Longitudinal Changes in Structural Connectivity in Young People at High Genetic Risk for Bipolar Disorder
Abstract
Objective:
Recent studies of patients with bipolar disorder or at high genetic risk reveal structural dysconnections among key brain networks supporting cognitive and affective processes. Understanding the longitudinal trajectories of these networks across the peak age range of bipolar disorder onset could inform mechanisms of illness onset or resilience.
Methods:
Longitudinal diffusion-weighted MRI and phenotypic data were acquired at baseline and after 2 years in 183 individuals ages 12–30 years in two cohorts: 97 unaffected individuals with a first-degree relative with bipolar disorder (the high-risk group) and 86 individuals with no family history of mental illness (the control group). Whole-brain structural networks were derived using tractography, and longitudinal changes in these networks were studied using network-based statistics and mixed linear models.
Results:
Both groups showed widespread longitudinal changes, comprising both increases and decreases in structural connectivity, consistent with a shared neurodevelopmental process. On top of these shared changes, high-risk participants showed weakening of connectivity in a network encompassing the left inferior and middle frontal areas, left striatal and thalamic structures, the left fusiform, and right parietal and occipital regions. Connections among these regions strengthened in the control group, whereas they weakened in the high-risk group, shifting toward a cohort with established bipolar disorder. There was marginal evidence for even greater network weakening in those who had their first manic or hypomanic episode before follow-up.
Conclusions:
Neurodevelopment from adolescence into early adulthood is associated with a substantial reorganization of structural brain networks. Differences in these maturational processes occur in a multisystem network in individuals at high genetic risk of bipolar disorder. This may represent a novel candidate to understand resilience and predict conversion to bipolar disorder.
Bipolar disorder is a serious and relatively common psychiatric disorder with a strong familial component, characterized by episodic disturbances in mood and cognition (1, 2). Research on the neurobiology of bipolar disorder has increasingly incorporated the study of brain networks, or connectomics (3–5). Recent structural and functional imaging studies of bipolar disorder have revealed connectivity disturbances centered on key emotional and cognitive hubs such as the insula and inferior frontal gyrus (6–10). Such dysconnections produce a loss of integration between emotional circuitry and cognitive control networks, mirroring the hallmark affective and neurocognitive disturbances of the disorder (5).
Interpretation of neurobiological differences in bipolar disorder is complex, as these differences may reflect biological risk factors, the consequences of the illness, or the impact of its pharmacological treatment (11). Unaffected first-degree relatives of patients with bipolar disorder have an odds ratio of ∼7–14 of developing bipolar disorder (12). Neuroimaging studies comprising young, unaffected bipolar disorder relatives are thus valuable, as they include high-risk individuals who will later convert to bipolar disorder but have not yet been exposed to the biological impacts of acute illness or psychotropic medication (13–15). Studies assessing white matter volume in individuals at high risk compared with control subjects have reported differences in the frontal gyrus (16), internal capsule (17), and corpus callosum (18), mirroring gray matter changes such as cortical thinning (19). Longitudinal studies show that neurobiological differences in those at high genetic risk evolve dynamically. For example, high-risk study participants show accelerated cortical thinning and volume reduction in the right frontal cortex, including the inferior frontal gyrus, lateral orbitofrontal cortex, frontal pole, and rostral middle frontal gyrus (19).
Recent studies of high-risk cohorts have also employed connectomics, using diffusion-weighted MRI sequences to study the connectivity and integrity of white matter. Studies using local diffusion-derived indices (such as fractional anisotropy) have reported widespread changes (20–22) as well as localized disturbances in specific white matter tracts such as the uncinate fasciculus, corticospinal tract, cingulum, and corpus callosum (23–27). Whole brain tractography allows reconstruction of structural brain networks, a more direct study of connectomics in high-risk cohorts (6). A recent study of young high-risk individuals found weaker structural connectivity in networks centered on the inferior frontal gyrus and insular cortex and stronger connectivity in a limbic network (10). Notably, brain network differences in high-risk cohorts were distinct from those in established bipolar disorder, although both included regions involved in emotional processing and affect regulation, such as the insula, hippocampus, amygdala, and cingulate cortices.
The age range of bipolar disorder onset overlaps with the final stages of brain development and the accompanying reorganization of structural brain networks (28). This reorganization happens in phases, shifting from sensorimotor loops in late childhood toward heteromodal and association cortex after adolescence (29). These changes plateau in early adulthood, hence showing a nonlinear, age-dependent profile (30), with behavioral correlates shifting from the acquisition of new skills toward the sophistication of behaviors and the maturation of cognitive control (31, 32). Conceptual models of neuropsychiatric illnesses such as bipolar disorder suggest that they be framed as disturbances in this process, that is, as “neurodevelopmental miswiring” (33).
Longitudinal studies are needed to disambiguate stable cross-sectional group differences from those that emerge in individuals during neurodevelopment or following illness onset. Only a small number of studies have investigated white matter changes in those at high risk of developing bipolar disorder. The Scottish Bipolar Family Study reported reduced fractional anisotropy across widespread brain regions over a 2-year period in individuals at risk of developing bipolar disorder who remained well, in those who developed major depressive disorder, and in control subjects. However, trajectories did not differ between groups (22). Our group recently reported a significant increase in the prevalence of periventricular hyperintensities in high-risk young adults and control subjects over a 2-year period (34). Again, no group differences in trajectories were evident.
Using whole-brain structural networks inferred from diffusion-weighted MRI, we studied connectomic changes over a 2-year interval, comparing young first-degree relatives of patients with bipolar disorder who did not have the disorder at baseline (the high-risk group) with control subjects from families without mental illness. Our cohort thus comprises adolescents and young adults who straddle the peak age of illness onset and who are themselves at high risk of bipolar disorder. We first report the linear and nonlinear reorganization of brain connectivity that occurs across the entire study cohort. We then report group differences in this reorganization, hypothesizing that these differences would be more pronounced in those high-risk individuals who experienced their first mood episode (a major depressive episode or a manic or hypomanic episode) between baseline and follow-up. We benchmark these changes against a group with established bipolar disorder, age-matched to our follow-up cohort. These analyses may help refine our understanding of neurodevelopmental processes in young people at high genetic risk of bipolar disorder and identify key protective factors and vulnerabilities.
Methods
Participants and Study Design
Participants comprised two groups of individuals 12–30 years of age who had baseline and follow-up scans: high-risk participants (N=97) who were first-degree relatives of a proband with a confirmed DSM-IV diagnosis of bipolar I or II disorder but who did not themselves have bipolar disorder at baseline, and control subjects (N=86) from families with no history of mental illness. In the high-risk group, 80% had parent probands and 20% had a sibling proband. Two control families and 17 high-risk families had more than one sibling in the sample (see the Supplementary Methods section in the online supplement for further details on recruitment, clinical assessments, exclusions, and characterization).
The study was conducted with approval by the University of New South Wales Human Research Ethics Committee (HREC Protocol 09/097) in Sydney, Australia. Written informed consent was obtained from all participants, with additional parental consent for participants age 16 or younger.
MRI Acquisition and Tractography
Diffusion MRI data were acquired using a 3-T Philips Achieva X MRI scanner. One acquisition of 32 directional diffusion-weighted images was performed (b=1,000 s/mm2, TR=7,767 ms, TE=68 ms), with the resulting images reconstructed to yield 1 mm×1 mm×2.5 mm voxels (see the online supplement for further details on acquisition and analysis). The preprocessing pipeline used to construct whole-brain structural networks from these data was similar to those used in our baseline study and elsewhere (10, 35, 36). In brief, the diffusion-weighted MRI sequence data were first preprocessed using MRtrix3 (https://github.com/MRtrix3/mrtrix3/releases/tag/3.0_RC3) plus FSL (version 5.0.11) and included denoising, eddy current and motion correction, and bias field correction (https://github.com/breakspear/diffusion-pipeline/tree/bipolarlongitudinal). The signal responses from multiple tissue types (white matter, cerebrospinal fluid) were estimated (37). Constrained spherical deconvolution (CSD) (37) and probabilistic tractography (iFOD2) (38) were then used to generate 5 million whole-brain streamlines representing structural connections between brain regions.
The standard anatomical automated labeling (AAL) template (39) was subdivided into 512 cortical and subcortical parcellation regions of approximately uniform size (40). Subject-specific parcellations were obtained through coregistration of each subject’s fractional anisotropy image to the FMRIB FA template and then combined with the individual’s whole brain tractography to generate weighted structural networks. Each weighted network edge corresponds to the total number of streamlines that intersect pairs of regions, adjusted by the distance between those regions (41).
To reduce false positive connections, the resulting connectivity matrices were thresholded using a group consistency approach (42). This ensures that all subjects have the same edges, differing only in their weight and thus avoiding a composite of weights and zeros across subjects in individual edges. Consistency-based thresholding also preserves the distant effect on edge strength that is evident when using direct histological methods (43). Following our previous cross-sectional study of this cohort (10), structural networks were thresholded with a connection density of 10% (see the online supplement for details on network thresholding).
Network-Based Statistics
For statistical inference, we used a general linear model in conjunction with network-based statistics (NBS) (44), a permutation-based method to control family-wise error (FWE) over the large number of edges in our whole brain structural networks.
To study longitudinal changes in connectivity across the entire cohort, two-sample one-tailed t tests were conducted for the main effect of time (baseline to follow-up). To test for group differences in these changes, one-tailed interaction tests between group and time were then calculated. One-tailed tests were performed to disambiguate connections that increase versus decrease with time or group. For each comparison, a base model was run that did not include age as a covariate. We also ran separate models that included age at time of scan as a covariate. As substantial longitudinal changes in connectivity were expected across the entire cohort (for both increases and decreases in this model), a conservative height threshold of t=3.5 was implemented for this model (corresponding to an uncorrected p<0.0004), while for the main interaction model a height threshold of t=3.0 was used, the default setting within NBS (corresponding to an uncorrected p<0.002). Topological inference (using network spatial extent) was then performed using permutation testing to control FWE at p<0.05. All models were run using 5,000 permutations.
Linear mixed-effects models were run to investigate potential confounding from current psychotropic medications, current mood episode (a binary yes/no), and current mood state (continuous Children’s Depression Inventory [CDI] [45], Montgomery-Åsberg Depression Rating Scale [MADRS] [46], and Young Mania Rating Scale [YMRS] [47] scores) on networks identified by NBS. Linear mixed-effects models were run with the mean network weight as the dependent variable and group (control, high-risk), time, or their interaction as fixed effects. Family relatedness was included as a random effect to accommodate within-family correlations arising from the inclusion of siblings from within the same family where they occurred. Separate models were run for each potential confounder.
The age range of our cohort extends across the final stages of cognitive and neurodevelopment, and hence to a plateauing of strengthening and pruning of developmentally active networks. To identify nonlinear age effects, quadratic polynomials were fitted to the strength of all weighted edges as a function of age, minimizing the sum of squares error. Edges showing a statistically significant positive or negative curvature were identified after a conservative Bonferroni correction across all edges in each group and time point (FWE-corrected p<0.05).
Structural Network Characterization and Control
Bipolar disorder can be conceptualized as a dysregulation of affect and cognitive control arising in emotional and executive circuitry (48, 49). Brain networks are formally “controllable,” in the sense that the activity states that they support can be manipulated, given appropriate inputs, from one stable state to another (see Figure 3A) (50, 51). Network controllability can be operationalized using linear control theory, yielding quantitative estimates of the amount of energy required to shift a stable complex system between different activity states (51, 52) and, conversely, the vulnerability of an unstable network to vacillation in the presence of intrinsic fluctuations. We calculated brain network controllability, following established algorithms (see the online supplement), focusing on the brain regions in subnetworks that showed longitudinal group differences. Network calculations and parcellation data are publicly available (https://github.com/AlistairPerry/CNHRLongitudinal). Linear mixed models were used with current psychotropic medications, current mood episode, and current mood state as nuisance covariates.
Clinical Conversion and Subgroup Analyses
We performed exploratory subgroup analyses to investigate the influence of a new psychiatric disorder on the high-risk contribution to any between-group effects. For these analyses, the high-risk group was divided according to the following criteria: 1) new onset of any mood episode (major depressive or manic/hypomanic episode); 2) new onset of a manic/hypomanic episode; or 3) new onset of any DSM-IV disorder from baseline to follow-up. To benchmark new against prior episodes, we additionally divided the high-risk cohort into 4) those with or without a lifetime mood episode at baseline. For these small-N subgroups, we used Bayesian repeated-measures analysis of variance (https://jasp-stats.org, 1 million samples) to determine the relative evidence in favor of an effect in subgroups (Bayes factor, BF).
Results
Demographic and Clinical Data
The high-risk and control groups did not significantly differ in age, IQ, sex distribution, or time between scans (Table 1; see also Figure S1 in the online supplement). Lifetime occurrences of at least one major depressive episode, lifetime DSM-IV diagnosis, or anxiety disorders were significantly higher in the high-risk than the control group, consistent with previous reports in high-risk populations (53, 54). Eighteen of the high-risk participants experienced a new onset of any DSM-IV disorder, and eight experienced a first mood episode from baseline to follow-up (see the online supplement). Of these, four had a first onset of a DSM-IV manic or hypomanic episode, three had new onset of a major depressive episode, and one had both. Hence, there were five converters to bipolar disorder.
| Characteristic | Control Group (N=86) | High-Risk Group (N=97) | Statistic | p | Pairwise Comparison | ||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Female | 46 | 53 | 57 | 59 | χ2=0.52 | 0.47 | |
| Mean | SD | Mean | SD | ||||
| Age (years) | 22.41 | 4.04 | 21.12 | 5.24 | t=1.88 | 0.06 | |
| Interscan interval (years) | 2.04 | 0.14 | 2.07 | 0.13 | t=1.73 | 0.09 | |
| IQ | 117.40 | 10.19 | 115.42 | 10.98 | t=1.25 | 0.21 | |
| N | % | N | % | ||||
| Lifetime DSM-IV diagnosis at baseline | |||||||
| Any diagnosis | 27 | 31 | 55 | 57 | χ2=11.08 | <0.001 | High risk > control |
| Major depressive disorder | 10 | 10 | 28 | 29 | χ2=9.57 | 0.002 | High risk > control |
| Any anxiety disorder | 10 | 12 | 24 | 25 | χ2=6.29 | 0.02 | High risk > control |
| Any behavioral disorder | 8 | 8.3 | |||||
| Any substance use disorder | 7 | 8.1 | 12 | 12 | χ2=0.88 | 0.35 | |
| Any other disordera | 3 | 3.5 | 11 | 11 | 0.054 | ||
| First onset of a new DSM-IV diagnosis between baseline and follow-up | |||||||
| Any diagnosis | 11 | 13 | 18 | 18 | χ2=1.14 | 0.29 | |
| Major depressive disordera | 7 | 8.1 | 4 | 4.1 | 0.35 | ||
| Bipolar I or II disorder | 5 | 5.2 | |||||
| Any affective disorder | 7 | 8.1 | 8 | 8.2 | χ2=0.001 | 0.98 | |
| Any anxiety disordera | 2 | 2.3 | 6 | 6.2 | 0.29 | ||
| Any behavioral disordera | 1 | 1.2 | 2 | 2.1 | 1.00 | ||
| Any substance use disordera | 3 | 3.5 | 4 | 4.1 | 1.00 | ||
| Any other disordera | 1 | 1.2 | 2 | 2.1 | 1.00 | ||
TABLE 1. Baseline and follow-up demographic and clinical data
Longitudinal Effects
The two-sample t tests for the main effect of time revealed a substantial reorganization of structural connectivity across both groups. With the default height threshold of t=3.0, some 850 edges (6.5% of total) were significantly stronger at follow-up than at baseline, and 1,145 (8.8% of total) were significantly weaker (FWE-corrected p<0.001; see Figure S3 in the online supplement). Large networks of significantly changing edges also survived more conservative search (height) thresholds (t=3.5) (Figure 1), eventually breaking into distinct networks at thresholds greater than t=4.0, although they continued to encompass distributed cortical systems. These effects occurred in almost all participants in both groups (Figure 1B). Longitudinally changing edges encompassed all major cortical systems and included substantial intra- and interhemispheric effects. The edges that weakened longitudinally (T1 > T2) were predominantly located over posterior and central regions, particularly the somatomotor cortex. Network edges that strengthened longitudinally (T2 > T1) were located more rostrally, with greater involvement of the cognitive control system. These networks showed a strong effect of sex, with females “leading” males in both networks—lesser weights at both time points in the network that decreased with age (p<0.005) and greater weights in the network that increased with age (p<0.003), consistent with the well-known tendency of females’ brains to mature more quickly than males’ (55, 56). There were no significant interaction effects (of time and sex, p>0.3), suggesting that this age-sex gap was relatively stable across the 2 years between baseline and follow-up.
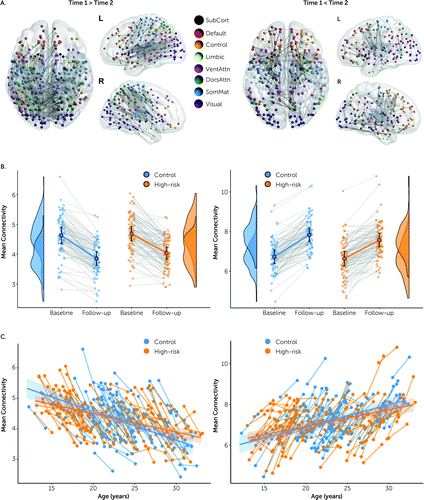
FIGURE 1. Longitudinal changes in structural connectivity across both high-risk and control groupsa
a In panel A, the distribution of edges shows a significant decrease (T1 > T2, left set of images) and increase (T2 > T1, right set of images) in strength. Colors show coding of nodes according to functional affiliation (72). SubCort=subcortical; Default=default mode network; VentAttn=ventral attention network; DorsAttn=dorsal attention network; SomMat=somatomotor network. Panel B shows the corresponding mean connectivity strength of this network in all individuals at both time points and the corresponding group distributions. Circles and error bars show the mean and 95% confidence interval. These networks derive from a relativity conservative height threshold of t=3.5. Networks with the network-based statistics default threshold of t=3.0 are provided in Figure S3 in the online supplement. Raincloud plots were generated using R software packages (73, 74). Panel C shows network connectivity strength in all individuals as a function of their age at baseline and follow-up, with corresponding group regression slopes and confidence intervals. Because of the collinearity with age, edges selected for a time effect also show an average age effect (both at and between T1 and T2) but are enriched across participants for the individual time effect. Hence, the individual time effects are, on average, steeper than the group-wise regressions.
Although the high-risk and control groups were age-matched, they covered a broad, developmentally active age range (12–30 years). This contrast aggregates temporal changes that encompass this entire age range and that are shared between both groups (Figure 1C). To identify edges that changed longitudinally regardless of age, we undertook a supplementary analysis after controlling for age at time of scan. This “age-invariant” longitudinal contrast revealed a smaller, discrete network of edges, connecting bilateral cortical midline structures including the bilateral middle and anterior cingulum, precuneus, and caudate (FWE-corrected p<0.024; see Figure S4 and Table S2 in the online supplement). This network captures the effect of an extra 2 years of neurodevelopment present in both groups by follow-up across the entire age range of our cohorts. The smaller and weaker nature of this age-regressed contrast thus suggests that longitudinal network changes shift dynamically across the brain at different stages of neurodevelopment.
Group Differences in Longitudinal Network Changes
Alongside these shared longitudinal changes, a discrete network of edges showed a significant group-by-time effect (t=3.0, FWE-corrected p=0.007) (Figure 2A). This network connects predominantly left-side inferior and lateral structures (left insula, inferior frontal gyrus, thalamus, caudate) via the left fusiform cortex to posterior and midline right-side regions (right superior and inferior occipital cortex, cuneus, precuneus, and thalamus; see Table S3 in the online supplement). Extracting the edge weights from the network comprising this interaction effect showed that this network increased in strength in the control group but decreased in the high-risk group (Figure 2B). This group-by-time effect shows an associated developmental increase in network strength with age in the control group but not the high-risk group (t=3.4, p=0.0007) (Figure 2C). Note that the group regressions cross at the age midpoint. This indicates that the T1–T2 gradient is balanced in magnitude across the younger and older participants in both groups.
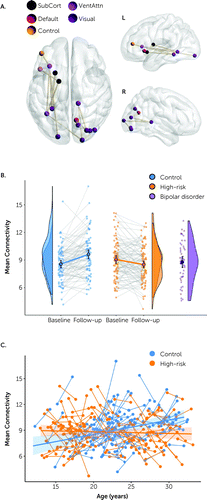
FIGURE 2. Group differences in longitudinal changes in structural connectivitya
aIn panel A, the anatomical distribution of edges shows a significant group-by-time effect across control and high-risk individuals. The color legend shows coding of nodes according to functional affiliation. SubCort=subcortical; Default=default mode network; VentAttn=ventral attention network. Panel B shows corresponding mean network connectivity in all individuals at both time points and the corresponding group distributions, benchmarked to a group with established bipolar disorder scanned at only one time point and age-matched at follow-up. Circles and error bars show the mean and 95% confidence interval. This network derives from the default network-based statistics height threshold of t=3.0. Panel C shows network connectivity strength in all high-risk and control individuals as a function of their age at baseline and follow-up, with corresponding group regression slopes and confidence intervals.
The effect replicated at different thresholds of network sparsity (see Figure S5 in the online supplement) and was virtually unchanged when age at scan was used as a covariate (see Table S3 in the online supplement).
To understand the clinical significance of these results, we extracted the mean weight of this network in a cohort of people with an established diagnosis of bipolar disorder, age-matched to the control and high-risk cohorts at the time of follow-up (see the Supplementary Methods section and Table S4 in the online supplement). Intriguingly, the network weights in the control cohort at baseline were similar to those of this bipolar disorder cohort (Figure 2B, purple), but increased substantially over time (p<0.001), differing from the bipolar disorder group by follow-up (p=0.03). In contrast, the network weights in the high-risk cohort crossed from slightly above to slightly below those of the bipolar disorder cohort from baseline to follow-up (see Table S5 in the online supplement), with moderate evidence favoring no difference in connectivity between the high-risk and bipolar disorder cohorts (BF10=0.25; see Table S5 in the supplement).
Controllability of this network also shows a significant group-by-time effect (p<0.005, Figure 3C), with effects mirroring the network weight effect—that is, increasing controllability in the control group contrasts with a decrease in the high-risk group. Current medications, current mood episode, and current mood state were not associated with mean edge weights in the network showing a group-by-time effect (p=0.665 for current medications; p=0.252 for MADRS score; p=0.111 for CDI score; p=0.599 for current depressive episode at baseline; p=0.780 for current depressive episode at follow-up; p=0.330 for current manic episode at follow-up), nor with network controllability (p=0.855 for current medications; p=0.492 for MADRS score; p=0.374 for CDI score; p=0.267 for current depressive episode at baseline; p=0.221 for current depressive episode at follow-up; p=0.079 for current manic episode at follow-up). There was no significant association between YMRS score (in those older than age 22) and mean edge weights in the interaction network in either group at either time point, although a positive correlation in the baseline high-risk group approached significance (r=0.293, p=0.056).
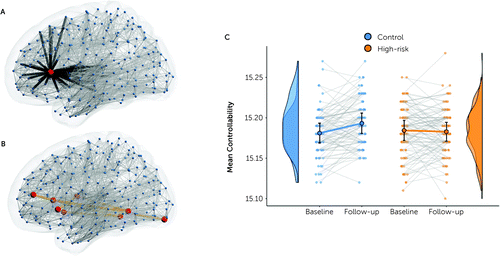
FIGURE 3. Group differences in longitudinal changes in network controllability for regions whose connections exhibited a group-by-time effecta
a Panel A shows average controllability for a single region (red circle), calculated as the average exogenous energy needed to control the transition from one brain state to another. In panel B, total network controllability was calculated as the sum of all controllabilities for regions (red circles) identified in the network-based statistics network (orange lines). Panel C shows network controllability in all individuals at both time points and the corresponding group distributions. Circles and error bars show the mean and 95% confidence interval.
Nonlinear Age Effects
The effect of age on edge strength was predominantly linear, with the mean weight of connectome edges falling well within 95% confidence intervals of a purely linear effect (see Figure S7A in the online supplement). Less than 1% of edges possessed nonlinear effects of age when considered individually: At baseline, 186 (0.71%) edges showed a significant nonlinear age-weight effect, of which 86 were concave down and 100 concave up (see Figure S7B in the online supplement). Similar numbers were observed at follow-up and within each group modeled separately. Of interest, these “nonlinear edges” were substantially stronger (see Figure S7C in the online supplement) and shorter (mean 13.1 mm) than the rest of the connectome (mean=44.1 mm; t=18.1, p<0.001). The nonlinearity of edges was consistent from baseline to follow-up (r=0.56, p<0.001), but not between groups (r=0.025, p>0.05). Of note, the nonlinearity of edges between baseline and follow-up was more consistent in the control group (r=0.60) than in the high-risk group (r=0.54, p<0.05), suggesting greater longitudinal heterogeneity in the high-risk cohort (see Figure S7D in the online supplement).
Clinical Conversion and Subgroup Analyses
Of the high-risk group, a small number (N=5) experienced a manic or hypomanic episode between baseline and follow-up, hence formally converting to bipolar disorder. In total, eight participants experienced any new mood episode and 18 high-risk participants developed any new DSM-IV disorder. The edge weights of the group-by-time network (Figure 2) showed a greater decrease over time in those high-risk participants who had a new onset of any mood episode since baseline (Figure 4A), particularly those who converted to bipolar disorder (Figure 4B), crossing from above to below the remaining high-risk participants. Those with a new onset of any DSM-IV disorder from baseline to follow-up showed a weaker difference (Figure 4C). There was no notable difference between those in the high-risk cohort who had and did not have not a lifetime mood episode at baseline (Figure 4D).
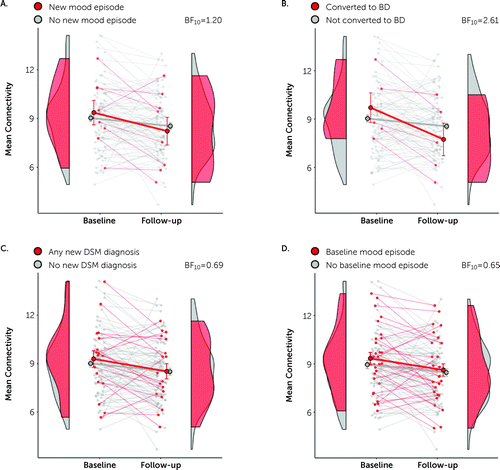
FIGURE 4. Mean edge weights of the longitudinal subnetwork in clinical subdivisions of the high-risk cohorta
a Participants are partitioned according to the presence (red) or absence (gray) of new onset of any mood episode (major depressive episode or manic or hypomanic episode) (N=8) (panel A); new onset of a manic or hypomanic episode (converted to bipolar disorder) (N=5) (panel B); new onset of any DSM-IV diagnosis (N=18) (panel C); and lifetime mood episode at baseline in the high-risk cohort (N=28) (panel D). BF=Bayes factor.
The corresponding effect sizes were 0.97 and 0.24, respectively, for those with and those without a new manic or hypomanic episode, and 0.52 and 0.27 for those with and without any new mood disorder. These effects yielded Bayes factors of 2.61 and 1.20, respectively, consistent with marginal evidence in favor of an effect of these new episodes (see Table S6 in the online supplement). In contrast, Bayes factors for those with and those without any new DSM-IV disorder (BF=0.69) or any lifetime mood episode at baseline (BF=0.65) suggest no subgroup differences for these factors.
The smaller effect size in the nonconverting high-risk group (effect size, 0.97 compared with 0.24 in 92 high-risk participants) remained after exclusion of the nonconverting participants who had any lifetime mood episode at baseline (effect size, 0.97 compared with 0.23 in 64 participants; see Table S6 in the online supplement). These results support the hypothesis that a stronger change in the weights of this network is unique to those high-risk participants with a new mood episode (especially a manic one) between baseline and follow-up.
Discussion
The peak incidence of bipolar disorder overlaps with the final stages of neurodevelopment in adolescents and young adults, a broad developmental phase that is associated with widespread longitudinal changes in structural connectivity. Consistent with the maturation of executive and cognitive control functions, connectivity in general tended to strengthen among central, midline, and rostral regions relative to posterior and central regions. Only very few changes were age invariant, consistent with well-established knowledge that cognitive and neural development occur in evolving waves of maturation (30, 31, 52, 57). These longitudinal changes are consistent with other brain network studies, which report both increases and decreases in structural and functional networks during this critical neurodevelopmental phase (28, 58–62). Embedded in these shared connectivity dynamics was a significant group-by-time interaction, comprising a distinct subnetwork whose connectivity increased in our control cohort but weakened in the high-risk cohort, shifting toward a comparative cohort with established bipolar disorder. Intriguingly, our results provide marginal evidence that this effect is enhanced in those who experience a first episode of any mood disorder, particularly a bipolar-disorder-defining first manic or hypomanic episode. There is no comparable evidence of an effect associated with a prior mood episode or any new DSM-IV diagnosis. These results provide novel insights into the longitudinal dynamics of structural connectivity in individuals who are at high risk of bipolar disorder and an intriguing preview of personalized outcome prediction in this relatively common clinical scenario.
The structural network where development differed for our high-risk cohort comprises left inferior and lateral regions connected to the thalamus and bilateral posterior cortex. Of particular interest, this network includes the left insula and left inferior frontal gyrus—regions that have frequently been highlighted in cross-sectional studies of high-risk (5, 7) and bipolar disorder (63) participants, including baseline reports on the present population (9, 10). These cortical hubs connect classic “limbic” regions such as the hypothalamus to cognitive control nodes, including the anterior cingulate and precuneus. Such connections underlie their integrative role in physiological, interoceptive, and executive functions (64–67) and their prominent role in anxiety and its dysregulation (68). The broader network involves multiple brain systems in this network, including visual, default, control, and subcortical regions, also consistent with structural changes in bipolar disorder (63).
Structural network changes identify candidate neurobiological markers of high genetic risk but do not, on their own, speak directly to the disturbances in affective and cognitive control that characterize bipolar disorder. Using computational methods, we found that the longitudinal network changes are associated with weaker network controllability, mirroring the emergence of affective dysregulation and disinhibition in bipolar disorder and its manic phenotype (69). Although network controllability is influenced by network strength, it also reflects higher-order network properties, including local cycles within the target subnetwork and longer loops that permeate the broader connectome (51). This loss of controllability thus shows how the subtle changes of edge strength within this network can have dynamic effects that propagate through the entire connectome, with brain-wide consequences.
There are several important caveats to our findings. Studying high-risk unaffected probands avoids the confounding effect of pharmacological treatment present in those with the established illness. Nonetheless, because we adopted an ecological approach (by not excluding those with any prior mood episode), a small number of participants in both groups were on pharmacotherapy. Current medications, current mood episode, and current mood state were not associated with the weighted strength of the group-by-time network. Other caveats of our study include the heterogeneous nature of high-risk cohorts, with some converting to the disorder while others remain well despite high genetic loading. Indeed, neurodevelopmental variability is a characteristic feature of healthy adolescent and young adult cohorts (30). Moreover, developing algorithms for prognostic prediction relies precisely on variance in longitudinal outcome. The substantial variability across our high-risk cohort in clinical outcome and network effects also indicates the presence of protective factors and variability in genetic risk among first-degree relatives of persons with bipolar disorder.
While our study is well powered to achieve the primary objectives (of a group-by-time effect), only a small number of participants developed a first mood episode between baseline and follow-up. We found marginal evidence for a stronger network difference in those who developed their index case of mania during our study. Although these analyses are based on Bayesian methods (which avoid the need for a point-wise threshold for inference), this observation clearly requires replication in a sufficiently powered study. However, extrapolating the present rate of conversion from high risk to bipolar disorder at 2 years suggests that a total cohort size of up to 1,000 would be required to obtain a sample size of 40 high-risk-to-bipolar-disorder clinical converters over a 2- to 3-year time frame. Studies of this size invariably require pooling across multiple sites, introducing nuisance variance arising from site-specific imaging platforms.
Longitudinal studies are uniquely placed to disambiguate associations from mechanism (using temporal precedence) and support data-driven enrichment and prediction algorithms. However, longitudinal studies carry their own challenges. The time lag from study design and ethics approval through baseline to complete follow-up acquisition is typically longer than current funding cycles and also outlives most imaging-based innovations. Although we used advanced tractography pipelines, advances in diffusion MRI sequences since our study inception have improved the reliability and accuracy of the derived structural connectomes (70). However, it is not possible to introduce these at follow-up in a longitudinal study because of the ensuing collinearity with follow-up characterization. The hardware, acquisition, and analysis pipelines were identical across both time points. Nonetheless, the role of the insula and inferior frontal gyrus is convergent with changes in other modalities (cortical thickness on structural MRI [19]) as well as their role in cross-sectional studies of high-risk and established bipolar disorder (5).
The acquisition of cognitive abilities mirrors neurobiological changes across developmentally active phases (32), with changes in the sensorimotor cortex in childhood yielding to the structural reorganization of the heteromodal cortex in adolescence (56). We found nonlinear changes (both slowing and accelerating) in a relatively small number of short, strong structural connections that were consistent across time points but unique between groups. The temporal stability of these “nonlinear edges” was lower in the high-risk group, suggesting greater neurodevelopmental heterogeneity, consistent with the variety of phenotypic outcomes. However, longitudinal changes in our data were (predominantly) linear, likely reflecting methodological choices imposed by the nature of our scan protocol. Recent developments in tractography, such as multishell acquisitions, structurally informed seeding, and anatomical filtering, may permit greater interrogation of nonlinear neurodevelopment in future longitudinal studies of high-risk individuals.
Persons with a first-degree relative with bipolar disorder often inquire about their own future risk of the disorder. Epidemiological studies show an overall odds ratio in the range of approximately 7–14 (12), with the incidence peaking in the third decade of life. Prediction algorithms combining phenotypic, neurobiological, and genetic information are urgently needed to better stratify individual risk prediction, identifying those who might benefit from early intervention rather than the present “watch and wait” approach (5, 71). For example, in light of predictive genetic and imaging ascertainment, initiation of a mood stabilizer for a depressive episode might be preferable to an antidepressant alone in an “ultra-high-risk” first-degree relative. Here we show distinct neurodevelopmental effects in structural connectivity in this population, with a qualitatively stronger effect in those who did experience a manic episode between baseline and follow-up. Further work with greater numbers of participants and with more follow-ups is required to explore the potential of these observations and establish a possible clinical role.
1. : Prevalence and correlates of bipolar spectrum disorder in the World Mental Health survey initiative. Arch Gen Psychiatry 2011; 68:241–251Crossref, Medline, Google Scholar
2. : Functional impairment and cognition in bipolar disorder. Psychiatr Q 2000; 71:309–329Crossref, Medline, Google Scholar
3. : Elucidating neural network functional connectivity abnormalities in bipolar disorder: toward a harmonized methodological approach. Biol Psychiatry Cogn Neurosci Neuroimaging 2016; 1:288–298Crossref, Medline, Google Scholar
4. : The role of intrinsic brain functional connectivity in vulnerability and resilience to bipolar disorder. Am J Psychiatry 2017; 174:1214–1222Link, Google Scholar
5. : Connectomics of bipolar disorder: a critical review, and evidence for dynamic instabilities within interoceptive networks. Mol Psychiatry 2019; 24:1296–1318Crossref, Medline, Google Scholar
6. : Structural brain network analysis in families multiply affected with bipolar I disorder. Psy Res 2015; 234:44–51Crossref, Medline, Google Scholar
7. : Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry 2012; 71:881–889Crossref, Medline, Google Scholar
8. : Dissociable functional connectivity changes during the Stroop task relating to risk, resilience, and disease expression in bipolar disorder. NeuroImage 2011; 57:576–582Crossref, Medline, Google Scholar
9. : Functional dysconnection of the inferior frontal gyrus in young people with bipolar disorder or at genetic high risk. Biol Psychiatry 2017; 81:718–727Crossref, Medline, Google Scholar
10. : Structural dysconnectivity of key cognitive and emotional hubs in young people at high genetic risk for bipolar disorder. Mol Psychiatry 2018; 23:413–421Crossref, Medline, Google Scholar
11. : The neural basis of familial risk and temperamental variation in individuals at high risk of bipolar disorder. Biol Psychiatry 2011; 70:343–349Crossref, Medline, Google Scholar
12. : Individual and familial risk factors for bipolar affective disorders in Denmark. Arch Gen Psychiatry 2003; 60:1209–1215Crossref, Medline, Google Scholar
13. : Age at onset in bipolar I affective disorder in the USA and Europe. World J Biol Psychiatry 2014; 15:369–376Crossref, Medline, Google Scholar
14. : Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. Oxford, UK, Oxford University Press, 2007Google Scholar
15. : Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 2007; 20:359–364Crossref, Medline, Google Scholar
16. : New structural brain imaging endophenotype in bipolar disorder. Mol Psychiatry 2012; 17:412–420Crossref, Medline, Google Scholar
17. : White matter density in patients with schizophrenia, bipolar disorder, and their unaffected relatives. Biol Psychiatry 2005; 58:254–257Crossref, Medline, Google Scholar
18. : Corpus callosum size and shape alterations in individuals with bipolar disorder and their first-degree relatives. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33:1050–1057Crossref, Medline, Google Scholar
19. : Accelerated cortical thinning and volume reduction over time in young people at high genetic risk for bipolar disorder. Psychol Med (Online ahead of print, September 7, 2020)Google Scholar
20. : White matter integrity in individuals at high genetic risk of bipolar disorder. Biol Psychiatry 2011; 70:350–356Crossref, Medline, Google Scholar
21. : Widespread white matter tract aberrations in youth with familial risk for bipolar disorder. Psychiatry Res 2015; 232:184–192Crossref, Medline, Google Scholar
22. : Diffusion tensor imaging correlates of early markers of depression in youth at high-familial risk for bipolar disorder. J Child Psychol Psychiatry 2018; 59:917–927Crossref, Medline, Google Scholar
23. : Reduced white matter integrity in sibling pairs discordant for bipolar disorder. Am J Psychiatry 2013; 170:1317–1325Link, Google Scholar
24. : Interhemispheric white matter integrity in young people with bipolar disorder and at high genetic risk. Psychol Med 2016; 46:2385–2396Crossref, Medline, Google Scholar
25. : White matter microstructure in youth with and at risk for bipolar disorder. Bipolar Disord 2020; 22:163–173Crossref, Medline, Google Scholar
26. : Fractional anisotropy of the uncinate fasciculus and cingulum in bipolar disorder type I, type II, unaffected siblings and healthy controls. Br J Psychiatry 2018; 213:548–554Crossref, Medline, Google Scholar
27. : White matter microstructural abnormalities in families multiply affected with bipolar I disorder: a diffusion tensor tractography study. Psychol Med 2014; 44:2139–2150Crossref, Medline, Google Scholar
28. : Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 2011; 31:10937–10947Crossref, Medline, Google Scholar
29. : Neurodevelopment of the association cortices: patterns, mechanisms, and implications for psychopathology. Neuron 2021; 109:2820–2846Crossref, Medline, Google Scholar
30. : Inter-individual variability in structural brain development from late childhood to young adulthood. NeuroImage 2021; 242:118450Crossref, Medline, Google Scholar
31. : Developmental changes in cognitive control through adolescence. Adv Child Dev Behav 2009; 37:233–278Crossref, Medline, Google Scholar
32. : Maturation of widely distributed brain function subserves cognitive development. NeuroImage 2001; 13:786–793Crossref, Medline, Google Scholar
33. : Unraveling the miswired connectome: a developmental perspective. Neuron 2014; 83:1335–1353Crossref, Medline, Google Scholar
34. : White matter hyperintensities in young individuals with bipolar disorder or at high genetic risk. J Affect Disord 2019; 245:228–236Crossref, Medline, Google Scholar
35. : The organisation of the elderly connectome. NeuroImage 2015; 114:414–426Crossref, Medline, Google Scholar
36. : The contribution of geometry to the human connectome. NeuroImage 2016; 124:379–393Crossref, Medline, Google Scholar
37. Dhollander T, Raffelt D, Connelly A: Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. ISMRM Workshop on Breaking the Barriers of Diffusion MRI, Lisbon, Portugal, 2016Google Scholar
38. Tournier JD, Calamante F, Connelly A: Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Proceedings of the 18th Annual Meeting of the International Society for Magnetic Resonance in Medicine (ISMRM), Paris, 2010Google Scholar
39. : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002; 15:273–289Crossref, Medline, Google Scholar
40. : Whole-brain anatomical networks: does the choice of nodes matter? NeuroImage 2010; 50:970–983Crossref, Medline, Google Scholar
41. : Mapping the structural core of human cerebral cortex. PLoS Biol 2008; 6:e159Crossref, Medline, Google Scholar
42. : Consistency-based thresholding of the human connectome. NeuroImage 2017; 145:118–129Crossref, Medline, Google Scholar
43. : Spatial embedding and wiring cost constrain the functional layout of the cortical network of rodents and primates. PLoS Biol 2016; 14:e1002512Crossref, Medline, Google Scholar
44. : Network-based statistic: identifying differences in brain networks. NeuroImage 2010; 53:1197–1207Crossref, Medline, Google Scholar
45. : Children’s Depression Inventory (CDI). New York, Multi-Health Systems, 1992Google Scholar
46. : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
47. : A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
48. : The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord 2007; 103:29–42Crossref, Medline, Google Scholar
49. : The human connectome: a structural description of the human brain. PLoS Comp Biol 2005; 1:e42Crossref, Medline, Google Scholar
50. Tang E, Pasqualetti F, Bassett DS: The control of brain activity across spatial and temporal scales. Bulletin of the American Physical Society, Los Angeles, American Physical Society, 2018Google Scholar
51. : Controllability of structural brain networks. Nat Commun 2015; 6:8414Crossref, Medline, Google Scholar
52. : Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999; 2:861–863Crossref, Medline, Google Scholar
53. : Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry 2009; 66:287–296Crossref, Medline, Google Scholar
54. : A high-risk study of bipolar disorder: childhood clinical phenotypes as precursors of major mood disorders. Arch Gen Psychiatry 2011; 68:1012–1020Crossref, Medline, Google Scholar
55. : Sex differences in brain maturation during childhood and adolescence. J Cereb Cortex 2001; 11:552–557Crossref, Medline, Google Scholar
56. : Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. NeuroImage 2014; 92:356–368Crossref, Medline, Google Scholar
57. : Structural and functional brain development and its relation to cognitive development. Biol Psychol 2000; 54:241–257Crossref, Medline, Google Scholar
58. : Developmental changes in brain network hub connectivity in late adolescence. J Neurosci 2015; 35:9078–9087Crossref, Medline, Google Scholar
59. : Development of frontoparietal connectivity predicts longitudinal symptom changes in young people with autism spectrum disorder. J Transl Psychiatry 2019; 9:86Crossref, Medline, Google Scholar
60. : A network neuroscience approach to typical and atypical brain development. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:754–766Crossref, Medline, Google Scholar
61. : Conservative and disruptive modes of adolescent change in human brain functional connectivity. J Proc Natl Acad Sci USA 2020; 117:3248–3253Crossref, Medline, Google Scholar
62. : A generative network model of neurodevelopmental diversity in structural brain organization. Nat Commun 2021; 12:4216Crossref, Medline, Google Scholar
63. : Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry 2018; 23:932–942Crossref, Medline, Google Scholar
64. : Diversity of the inferior frontal gyrus: a meta-analysis of neuroimaging studies. Behav Brain Res 2011; 225:341–347Crossref, Medline, Google Scholar
65. : Saliency, switching, attention, and control: a network model of insula function. Brain Struct Funct 2010; 214:655–667Crossref, Medline, Google Scholar
66. : Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron 2003; 40:655–664Crossref, Medline, Google Scholar
67. : Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry 2021; 178:761–770Link, Google Scholar
68. : An insular view of anxiety. Biol Psychiatry 2006; 60:383–387Crossref, Medline, Google Scholar
69. : Fronto-limbic dysconnectivity leads to impaired brain network controllability in young people with bipolar disorder and those at high genetic risk. Neuroimage Clin 2018; 19:71–81Crossref, Medline, Google Scholar
70. : Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. NeuroImage 2013; 80:80–104Crossref, Medline, Google Scholar
71. : Connectomic markers of disease expression, genetic risk, and resilience in bipolar disorder. Transl Psychiatry 2016; 6:e706Crossref, Medline, Google Scholar
72. : The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106:1125–1165Crossref, Medline, Google Scholar
73. : Raincloud plots: a multi-platform tool for robust data visualization. Wellcome Open Res 2019; 4:63Crossref, Medline, Google Scholar
74. van Langen J: Open-visualisations in R and Python (version 1.0.4). Zenodo, 2020.Google Scholar



