo T, Andreasen NC, Zeien G, Rajaprabhakaran R, Harris G, O’Leary D, Swayze V, Arndt S, Hichwa R, Ehrhardt J, Yuh WTC: Image registration issues in the analysis of multiple-injection 15O H2O PET studies: BRAINFIT. Proceedings of the Society of Photo-Optical Instrumentation Engineers 1994; 2168:423–430Google Scholar
39. Arndt S, Cizadlo T, Andreasen NC, Heckel D, Gold S, O’Leary DS: Tests for comparing images based on randomization and permutation methods. J Cereb Blood Flow 1996; 16:1271–1279Crossref, Medline, Google Scholar
40. Worsley K, Evans A, Marret S, Neelin P: A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 1992; 12:900–918Crossref, Medline, Google Scholar
41. Wiser AK, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Dysfunctional cortico-cerebellar circuits cause “cognitive dysmetria” in schizophrenia. Neuroreport 1998; 9:1895–1899Crossref, Medline, Google Scholar
42. Goldman-Rakic PS: Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge, in Handbook of Physiology, vol 5. Edited by Plum F, Mountcastle V. Bethesda, Md, American Physiological Society, 1987, pp 373–417Google Scholar
43. Frith CD, Dolan R: The role of prefrontal cortex in higher cognitive functions. Brain Res Cog Brain Res 1996; 5:175–181Crossref, Medline, Google Scholar
44. Andreasen NC, Nasrallah HA, Dunn V, Olson SC, Grove WM, Ehrhardt JC, Coffman JA, Crossett JH: Structural abnormalities in the frontal system in schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry 1986; 43:136–144Crossref, Medline, Google Scholar
45. Goldberg TE, Kelsoe JR, Weinberger DR, Pliskin NH, Kirwin PD, Berman KF: Performance of schizophrenic patients on putative neuropsychological tests of frontal lobe function. Int J Neurosci 1988; 42:51–58Crossref, Medline, Google Scholar
46. Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD: Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet 1997; 349:1730–1734Crossref, Medline, Google Scholar
47. Pearlson GD, Petty RG, Ross CA, Tien AY: Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology 1996; 14:1–7Google Scholar
48. Frith CD, Friston KJ, Liddle PF, Frackowiak RSJ: Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond 1991; 244:241–246Crossref, Medline, Google Scholar
49. Frith CD: The cognitive abnormalities underlying the symptomatology and the disability of patients with schizophrenia. Int Clin Psychopharmacol 1995; 10(suppl 3):87–98Google Scholar
50. Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE: A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci 1996; 16:808–812Medline, Google Scholar
51. Picard N, Strick PL: Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 1996; 6:342–353Crossref, Medline, Google Scholar
52. Halsband U, Ito N, Tanji J, Freund HJ: The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain 1993; 116:243–266Crossref, Medline, Google Scholar
53. Schr�der J, Wenz F, Schad LR, Baudendistel K, Knoop MV: Sensorimotor cortex and supplementary motor area changes in schizophrenia. Br J Psychiatry 1995; 167:197–201Crossref, Medline, Google Scholar
54. Pakkenberg B: Pronounced reduction of total neuron number in the mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 1990; 47:1023–1028Crossref, Medline, Google Scholar
55. Andreasen NC, Ehrhardt JC, Swayze VW II, Alliger RJ, Yuh WT, Cohen G, Ziebell S: Magnetic resonance imaging of the brain in schizophrenia: the pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry 1990; 47:35–44Crossref, Medline, Google Scholar
56. Buchsbaum MS, Someya T, Teng YC, Abel l, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE Jr: PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 1996; 153:191–199Link, Google Scholar
57. McGhie A, Chapman J: Disorders of attention and perception in early schizophrenia. Br J Med Psychol 1961; 34:103–116Crossref, Medline, Google Scholar
58. Jones EG: Cortical development and thalamic pathology in schizophrenia. Schizophr Bull 1997; 23:483–501Crossref, Medline, Google Scholar
59. Mesulam MM: A cortical network for directed attention and unilateral neglect. Ann Neurol 1981; 10:309–325Crossref, Medline, Google Scholar
60. Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R: Neurophysiological and neuropsychological evidence of attentional dysfunction in schizophrenia. Schizophr Res 1993; 10:131–141Crossref, Medline, Google Scholar
61. Paus T, Petrides M, Evans AC, Meyer E: Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol 1993; 70:453–469Crossref, Medline, Google Scholar
62. Haznedar MM, Buchsbaum MS, Luu C, Hazlett EA, Siegel BV Jr, Lohr J, Wu J, Haier RJ, Bunney WE Jr: Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry 1997; 154:682–684Link, Google Scholar
63. Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RS, Grasby PM: Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature 1995; 378:180–182Crossref, Medline, Google Scholar
64. Vrtunski PB, Simpson DM, Meltzer HY: Voluntary movement dysfunction in schizophrenics. Biol Psychiatry 1989; 25:529–539Crossref, Medline, Google Scholar
65. Sandyk R, Kay SR, Meriam AE: Atrophy of the cerebellar vermis: relevance to the symptoms of schizophrenia. Int J Neurosci 1991; 57:205–212Crossref, Medline, Google Scholar
66. Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR: Distributed neural system underlying the timing of movements. J Neurosci 1997; 17:5528–5535Medline, Google Scholar
67. Ito M: Movements and thought: identical control mechanisms by the cerebellum. Trends Neurosci 1993; 16:448–450Crossref, Medline, Google Scholar
68. Ivry RB: Cerebellar timing systems. Int Rev Neurobiol 1997; 41:555–573Crossref, Medline, Google Scholar
69. Middleton FA, Strick PL: Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 1994; 266:458–461Crossref, Medline, Google Scholar



