Minor Physical Anomalies in Schizophrenic Patients and Their Siblings
Abstract
Objective:The aim of this study was to assess the frequency and type of minor physical anomalies in schizophrenic patients and their normal siblings.Method:Sixty adult patients with schizophrenia, 21 siblings of these patients, and 75 normal comparison subjects were assessed through use of an extended scale consisting of the Waldrop scale and 23 other minor physical anomalies. Results:Patients had significantly more minor physical anomalies than comparison subjects in all body areas tested and also more minor physical anomalies in total than their siblings. Hand, eye, and mouth minor physical anomalies best discriminated patients from comparison subjects. Siblings had significantly more minor physical anomalies than normal comparison subjects. Sixty percent of the patients and 38% of the siblings, but only 5% of the comparison subjects, had a higher rate of minor physical anomalies (i.e., six or more). With the exception of ear minor physical anomalies, no association was found between minor physical anomalies in the patient and sibling in the same family.Conclusions:Higher levels of minor physical anomalies (especially in the eye, mouth, and hand/foot regions) characterize both schizophrenic patients and their normal siblings, but there is little similarity in these anomalies between patients and siblings in the same family. Thus, one or more genetic or shared environmental factors may increase the risk for development of both minor physical anomalies and schizophrenia in these families at large. Minor physical anomalies associated with schizophrenia are frequently found in, but are clearly not limited to, the head or facial region. The Waldrop scale identifies minor physical anomalies strongly associated with schizophrenia. Nevertheless, assessment of the new items clearly indicates that many additional minor physical anomalies are found in schizophrenic patients. Am J Psychiatry 1998; 155: 1695-1702
Minor physical anomalies are slight dysmorphic features representing subtle alterations in the development of various bodily structures in the mouth, eye, ear, global head, hand, and feet areas. Minor physical anomalies are considered to develop during the first and/or early second trimester of gestation (1,2). Because the bodily structures involved in the expression of minor physical anomalies typically share their embryonic origin with that of the brain (3), minor physical anomalies represent potentially valuable indices of disturbances in early neurodevelopment. Once formed, minor physical anomalies persist into adult life and are readily detected on simple visual examination of the particular body area. Because minor physical anomalies represent disturbed prenatal development, they have increasingly been studied in individuals with a range of mental, behavioral, and physical disorders. Minor physical anomalies have been found with increased frequency in autism, hyperactivity, epilepsy, learning disabilities, speech and hearing impairments, mental retardation, poor motor coordination (4), attention deficit disorder, fetal alcohol syndrome, cerebral palsy, and schizophrenia (5).
At least 11 different studies have shown higher rates of minor physical anomalies in adult schizophrenic samples (3–13), while one study showed a similar but nonsignificant trend in schizophrenic twins (14) and another failed to show a higher rate in patients (15). The strong predominance of positive findings in schizophrenia provides considerable general support for a neurodevelopmental model of schizophrenia.
Nevertheless, important questions remain about the relevance of minor physical anomalies for the etiology of schizophrenia. First, the origins of minor physical anomalies associated with schizophrenia remain unclear, and such anomalies may result from both genetic and environmental influences. The finding that minor physical anomalies are particularly common in schizophrenic patients with a family history of the disorder (13) represents evidence for a genetic effect. However, minor physical anomalies observed among monozygotic pairs discordant for schizophrenia were significantly related to maternal pregnancy complications (14), which speaks for the influence of environmental factors in minor physical anomalies in schizophrenic patients. An unanswered question concerns whether minor physical anomalies are “familial,” as might be reflected in a generally higher frequency of such anomalies in the mentally normal relatives of schizophrenic patients, a co-occurrence of minor physical anomalies in the patient and relative within the same family, or both. A high degree of within-pair similarity on minor physical anomaly level was observed in monozygotic twin pairs discordant for schizophrenia (14), as might be expected among identical twins, and the ill twin had only marginally higher (nonsignificant) rates of minor physical anomalies than the well co-twin. However, the only study that investigated minor physical anomalies in the siblings of schizophrenic patients did not show a significantly higher rate in siblings than in normal control subjects (7). Furthermore, rates of minor physical anomalies were not found to be higher in the offspring of women with psychoses (including schizophrenia) than in matched control subjects (16). Further investigation is needed of the possible familiality of minor physical anomalies in schizophrenia, evaluating both the levels of minor physical anomalies in patients, their relatives, and normal comparison subjects and the degree of individual similarity between patients and their relatives in level and type of minor physical anomalies.
A second question concerns the empirical effects resulting from the choice of instrument for measuring minor physical anomalies. With the exception of Lane et al. (11), all of the previous studies of minor physical anomalies in schizophrenia have used the Waldrop scale (17), with occasional modifications or omissions of items. The minor physical anomalies in that instrument originated from an unpublished study by Goldfarb and Botstein, who used those minor physical anomalies to distinguish schizophrenic from normal children (17). The Waldrop scale should thus be well suited for assessment of minor physical anomalies in adult patients. Nevertheless, the scale has been criticized for inherent limitations regarding both content and form. Several authors have recognized the need for a more extensive and well-operationalized scale (18, 19), since the study of minor physical anomalies in schizophrenia is still in an exploratory phase. An investigation directly comparing results from the Waldrop scale and from a parallel instrument consisting of other minor physical anomalies could potentially provide valuable information on the effects of using the Waldrop scale to measure minor physical anomalies in schizophrenic patients and other subjects.
A third question is whether the minor physical anomalies associated with schizophrenia have a characteristic localizing profile. Because higher rates of minor physical anomalies are seen in a range of other diseases, the question of specific localization is of direct relevance for whether minor physical anomalies are indicators of a generalized and nonspecific vulnerability to mental and physical abnormality, or are an expression of a specific regional disturbance related to a particular disease. Few previous studies have reported findings concerning individual minor physical anomalies in schizophrenic patients (possibly because of sample size limitations), but disorders of the mouth (palate) and perhaps also deviant head circumference have been a most frequent feature in schizophrenia (4dash;6, 11, 13). Lane et al. (11), in a carefully conducted study using both a new anthropometric scale and the Waldrop scale, concluded that there was direct evidence of disturbed craniofacial development in schizophrenia, closely associated with brain differentiation. If a craniofacial predominance could be confirmed for minor physical anomalies in other samples of schizophrenic patients, this would provide important evidence for a specific profile of early maldevelopment in individuals who later develop this psychosis.
The aim of the present study was to investigate the rate and topological profile of minor physical anomalies in a group of schizophrenic patients and their nonpsychotic siblings, as part of a multidisciplinary study of risk factors in the etiology of schizophrenia (20). The following hypotheses were tested: 1) minor physical anomalies are more common in schizophrenic patients than in their nonpsychotic siblings and normal comparison subjects, and are also more common in siblings than in normal comparison subjects; 2) a positive within-family relationship between the patient and the sibling is found for both total frequency and specific body area of minor physical anomalies; and 3) a higher rate of minor physical anomalies in schizophrenic patients than in comparison subjects is found predominantly in the head and facial regions (i.e., a craniofacial profile). Furthermore, we studied the effects on differences in minor physical anomaly rates across the three subject groups that resulted from using the Waldrop scale versus a parallel set of other minor physical anomalies.
METHOD
Subjects
The subjects were 60 patients with schizophrenia, 21 healthy siblings from 21 families of these patients, and 75 normal comparison subjects, as described in greater detail in Ismail et al. (20). All subjects were ethnically Caucasian. The 60 patients (44 men and 16 women) from the centralized psychiatric facilities in Malmö, Sweden, fulfilled DSM-III-R criteria for schizophrenia, were born in Scandinavia in 1941 or later, and had no history of psychoactive substance abuse as defined by DSM-III-R criteria, head trauma, or major neurological or somatic disorder with neurological components (e.g., multiple sclerosis). Mean length of illness since first psychotic episode was 14.8 years (SD=7.2, range=1–29), and mean age at time of study was 38.2 years (range=19–55).
Twenty-one siblings (one sibling per patient) who had no history of psychotic or affective disorder, head trauma, neurological disorder, somatic disorder with neurological components, or psychoactive substance abuse per DSM-III-R criteria were also studied. Participating siblings were interviewed with the Structured Clinical Interview for DSM-III-R (21) for schizotypal personality disorder. While a few siblings had isolated symptoms, no sibling met the complete DSM-III-R criteria for the disorder. The mean age of the participating siblings was 37.9 years (range=17–51).
A group of 75 normal comparison subjects (59 men, 16 women), selected from several occupational groups (e.g., firemen, hospital service personnel), was similar to the patient group with respect to educational level, age, and gender ratio. These subjects did not have a history of psychosis, affective disorder, schizophrenia-related personality disorder, head trauma, neurological disorder, somatic disorder with neurological components, psychoactive substance abuse per DSM-III-R criteria, or a family history of mental disorder. The mean age of the comparison subjects was 35.9 years (range=20–54).
No significant differences were found among schizophrenic, sibling, and normal comparison groups in current age or educational level. After complete description of the study to the subjects, written informed consent was obtained.
Assessment of Minor Physical Anomalies
Minor physical anomalies were assessed for each subject through use of an extended and standardized scale consisting of 41 items representing minor physical anomalies in six body areas: eyes (five items), ears (eight items), mouth (six items), global head (seven items), hands (eight items), and feet (seven items). The minor physical anomalies consisted of the 18 items from the Waldrop scale (22) and 23 new items based on other sources (1, 23, 24). The new items were chosen specifically because they belonged to the same body regions as the Waldrop scale items, thus not requiring the subject to remove further clothing for the examination. The total examination typically took 10–15 minutes per subject.
The majority of the 41 items (and all of the new items) were rated as being present or absent, while weighted scores were calculated for a number of Waldrop items. The weighted scores for head circumference and epicanthal distance were modified in accordance with Green et al. (6). Summary scores were calculated for the extended scale, the Waldrop scale per se, and the total new items per se, as well as for each body region within each scale. A copy of the minor physical anomaly manual and scoring system is available on request from Dr. McNeil.
All examinations were performed by the same examiner (B.I.). Interrater reliability was studied between two examiners (B.I. and S. Cardenal, M.D.), who co-examined 10 subjects independently of the current study group. The intraclass correlation coefficient for the extended minor physical anomaly scale score was 0.84 (F=11.22, df=9, 10, p<0.005).
Statistical Analysis
Because of the distribution of scores, group comparison was done by the Mann-Whitney U test, while pairwise comparison of the 21 patients and their 21 siblings was done by the Wilcoxon matched-pairs signed-ranks test. Within-family correlations on minor physical anomaly score between the patients and their siblings were calculated by Spearman rank correlation; the association between minor physical anomaly occurrence in a given body area in the patient and her or his sibling was tested by Fisher’s exact probability test, with odds ratios and 95% confidence intervals. Logistic regression (SPSS 6.1 [25]) was used to determine which particular minor physical anomalies best discriminated patients from comparison subjects, as described in detail later in the article. Statistical significance was defined as p≤0.01, two-tailed.
RESULTS
Group Differences Based on Extended Minor Physical Anomaly Scale
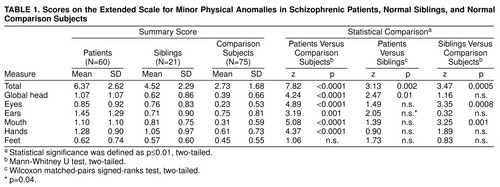
With the extended scale of 41 (Waldrop plus new) items, patients had significantly more minor physical anomalies than comparison subjects on both total minor physical anomalies and minor physical anomalies in each of the six body regions except the feet (table 1). The 21 patients with participating siblings also had significantly more minor physical anomalies in total and in global head characteristics than their siblings. The siblings had significantly higher scores than the comparison subjects on the total extended scale, as well as on minor physical anomalies in eye and mouth regions. The cutoff score that optimally discriminated the patients from comparison subjects (maximizing sensitivity and specificity for schizophrenia) was six or more minor physical anomalies. A score of 6 or more characterized only 5% of the comparison subjects but 60% of the patients and 38% of the siblings.
Group Differences Based on Waldrop and New Minor Physical Anomaly Scales
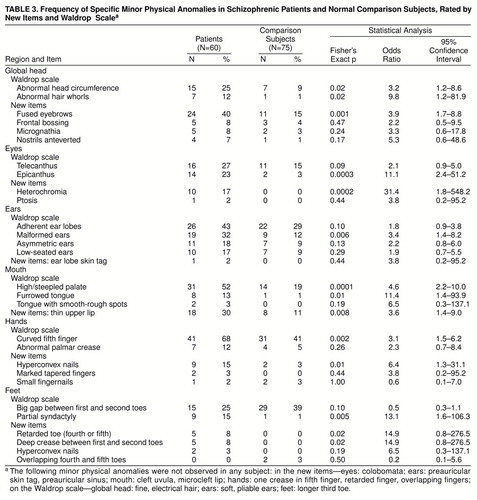
As shown in table 2, patients generally differed from comparison subjects as often on the new item scale as on the Waldrop scale. Significant group differences were found between patients and comparison subjects on the new item total scale, as well as on new item eye, mouth, global head, hands, and feet areas by themselves. Similarly, significant differences were found between patients and comparison subjects on the Waldrop scale in total and in eye, ear, mouth, global head, and hand areas by themselves. Minor physical anomalies in the ear and mouth were the only body areas in which greater patient-comparison subject differences were found with the Waldrop scale than with the new item scale (only one new ear item occurred in any group, and then with low frequency) (table 3). The patients also differed more from siblings on the new item scale than on the Waldrop scale for global head area.
In contrast, the siblings differed more from comparison subjects on the Waldrop scale than new item scale, showing significantly more minor physical anomalies than comparison subjects on both Waldrop scale total items and eye and mouth areas, but only on eye minor physical anomalies on the new item scale.
Within-Family Relationship on Total Minor Physical Anomalies
The correlations between total minor physical anomaly scores for the patient-sibling pair within each family were low and nonsignificant for the total extended scale (Spearman correlation, N=21; rs=0.02), the total Waldrop scale (N=21; rs=0.12), and the total new item scale (N=21; rs=0.25). Further, the presence of one or more minor physical anomalies (extended scale) in a given body region in the patient was—with one exception—not significantly related to the presence of one or more minor physical anomalies in the same body area in the patient’s sibling. Only ear malformations (extended scale) showed a significant co-occurrence in the patient and his or her sibling (N=21 patient-sibling pairs for all analyses; ears: Fisher’s exact p=0.01, odds ratio=24.8, 95% confidence interval=1.2–527.5; eyes: Fisher’s exact p=0.07, odds ratio=1.8, 95% confidence interval=0.3–11.3; mouth: Fisher’s exact p=1.00, odds ratio=1.1, 95% confidence interval=0.1–8.7; global head: Fisher’s exact p=0.55, odds ratio=0.3, 95% confidence interval=0.1–4.2; hands: Fisher’s exact p=1.00, odds ratio=1.1, 95% confidence interval=0.1–8.7; feet: Fisher’s exact p=0.06, odds ratio=10.0, 95% confidence interval=0.9–110.6).
Specific Minor Physical Anomalies in the Patients and Comparison Subjects
The patients had significantly higher rates than comparison subjects of (global head) fused eyebrows; (eye) epicanthus and heterochromia of iris; (ear) malformed ears; (mouth) high/steepled palate, furrowed tongue, and thin upper lip; (hand) curved fifth finger and hyperconvex fingernails; and (feet) partial syndactyly of toes. They also had strong (p≤0.02) trends toward more frequent (global head) abnormal head circumference and abnormal hair whorls; and (feet) retarded toe and deep crease between first and second toes (table 3).
Logistic regression was used in a manner similar to that of Lane et al. (11) in order to determine whether a specific discriminatory localization pattern could be discerned among the minor physical anomalies that were found with greater frequency in patients. The specific minor physical anomaly items selected for analysis with logistic regression were the 12 minor physical anomalies with odds ratios of 2.0 or higher and “lower” 95% confidence intervals greater than 1.0 (Fisher’s exact p≤0.05 for all items) (table 3) in comparison of patients and normal subjects: (global head) abnormal head circumference, abnormal hair whorls, and fused eyebrows; (eyes) epicanthus and heterochromia; (ears) malformed ears; (mouth) high/steepled palate, furrowed tongue, and thin upper lip; (hands) curved fifth finger and hyperconvex nails; and (feet) partial syndactyly. Logistic regression was performed separately on each of these body areas, using the above item or items from that area. These analyses showed that three of the 12 items (abnormal head circumference, heterochromia, and furrowed tongue) had odds ratios whose 95% confidence intervals transversed 1.0, and these three items were excluded from the subsequent regression analysis.
The remaining nine minor physical anomalies were then entered together into one logistic regression analysis, the results of which are presented in table 4. Three Waldrop items (in descending hierarchical order: curved fifth finger, epicanthus, and high/steepled palate) made significant (p≤0.01) independent contributions to prediction of patient-comparison group status, with two new minor physical anomaly items (hyperconvex fingernails and thin upper lip) showing p values of 0.03 and 0.05, respectively. These five items were all in the hand and facial (eye-mouth) regions. This model correctly classified 73% of the patients and 85% of the comparison subjects (overall classification=80%).
Specific Minor Physical Anomalies in the Siblings and Comparison Subjects
Siblings had significantly higher rates than comparison subjects of epicanthus (24% versus 3%; Fisher’s exact p=0.005; odds ratio=11.4; 95% confidence interval=2.0–64.1), high/steepled palate (52% versus 19%; Fisher’s exact p=0.004; odds ratio=4.8; 95% confidence interval=1.7–13.5), and partial syndactyly of toes (19% versus 1%; Fisher’s exact p=0.008; odds ratio=17.4; 95% confidence interval=1.8–165.9); heterochromia (10% versus 0%; odds ratio=19.4; 95% confidence interval=0.9–420.2) showed an exact p value of 0.05, and abnormal hair whorls (14% versus 1%; odds ratio=12.3; 95% confidence interval=1.2–125.7) an exact p value of 0.03.
DISCUSSION
The schizophrenic patient group showed a much higher level of minor physical anomalies than the normal comparison subjects, corroborating the results from previous research (e.g., references 3dash;5, 7, 11). It is important to keep in mind that these minor physical anomalies are fossilized imprints of early disturbance in embryonic development and are unaltered by the subsequent illness and its consequences. The optimal extended scale minor physical anomaly cutoff score (≥6) for discriminating patients from comparison cases was the same as the median and very close to the mean of scores in the patient group. This cutoff score yielded a specificity of 95% and sensitivity of 60%, a rate that is in the upper range of minor physical anomaly rates found in previous studies of schizophrenic groups (30%–75%) (26). The patient group’s mean total score on the Waldrop scale was 4.8 minor physical anomalies, which is very close to the mean of 4.2 minor physical anomalies predicted on the basis of results of 11 previous studies of schizophrenic patients (based on reference 3 plus table 6.1 in reference 18).
Both the Waldrop scale and the new item scale by themselves generally identified very significant higher rates of minor physical anomalies in patients than in comparison subjects (table 2). Significantly higher rates of minor physical anomalies were found in all body areas studied with either the Waldrop scale or the new item scale or both. While these differences were generally greater with the new minor physical anomaly item scale than with the Waldrop scale (table 2), the specific minor physical anomaly items making significant independent contributions to predicting patient versus comparison group status came from the Waldrop scale (table 4). Further, the significantly higher rate of minor physical anomalies in the normal siblings of schizophrenic patients than in comparison subjects was based primarily on the Waldrop scale rather than on the new item scale (table 2). In total, the results with the current battery suggest that the minor physical anomalies associated with schizophrenia are clearly not limited to those in the Waldrop scale (see also reference 11) but that the Waldrop scale appears to function well in identifying minor physical anomalies that are especially strongly associated with schizophrenia.
Our findings contrast with those of the only previous study of minor physical anomalies in siblings (7). In the current study, the normal siblings of schizophrenic patients had very significant higher rates of minor physical anomalies in total, as well as in eye and mouth regions. The specific minor physical anomalies with very significant increased rates in siblings were epicanthus (24%), high/steepled palate (52%), and partial syndactyly of toes (19%). These rates paralleled those in the total patient group (epicanthus: 23%, palate: 52%, syndactyly: 15%) and were notably higher than rates in comparison subjects (3%, 19%, and 1%, respectively). With the cutoff score that optimally discriminated patients from comparison subjects, 38% of the siblings showed notable rates of minor physical anomalies. This frequency is identical to that for their rate of total hard and soft neurological signs, based on a similar, empirically determined cutoff score that defined 5% of the comparison group as deviant (20). The higher rate of minor physical anomalies in the eye region in siblings is of special interest, since the neurological investigation of these siblings also showed high scores on eye coordination abnormalities (20). These results are in line with studies demonstrating eye movement abnormalities associated with schizophrenia (27). The significantly higher rates of minor physical anomalies in the mouth region in siblings (versus normal comparison subjects) are also interesting in light of a previous study showing a significant association between the rate of minor physical anomalies in this region in patients and a family history of schizophrenia in the patient group (3).
The sibling group’s higher rate of total minor physical anomalies, in scores for eye and mouth regions and in specific eye, mouth, and foot minor physical anomalies, is especially notable in that (with the exception of ear malformations) no association was found between the level or type of minor physical anomalies in the patient and in the sibling within the same family. Indeed, even the three specific minor physical anomalies that were especially increased among siblings (and also among patients) did not significantly co-occur in the patient and sibling in the same family (contingency analysis: epicanthus, Fisher’s exact p=1.00; high/steepled palate, Fisher’s exact p=0.20; syndactyly, Fisher’s exact p=1.00). Nevertheless, the patients also had significantly more minor physical anomalies than the siblings (table 1 and table 2). One interpretation of this combination of results is that one or more genetic or shared environmental factors increases the risk for developing both minor physical anomalies and schizophrenia in these families at large and that the increased rate of minor physical anomalies in patients (versus siblings) signals the increased effect of such factors in those who later become schizophrenic. Furthermore, the lack of relationship between patient and sibling minor physical anomalies within the particular family could speak against a reproductive defect in the specific mothers (or fathers) that leads to replication of the same malformation in several siblings in the same family (as is seen in some families in malformation samples).
The current findings do not appear to support the hypothesis that minor physical anomalies found in schizophrenic patients have a predominantly faciocranial topographical pattern, at least within the framework of the anomalies included. First, higher rates of minor physical anomalies in schizophrenic patients were found in all six of the body areas studied, both at a summary score and individual minor physical anomaly level (table 1– 3). Second, the individual minor physical anomalies that made significant independent contributions (p≤0.01) to predicting patient versus comparison subject status (logistic regression model) (table 4) were found in the hand (first rank), eye (second rank), and mouth (third rank), followed (nonsignificantly) by another hand item (fourth rank) and another mouth item (fifth rank). While the logistic regression analyses represent one possible model of the minor physical anomaly panorama in schizophrenia, the study results in total would suggest that the minor physical anomalies associated with schizophrenia are frequently found in, but are clearly not limited to, the head and facial region.
The finding of a higher rate of minor physical anomalies in the mouth region is especially interesting. High/steepled palate represents a microform of cleft palate, which is itself frequently associated with midline brain anomalies as in fetal alcohol syndrome. Further, midline brain anomalies such as enlarged cavum septi pellucidi and corpus callosum abnormalities have been found with increased incidence in patients with schizophrenia (28,29).
The high rate of minor physical anomalies in the hand region of patients merits further interest, since some groups of schizophrenic patients, including those in the current study (30), have shown aberrant dermatoglyphic patterns (31,32), which are also presumptive markers of prenatal neurodevelopmental disturbances.
With respect to limitations of the study, the sibling group was rather small, and the results for the siblings, especially for the co-occurrence of specific minor physical anomalies in sibling and patient pairs, should be considered tentative until they are retested on new groups. The findings for the new item scale should also be retested on new groups. As in all such studies, examination of subjects could not be done blindly with respect to patient versus nonpatient status. Nevertheless, examination of minor physical anomalies was done at a detailed item (rather than global) level, with acceptable interscorer reliability for the total examination, and with a notable range of scores across different subjects within each of the three groups.
In conclusion, the addition of new minor physical anomaly items to the examination seems to add valuable information concerning minor physical anomalies in schizophrenic patients. A question of continuing concern is the particular role of early maldevelopment in schizophrenia’s total etiological panorama. Investigation of the correlates of minor physical anomalies in these patients and their normal siblings may yield further knowledge about the developmental origins of this disease.
Received Dec. 12, 1997; revision received May 22, 1998; accepted May 29, 1998. From the Section for Epidemiology, Department of Community Medicine, Lund University, University Hospital, Malmö, Sweden. Address reprint requests to Dr. McNeil, Section for Epidemiology, Department of Community Medicine, University Hospital UMAS, S-205 02 Malmö, Sweden. Supported by grants from the Swedish Medical Research Council (3793), the Faculty of Medicine, Lund University, Sweden, the Söderström-König Foundation, Sweden, and the Theodore and Vada Stanley Foundation, United States of America.
 |
 |
 |
 |
1. Smith DW: Recognizable Patterns of Human Malformation. Philadelphia, WB Saunders, 1970Google Scholar
2. Warkany J: Congenital Malformations. Chicago, Year Book Medical, 1971Google Scholar
3. O’Callaghan E, Buckley P, Madigan C, Redmond O, Stack JP, Kinsella A, Larkin C, Ennis JT, Waddington JL: The relationship of minor physical anomalies and other putative indices of developmental disturbance in schizophrenia to abnormalities of cerebral structure on magnetic resonance imaging. Biol Psychiatry 1995; 38:516–524Crossref, Medline, Google Scholar
4. Lohr JB, Flynn K: Minor physical anomalies in schizophrenia and mood disorders. Schizophr Bull 1993; 19:551–556Crossref, Medline, Google Scholar
5. McGrath JJ, van Os J, Hoyos C, Jones PB, Harvey I, Murray RM: Minor physical anomalies in psychoses: associations with clinical and putative aetiological variables. Schizophr Res 1995; 18:9–20Crossref, Medline, Google Scholar
6. Green MF, Satz P, Gaier DJ, Ganzell S, Kharabi F: Minor physical anomalies in schizophrenia. Schizophr Bull 1989; 15:91–99Crossref, Medline, Google Scholar
7. Green MF, Satz P, Christenson C: Minor physical anomalies in schizophrenia patients, bipolar patients, and their siblings. Schizophr Bull 1994; 20:433–440Crossref, Medline, Google Scholar
8. Gualtieri CT, Adams A, Shen CD, Loiselle D: Minor physical anomalies in alcoholic and schizophrenic adults and hyperactive and autistic children. Am J Psychiatry 1982; 139:640–643Link, Google Scholar
9. Guy JD, Majorski LV, Wallace CJ, Guy MP: The incidence of minor physical anomalies in adult male schizophrenics. Schizophr Bull 1983; 9:571–582Crossref, Medline, Google Scholar
10. Lal R, Sharma S: Minor physical anomalies in schizophrenia. Indian J Psychiatry 1987; 29:119–122Medline, Google Scholar
10. Lane A, Kinsella A, Murphy P, Byrne M, Keenan J, Colgan K, Cassidy B, Sheppard N, Horgan R, Waddington JL, Larkin C, O’Callaghan E: The anthropometric assessment of dysmorphic features in schizophrenia as an index of its developmental origins. Psychol Med 1997; 27:1155–1164Crossref, Medline, Google Scholar
11. Nizamie SH, Nizamie A, Snagma MW, Sharma PL: Soft neurological signs and minor physical anomalies in schizophrenia. Indian J Psychiatry 1989; 31:230–237Medline, Google Scholar
12. O’Callaghan E, Larkin C, Kinsella A, Waddington JL: Familial, obstetric, and other clinical correlates of minor physical anomalies in schizophrenia. Am J Psychiatry 1991; 148:479–483Link, Google Scholar
13. Cantor-Graae E, McNeil TF, Torrey EF, Quinn P, Bowler A, Sjöström K, Rawlings R: Link between pregnancy complications and minor physical anomalies in monozygotic twins discordant for schizophrenia. Am J Psychiatry 1994; 151:1188–1193Link, Google Scholar
14. Alexander RC, Mukherjee S, Richter J, Kaufmann CA: Minor physical anomalies in schizophrenia. J Nerv Ment Dis 1994; 182:639–644Crossref, Medline, Google Scholar
15. McNeil TF, Blennow G, Lundberg L: Congenital malformations and structural developmental anomalies in groups at high risk for psychosis. Am J Psychiatry 1992; 149:57–61Link, Google Scholar
16. Waldrop MF, Halverson CF: Minor physical anomalies and hyperactive behavior in young children, in Exceptional Infant. Edited by Hellmuth J. New York, Brunner/Mazel, 1971, pp 343–381Google Scholar
17. Lane A, Larkin C, Waddington JL, O’Callaghan E: Dysmorphic features and schizophrenia, in The Neurodevelopmental Basis of Schizophrenia. Edited by Waddington JL, Buckley PF. London, Landes, 1996, pp 79–93Google Scholar
18. Murphy KC, Owen MJ: Minor physical anomalies and their relationship to the aetiology of schizophrenia. Br J Psychiatry 1996; 168:139–142Crossref, Medline, Google Scholar
19. Ismail B, Cantor-Graae E, McNeil TF: Neurological abnormalities in schizophrenic patients and their siblings. Am J Psychiatry 1998; 155:84–89Link, Google Scholar
20. Spitzer RL, Williams JBW, Gibbon M, First MB: Instruction Manual for the Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
21. Waldrop MF, Pedersen FA, Bell RQ: Minor physical anomalies and behavior in preschool children. Child Dev 1968; 39:391–400Crossref, Medline, Google Scholar
22. Leppig KA, Werler MM, Cann CI, Cook CA, Holmes LB: Predictive value of minor anomalies, 1: association with major malformations. J Pediatr 1987; 110:530–537Crossref, Google Scholar
23. Gorlin RJ, Chen MM, Levin SL: Syndromes of the Head and Neck. New York, Oxford University Press, 1990Google Scholar
24. Norusis MJ: SPSS for Windows Base System User"s Guide and Advanced Statistics, Release 6.0 and Update. Chicago, SPSS 1993, 1994Google Scholar
25. Kaplan HI, Sadock BJ: Concise Textbook of Clinical Psychiatry. Baltimore, Williams & Wilkins, 1996Google Scholar
26. Matthysse S, Holzman PS, Lange K: The genetic transmission of schizophrenia: application of Mendelian latent trait structures analysis to eye tracking dysfunctions in schizophrenia and affective disorders. J Psychiatr Res 1986; 20:57–76Crossref, Medline, Google Scholar
27. DeLisi LE, Hoff AL, Kushner M, Degreef G: Increased prevalence of cavum septum pellucidum in schizophrenia. Psychiatr Res Neuroimaging 1993; 50:193–199Crossref, Medline, Google Scholar
28. Swayze VW II, Andreasen NC, Ehrhardt JC, Yuh WT, Alliger RJ, Cohen GA: Developmental abnormalities of the corpus callosum in schizophrenia. Arch Neurol 1990; 47:805–808Crossref, Medline, Google Scholar
29. Cantor-Graae E, Ismail B, McNeil TF: Neonatal head circumference and related indices of disturbed fetal development in schizophrenic patients. Schizophr Res 1998; 32:191-199Crossref, Medline, Google Scholar
30. Mellor CS: Dermatoglyphic evidence of fluctuating asymmetry in schizophrenia. Br J Psychiatry 1992; 160:467–472Crossref, Medline, Google Scholar
31. Bracha HS, Torrey EF, Gottesman II, Bigelow LB, Cunniff C: Second-trimester markers of fetal size in schizophrenia: a study of monozygotic twins. Am J Psychiatry 1992; 149:1355–1361Link, Google Scholar



