Olanzapine Versus Lithium in the Maintenance Treatment of Bipolar Disorder: A 12-Month, Randomized, Double-Blind, Controlled Clinical Trial
Abstract
OBJECTIVE: The authors compared the efficacy of olanzapine and lithium in the prevention of mood episode relapse/recurrence. METHOD: Patients with a diagnosis of bipolar disorder (manic/mixed), a history of two or more manic or mixed episodes within 6 years, and a Young Mania Rating Scale total score ≥20 entered the study and received open-label cotreatment with olanzapine and lithium for 6–12 weeks. Those meeting symptomatic remission criteria (Young Mania Rating Scale score ≤12; 21-item Hamilton depression scale score ≤8) were randomly assigned to 52 weeks of double-blind monotherapy with olanzapine, 5–20 mg/day (N=217), or lithium (target blood level: 0.6–1.2 meq/liter) (N=214). RESULTS: Symptomatic relapse/recurrence (score ≥15 on either the Young Mania Rating Scale or Hamilton depression scale) occurred in 30.0% of olanzapine-treated and 38.8% of lithium-treated patients. The noninferiority of olanzapine relative to lithium (primary objective) in preventing relapse/recurrence was met, since the lower limit of the 95% confidence interval on the 8.8% risk difference (–0.1% to 17.8%) exceeded the predefined noninferiority margin (–7.3%). Secondary results showed that compared with lithium, olanzapine had significantly lower risks of manic episode and mixed episode relapse/recurrence. Depression relapse/recurrence occurred in 15.7% of olanzapine-treated and 10.7% of lithium-treated patients. Mean weight gain during open-label cotreatment was 2.7 kg; during double-blind monotherapy, weight gain was significantly greater with olanzapine (1.8 kg) than with lithium (–1.4 kg). CONCLUSIONS: These results suggest that olanzapine was significantly more effective than lithium in preventing manic and mixed episode relapse/recurrence in patients acutely stabilized with olanzapine and lithium cotreatment. Both agents were comparable in preventing depression relapse/recurrence.
Despite ongoing maintenance therapy, patients with bipolar disorder will experience frequent fluctuations in symptom severity and multiple relapses. Prospective naturalistic studies examining relapse have reported relapse risks ranging from 44% in 1 year (1) to 73%–88.7% over 4–5 years (2, 3). To date, a limited number of therapeutic agents have been available for the long-term treatment of bipolar disorder. Lithium has been the mainstay of maintenance therapy for >30 years. It is the most extensively studied mood stabilizer and has the best overall efficacy for the prophylactic treatment of bipolar disorder. Recent meta-analyses show it to be superior to placebo in the prevention of relapse (4) and to reduce the risk of relapse 3.6-fold (5). The anticonvulsant agents valproate (6), lamotrigine (7), and carbamazepine (8) also have been used as maintenance therapies.
Olanzapine has shown superiority to placebo in treating acute manic episodes in patients with bipolar I disorder (9, 10). Furthermore, in a 47-week comparative trial of olanzapine versus valproate, rates of mood episode following acute remission of mania were comparable between the groups (11), suggesting that olanzapine may be effective in the prevention of bipolar disorder relapse/recurrence.
This trial compared the efficacy of olanzapine and lithium for the prevention of mood episode relapse/recurrence. For simplicity, the term recurrence will be used throughout the text.
Method
Patients
Patients enrolled in this study were ≥18 years of age and met DSM-IV criteria for bipolar disorder (current episode manic or mixed) as determined with the Structured Clinical Interview for DSM-IV, Patient Version. Patients were required to have a Young Mania Rating Scale total score ≥20 at baseline and a history of at least two manic or mixed episodes in the preceding 6 years. Patients were excluded from the study if they had a serious, unstable medical illness; met DSM-IV substance dependence criteria (nicotine or caffeine excepted) within the past 30 days; had been treated with a depot neuroleptic within 6 weeks of random assignment; or were considered a serious suicide risk. Patients were also excluded if they had a history of intolerance, or lack of response, to an adequate trial of lithium or olanzapine as determined by the investigator. After the study was completely described to the patients, written informed consent was obtained. The study was approved by the appropriate ethics review boards.
Study Design
Patients were recruited from 87 inpatient and outpatient settings across Western Europe, Canada, South Africa, Israel, Australia, and New Zealand between August 1999 and June 2002. This randomized, double-blind, controlled trial consisted of four study periods: 1) screening (two clinic visits over 2–7 days), 2) open-label cotreatment (6–12 weeks; twice-weekly visits for the first 2 weeks, weekly thereafter), 3) double-blind taper (4 weeks; weekly visits), and 4) double-blind monotherapy (48 weeks; biweekly during the first 4 weeks, monthly thereafter). Eligible patients began open-label cotreatment with olanzapine, 15 mg/day, and lithium, 600 mg/day. Allowed dosages of olanzapine were 5–20 mg/day. Investigators were required to optimize lithium dose and reach a target blood level of 0.6–1.2 meq/liter by week 4 during this period.
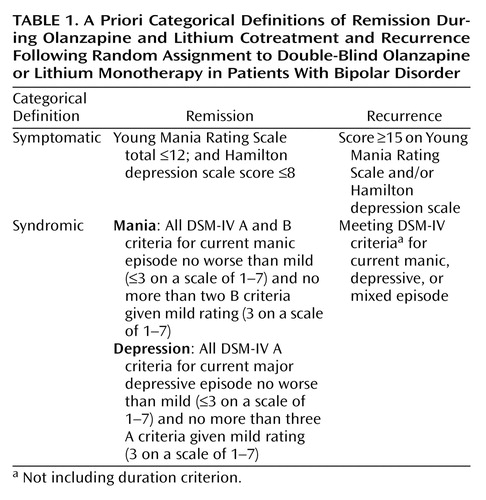
Patients who met symptomatic remission criteria (Table 1) during the open-label cotreatment period were randomly reassigned in a 1:1 ratio by means of a unique drug kit number (via a call-in Interactive Voice Response System) to monotherapy with either olanzapine or lithium. All patients, study site personnel, and sponsor investigators were blind to randomization codes. During the double-blind taper period, patients remained on their current dose of randomly assigned treatment, and the dose of the discontinued drug was tapered in a blinded, a priori-determined, step-wise manner over 4 weeks.
Lithium levels were monitored every 2 weeks during the double-blind taper period and monthly during double-blind maintenance monotherapy. If the serum level of lithium deviated from the therapeutic range during these study periods, the investigator was to adjust the dose of lithium to reestablish blood levels within the therapeutic range, with a goal of reaching this range within 30 days. Serum levels ranging from 0.6–1.2 meq/liter were considered within normal limits. Maintenance of the blind associated with blood draws has been described (12). Briefly, all patients randomly assigned to olanzapine also had blood drawn. For every outlier report generated for a lithium patient, a sham lithium outlier report was sent to an olanzapine patient. Thus, reports to investigative sites indicating that the lithium dose should be adjusted did not unmask the randomized assignment.
Concomitant Medications
Patients who entered the study receiving psychotropic medications (including anticonvulsants, typical or atypical antipsychotics [oral or intramuscular], or antidepressants) were gradually discontinued from these medications at the discretion of the investigator during the first 3 weeks of the open-label cotreatment period. However, oral or intramuscular haloperidol and zuclopenthixol were permitted for extreme agitation during the open-label period. Benzodiazepines were allowed according to the following guidelines. The maximum dose from the screening period through the first 6 weeks of the open-label cotreatment period was 8 mg/day in lorazepam equivalents and 6 mg/day in lorazepam equivalents for the remainder of the open-label period and during the first 2 weeks of the taper period. It was further decreased to 4 mg/day for the remaining 2 weeks of the taper period and then to 2 mg/day (for not more than 60 cumulative days) for the double-blind monotherapy period. Patients were permitted concomitant medication for treatment-emergent extrapyramidal symptoms (biperiden or benztropine mesylate, ≤6 mg/day; trihexyphenidyl, ≤12 mg/day). However, prophylactic use of anticholinergics for extrapyramidal symptoms was not allowed.
Assessments
Recurrence and severity of illness were assessed with the Young Mania Rating Scale and the 21-item Hamilton depression scale; raters were trained and certified to use these scales. A minimum reliability score (intraclass correlation [ICC]) of 0.75 was required for certification; raters who failed to achieve an ICC of ≥0.75 were retrained and tested again. Four hundred fifty-two raters were certified to use the Young Mania Rating Scale and Hamilton depression scale for this study, with average ICCs of 0.88 (Hamilton depression scale) and 0.90 (Young Mania Rating Scale). The vast majority of raters achieved an ICC of ≥0.85 (78.8% for the Hamilton depression scale and 77.4% for the Young Mania Rating Scale). Patient safety was evaluated through standard clinical observations, and extrapyramidal symptoms were assessed with the Simpson-Angus Rating Scale, the Barnes Rating Scale for Drug-Induced Akathisia, and the Abnormal Involuntary Movement Scale. Criteria for treatment-emergent extrapyramidal symptoms have been described previously (12).
Statistical Methods
The primary objective of the study was the assessment of olanzapine’s noninferiority to lithium in the risk of symptomatic mood episode recurrence. It was estimated that it would require that 200 patients in symptomatic remission be randomly assigned to each therapy to provide 80% power to detect the protocol-defined margin of noninferiority for the reduction in absolute risk for olanzapine relative to lithium: a 95% confidence interval lower limit at or below –0.073 (–7.3%). The calculation assumed an expected risk difference of 0.045 (0.319 for olanzapine, 0.364 for lithium). Differences in recurrence risk were tested by using Fisher’s exact test, and the 95% confidence interval about the absolute risk reduction was computed using the normal approximation. Odds ratios with 95% confidence intervals are also presented.
Categorical baseline characteristics (as well as adverse event incidence rates and other categorical outcomes) were compared between treatments with Fisher’s exact test. For continuous baseline data, Wilcoxon rank-sum tests or analyses of variance (ANOVAs) were used. ANOVA models (with terms for treatment, country, and the treatment-by-country interaction) were also used to analyze the last observation carried forward baseline-to-endpoint changes for the continuous safety measures (laboratory analytes, vital signs, and weight). Time-to-event data were analyzed by using Kaplan-Meier estimated survival curves with comparisons made by using log-rank tests. A logistic regression analysis was performed to examine the impact of quantitative length of remission before treatment assignment on the difference between treatments in mood episode recurrence risk (test of interaction between treatment and length of remission). Analyses were completed on an intent-to-treat basis; SAS software version 6 (Cary, N.C., SAS Institute) was used to perform all analyses; treatment effects were tested at the two-sided alpha level of 0.05 and interactions at a level of 0.10 as the protocol specified.
Results
Patient Disposition and Characteristics
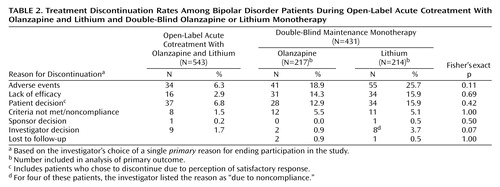
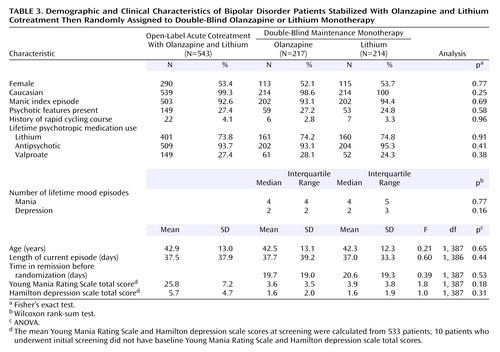
A total of 543 patients were enrolled during the open-label period and received lithium/olanzapine cotreatment. Ultimately, 431 (79.4%) achieved symptomatic (protocol-defined) remission criteria during the open-label period and were randomly assigned to double-blind maintenance monotherapy with olanzapine (N=217) or lithium (N=214). The most common reasons for discontinuation during the open-label period were patient decision and adverse events (Table 2). Of the patients achieving symptomatic remission, 91.6% (N=395) achieved syndromic remission. One hundred seventy-one patients completed the double-blind maintenance period, with significantly more olanzapine-treated than lithium-treated patients completing the trial (46.5% [N=101] versus 32.7% [N=70], respectively; p=0.004, Fisher’s exact test). The most common reasons for discontinuation during the double-blind maintenance period were adverse events, lack of efficacy, and patient decision (Table 2). There were no significant between-group differences in reasons for premature discontinuation. The estimated median time to discontinuation was 303 and 207 days for olanzapine- and lithium-treated patients, respectively, and the time to discontinuation for any reason was significantly earlier for patients receiving lithium (χ2=5.7, df=1, p<0.02, log-rank test). Because symptomatic recurrence in a few cases did not necessarily result in immediate discontinuation from the trial, a post hoc tabulation of the number of completers with sustained remission throughout the double-blind phase of the study also shows significantly higher completion with sustained remission for olanzapine (43.3% [N=94 of 217]) than lithium (28.5% [N=61 of 214]) (p=0.002, Fisher’s exact test). If those patients who had a recurrence were also counted as completers, then completion rates were 73.3% for olanzapine and 67.3% for lithium (p=0.206, Fisher’s exact test).
Patient characteristics are presented in Table 3. Approximately 93% of the patients had a manic index episode, and 26% were experiencing psychotic features. Among patients randomly assigned to a treatment condition, 72.2% were hospitalized for treatment of their index episode at the time they entered into the open-label cotreatment phase (lithium=74.8%; olanzapine=69.6%) (p=0.24, Fisher’s exact test). Overall, treatment groups were comparable with respect to demographic and clinical characteristics.
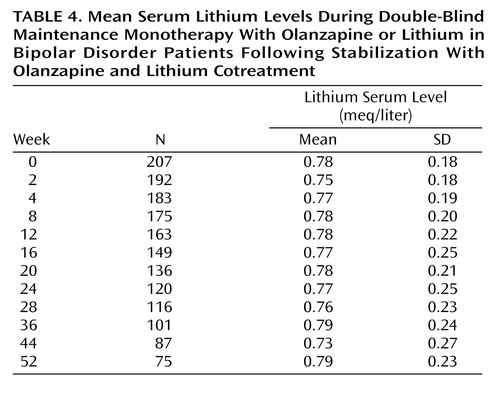
The mean doses of olanzapine and lithium, respectively, during the open-label period were 13.5 mg/day (SD=4.0) and 1003.3 mg/day (SD=267.0) (mean serum level=0.697 meq/liter, SD=0.14). During the open-label phase, investigators were to titrate the lithium dose to attain therapeutic levels by week 4. Considering the post-titration stabilization period only (from week 4 to random assignment), the mean dose of lithium was 1097.0 mg (SD=277.0), and the mean lithium serum level was 0.76 meq/liter (SD=0.14). For the double-blind period, the mean dose was 11.9 mg (SD=4.4) for olanzapine and 1102.7 mg (SD=270.3) for lithium (mean serum level=0.76 meq/liter, SD=0.14). The mean lithium serum levels across the double-blind phase are shown in Table 4. Benzodiazepines were used by significantly more lithium-treated patients (52.3%) than olanzapine-treated patients (35.5%) (p<0.001, Fisher’s exact test) during the double-blind maintenance period. Anticholinergics were used by 7.4% and 8.4% of olanzapine-treated and lithium-treated patients, respectively (p=0.72, Fisher’s exact test).
Incidence of and Time to Mood Episodes
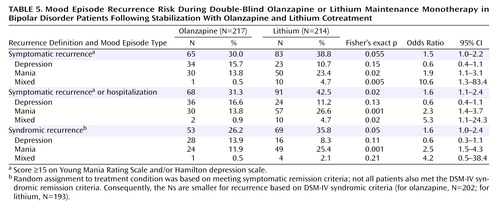
Symptomatic recurrence of any mood episode following remission of mania or depression was observed in 38.8% of lithium-treated and 30.0% of olanzapine-treated patients (Table 5). Statistical noninferiority of olanzapine relative to lithium was established because the 95% confidence interval about the observed 8.8% absolute risk reduction (–0.1% to 17.8%) excludes the predefined margin of noninferiority (–7.3%). Considering pole-specific recurrences, olanzapine and lithium did not differ significantly in the proportion of patients who had a depressive recurrence. However, significantly fewer olanzapine-treated patients had recurrence of manic or mixed episodes compared with lithium-treated patients. Time to symptomatic recurrence to any mood episode was not significantly different between treatments (Figure 1).
Recurrence was further assessed as 1) meeting symptomatic recurrence criteria or hospitalization for a mood episode and 2) meeting DSM-IV criteria for syndromic recurrence after having met syndromic criteria for remission. Rates of recurrence and odds ratios are presented in Table 5. Considering both these criteria, significantly fewer olanzapine-treated patients experienced a mood episode recurrence compared with lithium-treated patients. Furthermore, as shown in Figure 1, time until mood episode recurrence was significantly longer for olanzapine-treated patients.
Significantly fewer olanzapine-treated patients (14.3% [N=31 of 217]) were hospitalized for a mood episode during the double-blind period compared with lithium-treated patients (22.9% [N=49 of 214]) (p<0.03, Fisher’s exact test), and time to hospitalization was significantly longer for the olanzapine group (Figure 2). For both groups, the majority of hospitalizations was for recurrence of mania.
Although a documented history of intolerance or lack of response to an adequate trial of olanzapine or lithium was an exclusion criterion, 206 such patients entered the study on lithium regimens. One hundred sixty-four were subsequently randomly assigned to double-blind treatment with lithium (N=80) or olanzapine (N=84). Symptomatic recurrence criteria were met by 46.3% (N=37) of those given lithium and 34.5% (N=29) of those given olanzapine (p=0.152). The impact of lithium use at study entry was examined further to assess the potential bias favoring olanzapine by comparing the differential rates of recurrence among those who were and were not taking lithium at entry. Among patients not taking lithium at study entry, there was a 7.2% recurrence rate advantage for olanzapine (27.1%) over lithium (34.3%). Among patients taking lithium at study entry, there was an 11.8% advantage for olanzapine (34.5%) over lithium (46.3%). The differential advantage was not significantly different (p=0.724, Breslow-Day test). Additionally, the noninferiority of olanzapine relative to lithium can be shown in each lithium-use-at-entry subgroup, since the two-sided 95% confidence intervals around the risk differences did not cover the predefined –7.3% margin of noninferiority.
Lithium Levels
Lithium levels were obtained for 211 of 214 patients, and 171 (81%) maintained lithium levels within the therapeutic range or were brought back into the range within the mandated 30-day timeframe. Of the 40 patients whose serum levels were not brought back into the range within the 30-day timeframe, 32 had low serum levels, and eight had high serum levels. Among patients with low serum levels, seven (21.9%) experienced a recurrence, whereas 76 (42.5%) of the 179 within the range or with high serum levels experienced a recurrence.
There was no difference in recurrence rates between lithium-treated patients with high (≥0.8 meq/liter) versus low (<0.8 meq/liter) lithium serum levels (39.0% [N=32 of 82] and 39.5% [N=51 of 129], respectively; p=1.00). Furthermore, in the 83 lithium-treated patients who experienced a recurrence, the mean lithium serum levels were slightly higher (mean=0.78 meq/liter, SD=0.11) than in the 128 lithium-treated patients who did not recur (mean=0.75 meq/liter, SD=0.16), but the difference was not statistically significant (t=1.7, df=209, p=0.09). Considering the 83 patients who met recurrence criteria, the mean lithium level before recurrence was 0.73 meq/liter (SD=0.30); however, for 15 of these (18.1%), the before-recurrence lithium level was <0.6 meq/liter. Excluding these 15 patients with low lithium level before recurrence provides an adjusted overall rate of recurrence of 34.2% (68 of 199) for the lithium therapy group compared with 30.0% for the olanzapine group (p=0.40). The two-sided 95% confidence interval on this observed 4.2% risk difference (–4.8% to 13.2%) is consistent with the noninferiority of olanzapine relative to lithium.
We further assessed whether lithium levels were a factor in study completion/disposition, using revised disposition categories in which recurrence superseded any other reported reason for discontinuation. The mean lithium serum level for recurring lithium patients was 0.78 meq/liter (SD=0.11) and was 0.79 meq/liter (SD=0.11) for patients who completed the study in sustained remission. The lowest mean lithium level was found among patients who discontinued due to not meeting protocol criteria or noncompliance (N=10, mean=0.57 meq/liter, SD=0.27). Among other reasonably sized (N>8) disposition groups, mean lithium levels were 0.72 meq/liter (SD=0.13) for those discontinuing due to patient decision (N=25), 0.85 meq/liter (SD=0.12) for those discontinuing due to an adverse event (N=11), and 0.75 meq/liter (SD=0.15) for those discontinuing due to lack of efficacy (N=9).
To determine whether variations in lithium levels contributed to differences in recurrence risk at various times during the study, we examined mean lithium serum levels for early recurrences (<150 days) and for late recurrences (>150 days). The mean serum lithium level for early recurrences (N=49) was 0.78 meq/liter (SD=0.11). For those patients who had a late recurrence, the mean serum level up to 150 days was 0.78 meq/liter (SD=0.11 [N=34]) and after 150 days, 0.76 meq/liter (SD=0.12 [N=32]).
Adverse Events
One patient committed suicide during the open-label phase of this study. During the double-blind period, two patients randomly assigned to lithium died. One of these patients committed suicide, the other died of accidental causes.
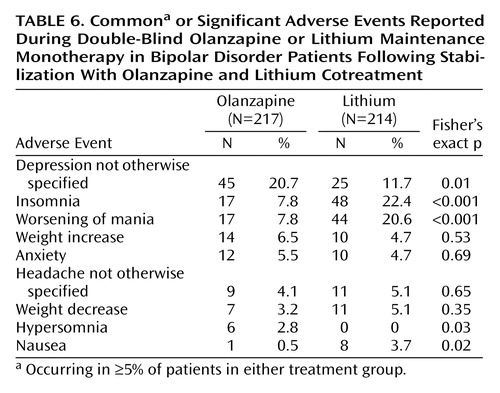
Thirty-four patients (6.3%) discontinued treatment during the open-label period due to an adverse event. Common (≥5%) treatment-emergent adverse events during this period were increased weight (10.3%), tremor (9.8%), sedation (7.2%), somnolence (6.8%), and insomnia (5%). During the double-blind period, adverse events led to the withdrawal of 41 patients in the olanzapine group (18.9%) and 55 patients in the lithium group (25.7%). Common or significant treatment-emergent adverse events occurring during double-blind monotherapy are reported in Table 6. We examined the occurrence of depression as a treatment-emergent adverse event in relation to depressive relapse among the olanzapine-treated patients. Of 45 olanzapine-treated patients with treatment-emergent depression, 23 met relapse criteria. Most (73.9%) of these 23 olanzapine-treated patients had Hamilton depression scale total scores ≥15 at the time of the reported emergence of depressive symptoms, and most (65.2%) met relapse criteria within 14 days of the event onset. Similarly, we examined the occurrence of insomnia as a treatment-emergent adverse event in relation to manic symptoms among the lithium-treated patients. Overall, 22/48 patients with insomnia met relapse criteria, but only two cases of relapse were within 1 week of insomnia emergence, and most (63%) had event onset >14 days before relapse. In addition, elevated mood (Young Mania Rating Scale item 1) was absent in 33 patients near the emergence of insomnia; across all 48 patients, the mean Young Mania Rating Scale item 1 score was 0.49 (SD=0.86). Sleep (Young Mania Rating Scale item 4), however, was reduced; 24 patients (51%) had scores of ≥2 (sleeping less than normal by more than 1 hour) near when treatment-emergent insomnia was reported as an adverse event.
Extrapyramidal Symptom Ratings
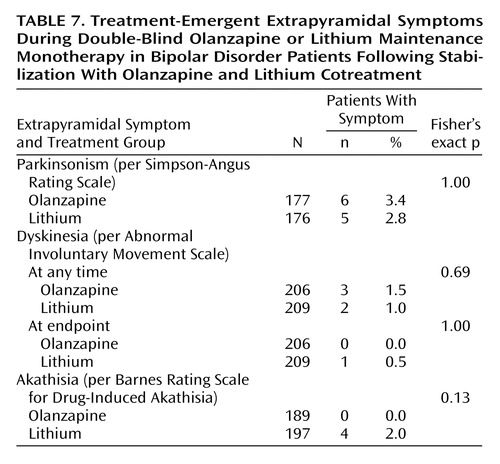
Extrapyramidal symptoms were monitored as patient-reported treatment-emergent events (data not shown), rating scale-defined treatment-emergent events (see Method section), and as mean change in scores on rating scales (data not shown). Irrespective of the means of assessment, changes in and incidences of extrapyramidal symptoms were small and did not differ statistically between treatment groups (Table 7).
Vital Signs, Weight, and Laboratory Measures
There were no statistically significant differences between treatments in the incidence rates of potentially clinically relevant changes in vital signs during double-blind therapy. Mean weight gain during the open-label period was 2.74 kg (SD=3.8), and 148 (27.8%) of 532 experienced ≥7% change from baseline. Mean change in weight during the double-blind period was significantly greater for the olanzapine group (mean=1.8 kg, SD=5.8) than in the lithium group (mean=–1.4 kg, SD=5.0) (F=21.2, df=1, 385, p<0.001). Significantly more olanzapine-treated patients had ≥7% increase in weight than lithium-treated patients (29.8% [N=64] versus 9.8% [N=21], respectively) (p<0.001, Fisher’s exact test).
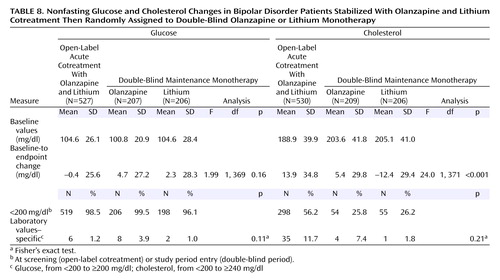
No statistically significant differences occurred between treatment groups in the rates of potentially clinically relevant changes in laboratory measures. More detailed analyses on nonfasting glucose and cholesterol outcomes are presented in Table 8. During double-blind treatment, the mean baseline-to-endpoint change in cholesterol was greater for patients treated with olanzapine compared with lithium-treated patients. However, no significant differences occurred in incidence rates of potentially clinically relevant increases in nonfasting glucose or cholesterol between treatment groups.
Discussion
This is the first double-blind, randomized, controlled study to investigate the potential of an atypical antipsychotic to prevent recurrence of bipolar disorder in comparison with any active treatment. Olanzapine and lithium did not statistically differ in preventing mood episode recurrence according to symptomatic rating scale criteria. However, olanzapine was significantly more effective than lithium in preventing recurrence of manic and mixed episodes. Olanzapine’s superiority to lithium in the prevention of mania recurrence is important because in a rigorous recently conducted meta-analysis (4), prevention of mania recurrence had been identified as a particular strength of lithium. Prevention of depression recurrence was similar between the treatments. Time until premature discontinuation for any reason occurred significantly earlier for lithium-treated patients; the estimated median time to discontinuation was approximately 100 days sooner than with olanzapine.
Lithium is the most extensively studied mood stabilizer; meta-analyses have indicated that it is superior to placebo in the prevention of relapse (4) and reduces the risk of relapse 3.6-fold (5). Among placebo-controlled trials that have addressed potential bias associated with rapid withdrawal of lithium, rates of relapse with lithium were 31% (Bowden et al.’s 12-month study [6]), 36% (12-month outcomes reported by Prien et al. [13]), and 40.9% (Bowden et al.’s 18-month study [7]). In these studies, lithium was superior to placebo in preventing bipolar disorder relapse among patients who had a manic index episode. Consistent with these reports, our results showing a recurrence rate of 38.8% after 48 weeks of lithium monotherapy further support the prophylactic efficacy of lithium in bipolar disorder.
Three design measures were taken to minimize bias for either treatment. First, patients with a history of nonresponse, or lack of tolerance, to lithium or olanzapine were excluded from the study. Even though a number of patients were taking lithium at study entry, which may have given a potential selection bias advantage in favor of olanzapine in preventing recurrence, the putative advantage difference was not significantly different (p=0.724). Second, the study design included a 4-week taper period as a means of preventing recurrences associated with the abrupt withdrawal of lithium (14). Third, the enriched design of treating patients during their index episode with a combined regimen of lithium and olanzapine ensured that randomly assigned patients were not preselected to respond preferentially to one treatment. Also note that small proportions of mixed-episode bipolar patients or patients with a history of a rapid-cycling course were enrolled in this study, which may reflect the exclusion of patient subtypes who may respond poorly to lithium (15). Last, serum levels of lithium were comparable between those individuals who did and did not have a recurrence; lithium levels also did not appear to decrease at 150 days, a time when recurrence with lithium appeared to accelerate.
Both olanzapine and lithium were generally well tolerated. Few extrapyramidal symptom events, whether measured subjectively or objectively, occurred during this 52-week study. Weight gain was significantly greater in the olanzapine group. The pattern of weight gain was comparable with previous observations (11, 16); most of the weight gain occurred early (open-label period), followed by an additional 1.8 kg gained during the maintenance period.
No significant differences occurred between the groups in mean baseline-to-endpoint changes in nonfasting glucose or incidences of nonfasting glucose levels ≥200 mg/dl. It is noteworthy, however, that this study may not have had sufficient power to determine treatment differences in these adverse events. It is also noteworthy that 43.8% of patients had a nonfasting cholesterol level at screening that exceeded 200 mg/dl. Measurements of the effects of atypical antipsychotics on glucose and cholesterol are important both because of apparent increased rates of diabetes among patients with bipolar disorder and case reports of diabetes among patients treated with atypical antipsychotic agents.
Several limitations in this study warrant discussion. There was no placebo arm in this trial, but both lithium (17) and olanzapine (18) have demonstrated superior relapse prevention relative to placebo. Assessment of previous response to lithium or olanzapine was collected retrospectively and, as such, may have questionable reliability. Retrospective collection of information is a limitation of all studies that attempt to set exclusion criteria to prevent bias associated with known lack of response to a comparator. This topic is an issue of all contemporary active-controlled clinical trials and deserves more attention by clinical trial methodologists.
In recent meta-analyses examining the effectiveness of lithium for relapse prevention, lithium levels for six trials ranged between 0.5 and 1.4 meq/liter (17). Included in these meta-analyses were two 1973 studies of Prien et al. (13), which reported median lithium levels of 0.7 and 0.8 meq/liter; the 1971 study of Coppen et al. (19), which reported a mean lithium level of 0.93 meq/liter; the 2000 study of Bowden et al. (20), which compared valproate, lithium, and placebo and reported a mean level of lithium of 1.0 meq/liter at day 30; and the 2003 study of Calabrese et al. (21), which compared lamotrigine, placebo, and lithium in patients with an index episode of depression and reported a mean lithium level of 0.8 meq/liter. With the exception of the Bowden et al. study (in which lithium did not separate from placebo), the mean (0.76 meq/liter) and median (0.8 meq/liter) lithium levels reported in this trial are consistent with those reported in the literature. However, it is possible that some patients may not have been maintained on optimum therapeutic levels, which may represent a limitation of the study.
The generalizability of this study is limited to 52 weeks and mainly to individuals with a recent manic episode, since there were few patients with a mixed index episode or a history of rapid cycling, and entry criteria excluded patients with an index episode of depression. In addition, the results can only be generalized to patients stabilized with a combined regimen of olanzapine and lithium and may not be applicable to those stabilized with other mood stabilizers or combinations of mood stabilizers. The interpretation of recurrence on the basis of DSM-IV syndromic criteria may be limited because the definition of recurrence did not include a duration criterion.
It is noteworthy that this study may not have had sufficient power to determine treatment differences in rare adverse events and that assessment of the potential impact of treatment on glucose homeostasis is limited in this study because glucose and lipid measurements were nonfasting. Another limitation is that more than half of the patients withdrew prematurely from the study. High discontinuation rates in maintenance studies are common. Bowden et al. (6) reported dropout rates of 62% and 76% among patients treated with divalproex and lithium for 52 weeks, and dropout rates of 95%, 98%, and 100% were reported for patients treated with lamotrigine, lithium, and placebo for 18 months (7). Finally, the time in remission before random assignment was relatively short; however, when early remitters were excluded from the analyses, risks of recurrence were similar to the previous values.
The results of this trial are consistent with previous studies demonstrating the efficacy of lithium in relapse prevention in bipolar disorder and suggest that olanzapine may also be effective in the prevention of relapse/recurrence in this disorder. Additional independent studies are needed to confirm these results.
Acknowledgments
The following countries and individuals participated in the HGHT clinical trial: Australia: Prof. P. Morris, Dr. R. Newton, Dr. D. Tannenbaum; Austria: Prof. S. Kasper, Dr. M. Schmitz, Prof. C. Simhandl; Belgium: Dr. G. De Bruecker, Dr. H. Bryon, Dr. B. Gillain; Bulgaria: Dr. S. Haralanov, Prof. V. Milanova, Dr. O. Tanchev, Prof. S. Todorov; Canada: Dr. P. Carr, Dr. L. N. Yatham; Croatia: Dr. V. Jukic, Dr. N. Mandic, Prof. L. Moro; Czech Republic: Dr. E. Bockova, Dr. J. Boucek, Dr. V. Hanuskova, Dr. M. Marsalek, Dr. M. Poricky, Dr. D. Seifertova; Denmark: Dr. N. R. Hansen; Finland: Dr. H. J. Koponen, Dr. I. Larmo, Dr. K. Lehtinen, Dr. V. Nevalainen, Dr. R. Riihikangas; Germany: Dr. A. Berghöfer, A/Prof. P. Bräunig, Prof. E. Haen, Prof. F. A. Henn, Prof. W. Maier, Prof. A. Marneros, Prof. H. J. Moeller, Prof. M. Schmauß; Hungary: Dr. L. Haraszti, Dr. L. Mod, Dr. G. Ostorharics-Horvath, Prof. Z. Rihmer, Dr. G. Vincze; Ireland: Dr. V. O’Keane, Dr. D. Walshe; Israel: Prof. H. P. Belmaker; Italy: Prof. C. Bellantuono, Prof. F. Bogetto, Prof. G. B. Cassano, Prof. A. Koukopoulos, Prof. C. Maggini, Prof. G. Minnai, Prof. G.M. Muscettola; Lithuania: Dr. B. Burba, Dr. V. Maciulis, Dr. L. E. Radavicius; the Netherlands: Prof. H. F. Kraan, Dr. J. J. Van Egmond; New Zealand: Prof. T. Silverstone; Norway: Dr. O. Augland, Dr. B. Stubhaug; Poland: Dr. W. Chrzanowski, Dr. A. Czernikiewicz, Dr. J. Janczewski, Dr. J. Laczkowski, Dr. J.K. Rybakowski; Romania: Prof. P. Boisteanu, Dr. I. A. Grigoriu, Dr. A. S. Ionescu; Russia: Prof. L.M. Bardenstein, Prof. N. G. Neznanov, Prof. G. P. Panteleyeva, Prof. A. B. Smulevich; Slovak Republic: Dr. E. Palova, Dr. L. Vavrusova, Dr. L. Vircik; Slovenia: A/Prof. S. Ziherl; South Africa: Dr. C. Grobler; Sweden: Prof. R. Adolfsson, Dr. G. Arnell, Dr. L. Haggstrom, Dr. C. Rolleri, Dr. P. Skeppar; Switzerland: Dr. B. Blajev; Turkey: Prof. T. Oral; United Kingdom: Dr. C. M. Bonthala, Dr. A. Gregoire, Dr. D. Patience, Dr. S. Vethanayagam.
 |
 |
 |
 |
 |
 |
 |
 |
Presented in part at the Stanley Foundation Conference on Bipolar Disorder, Freiburg, Germany, Sept. 12–14, 2002; the 41st annual meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, Dec. 8–12, 2002; the 156th annual meeting of the American Psychiatric Association, San Francisco, May 17–22, 2003; and the fifth International Conference for Bipolar Disorders, Pittsburgh, June 12–14, 2003. Received Oct. 30, 2003; revisions received June 17 and Aug. 2, 2004; accepted Aug. 11, 2004. From Lilly Research Laboratories; the Harvard Medical School Department of Psychiatry, McLean Hospital, Belmont, Mass.; the University of Munich Department of Psychiatry, Munich; the Department of Psychiatry, Case Western Reserve University, Cleveland; the Harvard Medical School Department of Psychiatry, Massachusetts General Hospital, Boston; the Department of Psychiatry, University of British Columbia, Vancouver; Former Research Group Clinical Psychopharmacology, Freie Universität Berlin, Germany; Centro Lucio Bini, Rome, Italy; the Department of Psychiatry, University of Pisa, Italy; Mood Disorders Research Unit, Aarhus University Hospital, Aarhus, Denmark; Lilly Area Medical Center, Vienna, Austria; and the Department of Psychiatry, University of Texas Health Sciences Center, San Antonio. Address correspondence and reprint requests to Dr. Tohen, Lilly Research Laboratories, Indianapolis, IN 46285; [email protected] (e-mail). This study was sponsored by Lilly Research Laboratories.
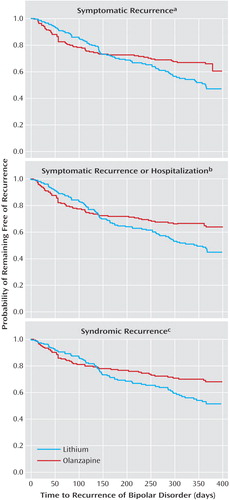
Figure 1. Time Until Mood Episode Recurrence Among Bipolar Disorder Patients Randomly Assigned to Double-Blind Olanzapine or Lithium Monotherapy Following Stabilization With Olanzapine and Lithium Cotreatment
aRecurrence defined as score ≥15 on the Young Mania Rating Scale and/or Hamilton depression scale. Time until recurrence longer for the olanzapine group than for the lithium group, but the difference was not significant (χ2=3.4, df=1, p=0.07, log-rank test).
bRecurrence defined as score ≥15 on the Young Mania Rating Scale and/or Hamilton depression scale or hospitalization for a mood episode. Time until recurrence significantly longer for the olanzapine group than for the lithium group (χ2=4.6, df=1, p=0.03, log-rank test).
cRecurrence defined as meeting DSM-IV criteria (other than the duration criterion) for current manic, mixed, or depressive episode. Time until recurrence significantly longer for the olanzapine group than for the lithium group (χ2=4.0, df=1, p=0.04, log-rank test).
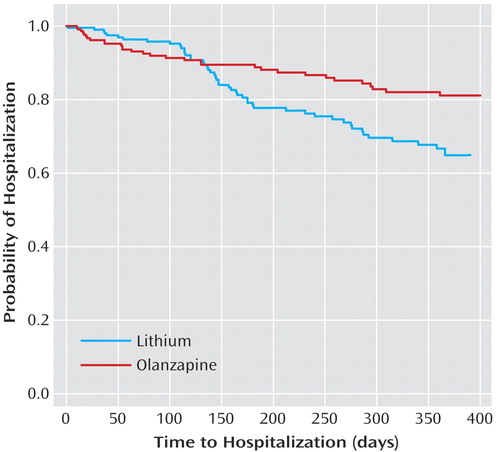
Figure 2. Time Until Hospitalization Among Bipolar Disorder Patients Randomly Assigned to Double-Blind Olanzapine or Lithium Monotherapy Following Stabilization With Olanzapine and Lithium Cotreatmenta
aTime until hospitalization significantly longer for the olanzapine group than for the lithium group (χ2=6.2, df=1, p=0.01, log-rank test).
1. Coryell W, Keller M, Endicott J, Andreasen N, Clayton P, Hirschfeld R: Bipolar II illness: course and outcome over a five-year period. Psychol Med 1989; 19:129–141Crossref, Medline, Google Scholar
2. Tohen M, Waternaux CM, Tsuang MT: Outcome in mania: a 4-year prospective follow-up of 75 patients utilizing survival analysis. Arch Gen Psychiatry 1990; 47:1106–1111Crossref, Medline, Google Scholar
3. Tohen M, Zarate CA Jr, Hennen J, Khalsa H-MK, Strakowski SM, Gebre-Medhin P, Salvatore P, Baldessarini RJ: The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry 2003; 160:2099–2107Link, Google Scholar
4. Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM: Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry 2004; 161:217–222; correction, 161:1517Link, Google Scholar
5. Baldessarini RJ, Tondo L: Does lithium treatment still work? evidence of stable responses over three decades. Arch Gen Psychiatry 2000; 57:187–190Crossref, Medline, Google Scholar
6. Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, Pope HG Jr, Chou JC, Keck PE Jr, Rhodes LJ, Swann AC, Hirschfeld RM, Wozniak PJ (Divalproex Maintenance Study Group): A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Arch Gen Psychiatry 2003; 57:481–489Crossref, Google Scholar
7. Bowden CL, Calabrese JR, Sachs G, Yatham LN, Asghar SA, Hompland M, Montgomery P, Earl N, Smoot TM, DeVeaugh-Geiss J: A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry 2003; 60:392–400Crossref, Medline, Google Scholar
8. Hartong EG, Moleman P, Hoogduin CA, Broekman TG, Nolen WA (LitCar Group): Prophylactic efficacy of lithium versus carbamazepine in treatment-naive bipolar patients. J Clin Psychiatry 2003; 64:144–151Crossref, Medline, Google Scholar
9. Tohen M, Sanger TM, McElroy SL, Tollefson GD, Chengappa KNR, Daniel DG, Petty F, Centorrino F, Wang R, Grundy SL, Greaney MG, Jacobs TG, David SR, Toma V (Olanzapine HGEH Study Group): Olanzapine versus placebo in the treatment of acute mania. Am J Psychiatry 1999; 156:702–709Abstract, Google Scholar
10. Tohen M, Jacobs TG, Grundy SL, McElroy SL, Banov MC, Janicak PG, Sanger T, Risser R, Zhang F, Toma V, Francis J, Tollefson GD, Breier A: Efficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled study. The Olanzapine HGGW Study Group. Arch Gen Psychiatry 2000; 57:841–849; correction, 2002; 59:91Google Scholar
11. Tohen M, Ketter TA, Zarate CA, Suppes T, Frye M, Altshuler L, Zajecka J, Schuh LM, Risser RC, Brown E, Baker RW: Olanzapine versus divalproex sodium for the treatment of acute mania and maintenance of remission: a 47-week study. Am J Psychiatry 2003; 160:1263–1271Link, Google Scholar
12. Tohen M, Baker RW, Altshuler LL, Zarate CA, Suppes T, Ketter TA, Milton DR, Risser R, Gilmore JA, Breier A, Tollefson GA: Olanzapine versus divalproex in the treatment of acute mania. Am J Psychiatry 2002; 159:1011–1017Link, Google Scholar
13. Prien RF, Caffey EM Jr, Klett CJ: Prophylactic efficacy of lithium carbonate in manic-depressive illness: report of the Veterans Administration and National Institute of Mental Health Collaborative Study Group. Arch Gen Psychiatry 1973; 28:337–341Crossref, Medline, Google Scholar
14. Goodwin GM: Recurrence of mania after lithium withdrawal: implications for the use of lithium in the treatment of bipolar affective disorder. Br J Psychiatry 1994; 164:149–152Crossref, Medline, Google Scholar
15. Baldessarini RJ, Tondo L, Floris G, Hennen J: Effects of rapid cycling on response to lithium maintenance treatment in 360 bipolar I and II disorder patients. J Affect Disord 2000; 61:13–22Crossref, Medline, Google Scholar
16. Kinon BJ, Basson BR, Gilmore JA, Tollefson GD: Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. J Clin Psychiatry 2001; 62:92–100Crossref, Medline, Google Scholar
17. Burgess S, Geddes J, Hawton K, Townsend E, Jamison K, Goodwin G: Lithium for Maintenance Treatment of Mood Disorders (Cochrane Review). Oxford, UK, The Cochrane Library, 2002Google Scholar
18. Tohen MF, Bowden C, Calabrese J, Chou JC, Jacobs T, Baker RW, Williamson D, Evans AR: Olanzapine’s efficacy for relapse prevention in bipolar disorder: a randomized, double-blind, placebo-controlled clinical trial. Eur Neuropsychopharmacol 2003; 13(suppl 4):S212Google Scholar
19. Coppen A, Noguera R, Bailey J, Burns BH, Swani MS, Hare EH, Gardner R, Maggs R: Prophylactic lithium in affective disorders: controlled trial. Lancet 1971; 2:275–279Crossref, Medline, Google Scholar
20. Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, Pope HG Jr, Chou JC, Keck PE Jr, Rhodes LJ, Swann AC, Hirschfeld RM, Wozniak PJ (Divalproex Maintenance Study Group): A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Arch Gen Psychiatry 2000; 57:481–489Crossref, Medline, Google Scholar
21. Calabrese JR, Bowden CL, Sachs G, Yatham LN, Behnke K, Mehtonen OP, Montgomery P, Ascher J, Paska W, Earl N, DeVeaugh-Geiss J (Lamictal 605 Study Group): A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry 2003; 64:1013–1024Crossref, Medline, Google Scholar



