Disparities in Screening and Treatment of Cardiovascular Diseases in Patients With Mental Disorders Across the World: Systematic Review and Meta-Analysis of 47 Observational Studies
Abstract
Objective:
This study used meta-analysis to assess disparities in cardiovascular disease (CVD) screening and treatment in people with mental disorders, a group that has elevated CVD incidence and mortality.
Methods:
The authors searched PubMed and PsycInfo through July 31, 2020, and conducted a random-effect meta-analysis of observational studies comparing CVD screening and treatment in people with and without mental disorders. The primary outcome was odds ratios for CVD screening and treatment. Sensitivity analyses on screening and treatment separately and on specific procedures, subgroup analyses by country, and by controlling for confounding by indication, as well as meta-regressions, were also run, and publication bias and quality were assessed.
Results:
Forty-seven studies (N=24,400,452 patients, of whom 1,283,602 had mental disorders) from North America (k=26), Europe (k=16), Asia (k=4), and Australia (k=1) were meta-analyzed. Lower rates of screening or treatment in patients with mental disorders emerged for any CVD (k=47, odds ratio=0.773, 95% CI=0.742, 0.804), coronary artery disease (k=34, odds ratio=0.734, 95% CI=0.690, 0.781), cerebrovascular disease (k=8, odds ratio=0.810, 95% CI=0.779, 0.842), and other mixed CVDs (k=11, odds ratio=0.839, 95% CI=0.761, 0.924). Significant disparities emerged for any screening, any intervention, catheterization or revascularization in coronary artery disease, intravenous thrombolysis for stroke, and treatment with any and with specific medications for CVD across all mental disorders (except for CVD medications in mood disorders). Disparities were largest for schizophrenia, and they differed across countries. Median study quality was high (Newcastle-Ottawa Scale score, 8); higher-quality studies found larger disparities, and publication bias did not affect results.
Conclusions:
People with mental disorders, and those with schizophrenia in particular, receive less screening and lower-quality treatment for CVD. It is of paramount importance to address underprescribing of CVD medications and underutilization of diagnostic and therapeutic procedures across all mental disorders.
People with mental disorders (schizophrenia spectrum disorders, bipolar disorder, and depressive disorders, among others) have poorer physical health than the general population (1, 2), with a higher burden of risk factors for cardiovascular diseases (CVDs), diabetes (3), metabolic syndrome (4), poor nutritional habits (5, 6), more sedentary behavior (7), and smoking (1). Pharmacological treatment of mental disorders, including second-generation antipsychotics, also contribute to poor metabolic status (8). According to a large-scale meta-analysis (9), people with mental disorders have a high prevalence of CVD (9.9%), and among those with severe mental disorders, the incidence of CVD is roughly 80% higher than in the general population. Mental disorders appear to be independent risk factors for cardiovascular disease, and a variety of putative causal mechanisms may explain this (10). This independent relationship has been best studied and established for depression (11), although there is compelling evidence of independent associations, from prospective studies, of other mental disorders with cardiovascular disease and mortality, especially for bipolar disorder and schizophrenia (9, 12), and less so for the anxiety disorders (10, 12).
Several medical conditions and CVDs contribute to a large extent to the reduction by 10–20 years in longevity among people with mental disorders (which is only partially due to suicide, which accounts for the largest relative mortality risk, but has lower prevalence than CVD) (13–17). Beyond increased incidence, the stage at which medical comorbidities are diagnosed and the quality and timeliness of care play a role in determining disease course and outcome. For instance, while the overall incidence of cancer in people with mental disorders is similar to that of the general population, mortality from cancer in both sexes is elevated in people with mental disorders (18), with higher fatality rates for cervical, breast, and overall cancer compared with the general population (increased mortality risks of 100%, 23%, 50%, respectively) (19, 20). Such increased cancer fatality among people with mental disorders might be explained by poor cancer screening, barriers to access to treatment, or lower-quality treatment. While access and treatment quality remain to be investigated, disparities in cancer screening have been demonstrated. A recent meta-analysis (21) that included 4,717,839 individuals (501,559 of whom had mental disorders) showed that people with mental disorders are less likely to be screened for any, breast, cervical, or prostate cancer (odds ratios, 0.76, 0.65, 0.89, and 0.78, respectively), and women with schizophrenia in particular have lower screening rates.
Similarly, disparities in CVD screening may also exist for people with mental disorders (21, 22). Moreover, consistent with general medical care (23, 24), the problem may go beyond CVD screening and extend to CVD treatment. Disparities in CVD screening and treatment may in part explain why people with mental disorders show an approximate 80% higher risk of CVD-related death compared with the general population (9, 25, 26).
A rigorous synthesis of the available evidence is paramount to determine whether disparities of this kind exist and to assess their type and extent. However, no recent comprehensive meta-analysis, without restriction in types of CVDs or types of mental disorders, has investigated disparities in CVD screening and treatment. To the best of our knowledge, the latest systematic reviews on disparities in medical prescriptions in people with mental illness compared with the general population was published by Mitchell et al., in 2012 (23), with the last search conducted in 2010. Since then, a number of studies have been published, reporting alarming disparities in CVD screening and treatment between people with and without mental disorders (27). Furthermore, assessing sources of bias in observational evidence is crucial (28). Particularly, confounding by indication—that is, not accounting for the expected higher frequency of screening and treatment for CVD in groups with a higher base rate of CVD (i.e., people with mental illness)—can lead to underestimation of disparities in observational evidence (29, 30). Conversely, given increased rates of undetected and untreated CVD in individuals with mental disorders, studies comparing CVD screening and treatment without restricting the selection criteria to people with underlying CVD might also underestimate screening and treatment disparities.
Based on the above, we conducted a systematic review and meta-analysis of studies measuring disparities in CVD screening and treatment between people with and without mental disorders. Our hypothesis was that significant disparities exist, with both reduced screening and treatment of CVD, both across CVDs and mental disorder groups.
Methods
Search and Inclusion Criteria
We followed an a priori protocol (https://osf.io/b8rvs/), adhering to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) (31) (see Table S1 in the online supplement) and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) (32) guidelines (see Table S2 in the online supplement). We performed an electronic search from database inception and without language restriction on July 31, 2020, via Ovid, searching MEDLINE and PsycInfo, using the following search terms: (schizophrenia or schizoaffective or psychos* or depress* or bipolar or mania or mental) and (limit* or unequal* or barrier* or access or inequit* or disparit*) and (myocardial or coronary or stroke or thrombolysis or ischemic or heart or cardiac or revasc* or screening or exercise test)).ab,ti. not (review or meta-analysis).mp. [mp=ti, ab, ot, nm, hw, fx, kf, ox, px, rx, ui, an, sy, tc, id, tm, mh] limited to Humans, Adults. In addition to systematic database searches, reference lists of previous reviews and included studies were searched manually.
Inclusion criteria were observational study design, published in any language, focusing on screening, diagnosis, and treatment of CVDs, in people with mental disorders, defined according to structured criteria, validated scales, or clinical records, and reporting comparative effect sizes of CVD screening or treatment between people with mental disorders and the general population, or raw frequencies in both groups. Hospitalization for CVD was not considered, as this does not automatically indicate quality of care.
Two authors (L.P., M.D.) independently conducted the searches and selected eligible papers. Any disagreement was resolved by consensus, or by a third author (M.S.).
Data Extraction
The following information was extracted into a predefined spreadsheet: author, year, country, study design, diagnostic criteria for CVDs and mental disorders, specific CVD and mental disorder, specific procedure (e.g., coronary catheterization, intravenous thrombolysis), age, sex, presence of confounding by indication (i.e., assessing treatment for CVD without restricting to people with CVD), association measures quantifying disparities in CVD screening or treatment between the two groups, and raw frequencies from which the association measure could be computed. When studies provided unadjusted and adjusted effect sizes, we considered the adjusted (or the most adjusted) effect size. When studies provided association measures at different time points, we considered the result at the longest time point. Two authors (L.P., M.D.) independently extracted data. Any disagreement was resolved by consensus, or by a third author (M.S.).
Quality Assessment
Two authors (L.P., M.D.) independently assessed the quality of the included studies, using the Newcastle-Ottawa Scale, with a score ≥7 (out of 9) indicating high quality (33).
Meta-Analysis
We performed a random-effect meta-analysis (34, 35), using the Comprehensive Meta-Analysis software package, version 2 (https://www.meta-analysis.com/). We calculated the odds ratio and 95% confidence interval of any CVD screening or treatment in people with mental disorders compared with the general population (primary outcome). When multiple outcomes were reported in one study, we considered the mean estimate to avoid double-counting participants (and artificially inflating sample size). In sensitivity analyses, we analyzed screening and treatment separately, across mental disorders and across specific procedures (catheterization in coronary artery disease [CAD], revascularization in CAD, intravenous thrombolysis in stroke, medications in any CVD) in people with mental disorders. Finally, subgroup analyses were conducted by country, and by presence of confounding by indication, across mental disorders.
Heterogeneity was assessed with the I2 statistic (with high heterogeneity being indicated by an I2 value ≥50%) (36). Publication bias was assessed via visual inspection of funnel plots and Egger’s bias test (37). We also calculated the fail-safe number (estimated number of studies needed to move the effect size from significant to nonsignificant), and trim-and-fill adjusted analysis (38) in case of publication bias (Egger’s test p value <0.1). Random-effect meta-regression was conducted to explore sex, age, sample size, and quality of included studies as potential moderators of the primary outcome.
Results
Search Results and Study Characteristics
Search results and the study selection process are outlined in Figure 1. Of 5,074 initial hits and 21 additional records identified through manual search, and after removing duplicates, we screened 5,095 studies at the title and abstract level, of which we selected 116 for full-text assessment. We excluded 69 studies after full-text assessment (see Table S3 in the online supplement), and ultimately included 47 studies.
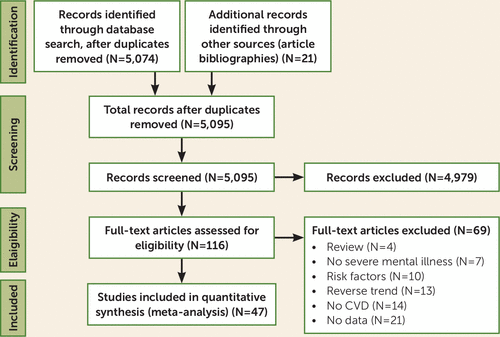
FIGURE 1. PRISMA flowchart for a systematic review and meta-analysis of disparities in screening and treatment of cardiovascular diseases in patients with mental disorders
Detailed characteristics and references of included studies are reported in Table S4 in the online supplement. Overall, this meta-analysis reports data from 24,400,452 subjects, including 1,283,602 with mental disorders. The mean or median age was >65 years in 15 studies. Among people with mental disorders, 873,268 (68.0%) were diagnosed with mood disorders, 279,177 (21.8%) with schizophrenia spectrum disorders, and 131,157 (10.2%) with other mental disorders (anxiety disorders, dementia, personality disorders, posttraumatic stress disorder, substance use disorders, and other, mixed disorders). Thirty studies (63.8%) included subjects with different disorders, 13 (27.7%) included subjects with schizophrenia spectrum disorders, and four (8.5%) included subjects with mood disorders. Twenty-eight studies (59.6%) included subjects with CAD, including acute myocardial infarction, 15 (31.9%) with different CVDs, and four (8.5%) with cerebrovascular disease (CBVD), including stroke.
All populated continents except Africa and South America were represented. Twenty-one studies were conducted in the United States, seven in Denmark, five in Canada, three in the United Kingdom, two in Finland, two in Israel, two in Norway, and one each in France, Taiwan, Australia, Hong Kong, and the Netherlands. Overall, five studies were affected by confounding by indication. The quality of included studies was high (Newcastle-Ottawa Scale score ≥7) in 41 studies, with a median score of 8 (IQR=7–8) (see Table S5 in the online supplement).
Primary Outcome: Screening or Treatment for CVD
The main results for the primary outcome are reported in Table 1. Patients with mental disorders had significantly lower rates of screening or treatment for any CVD (k=47, odds ratio=0.773, 95% CI=0.742, 0.805, p<0.001), for CAD (k=34, odds ratio=0.734, 95% CI=0.690, 0.781, p<0.001), for CBVD (k=8, odds ratio=0.810, 95% CI=0.779, 0.842, p<0.001), and for mixed CVDs (k=11, odds ratio=0.839, 95% CI=0.761, 0.924, p<0.001). Disparities were confirmed for patients with mood disorders for any CVD (k=19, odds ratio=0.842, 95% CI=0.786, 0.901, p<0.001), for CAD (k=13, odds ratio=0.827, 95% CI=0.750, 0.911, p<0.001), and for CBVD (k=4, odds ratio=0.811, 95% CI=0.768, 0.857, p<0.001), but not for mixed CVDs (p=0.397). Larger disparities emerged for patients with schizophrenia spectrum disorders for any CVD (k=29, odds ratio=0.615, 95% CI=0.564, 0.671, p<0.001), for CAD (k=21, odds ratio=0.564, 95% CI=0.513, 0.620, p<0.001), for CBVD (k=5, odds ratio=0.717, 95% CI=0.626, 0.821, p<0.001), and for mixed CVDs (k=6, odds ratio=0.764, 95% CI=0.674, 0.866, p<0.001). Finally, for patients with mixed mental disorders, disparities were also confirmed for any CVD (k=25, odds ratio=0.836, 95% CI=0.787, 0.887, p<0.001), for CAD (k=18, odds ratio=0.819, 95% CI=0.760, 0.883, p<0.001), and for CBVD (k=4, odds ratio=0.849, 95% CI=0.808, 0.892, p<0.001), but not for any mixed CVDs (p=0.111).
| Cardiovascular Disease and Mental Disorder | Publications and Samples (k/k) | Odds Ratio | 95% CI | p | I2 | Egger’s Test p | Fail-Safe N | Effect Size After Trim and Filla | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Any cardiovascular disease (primary outcome) | |||||||||
| Any | 47/90 | 0.773 | 0.742, 0.805 | <0.001 | 93.577 | 0.265 | 8,599 | ||
| Mood disorders | 19/26 | 0.842 | 0.786, 0.901 | <0.001 | 93.532 | 0.876 | 1,484 | ||
| Schizophrenia spectrum disorders | 29/30 | 0.615 | 0.564, 0.671 | <0.001 | 93.237 | 0.018 | 7,967 | 0.625 | 0.573, 0.682 |
| Mixedb | 25/34 | 0.836 | 0.787, 0.887 | <0.001 | 91.485 | 0.411 | 2,714 | ||
| Acute myocardial infarction, ischemic heart disease | |||||||||
| Any | 34/63 | 0.733 | 0.690, 0.781 | <0.001 | 94.830 | 0.122 | 8,716 | ||
| Mood disorders | 13/15 | 0.827 | 0.750, 0.911 | <0.001 | 90.884 | 0.457 | 339 | ||
| Schizophrenia spectrum disorders | 21/21 | 0.564 | 0.513, 0.620 | <0.001 | 84.590 | 0.212 | 4,617 | ||
| Mixedb | 18/26 | 0.819 | 0.760, 0.883 | <0.001 | 92.314 | 0.524 | 1,750 | ||
| Cerebrovascular disease, stroke, transient ischemic attack | |||||||||
| Any | 8/19 | 0.810 | 0.779, 0.842 | <0.001 | 70.617 | 0.625 | 1,045 | ||
| Mood disorders | 4/6 | 0.811 | 0.768, 0.857 | <0.001 | 57.463 | 0.341 | 364 | ||
| Schizophrenia spectrum disorders | 5/5 | 0.717 | 0.626, 0.821 | <0.001 | 45.877 | 0.141 | 151 | ||
| Mixedb | 4/8 | 0.849 | 0.808, 0.892 | <0.001 | 29.354 | 0.936 | 54 | ||
| Mixed cardiovascular disease | |||||||||
| Any | 11/16 | 0.839 | 0.761, 0.924 | <0.001 | 83.160 | 0.742 | 234 | ||
| Mood disorders | 4/4 | 0.933 | 0.794, 1.096 | 0.397 | 33.299 | NPc | |||
| Schizophrenia spectrum disorders | 6/6 | 0.764 | 0.674, 0.866 | <0.001 | 68.076 | 0.348 | 151 | ||
| Mixedb | 6/6 | 0.869 | 0.731, 1.033 | 0.111 | 85.198 | 0.231 | 13 | ||
TABLE 1. Disparities in any procedure (screening or treatment) for any and specific cardiovascular diseases across mental disorders in a systematic review and meta-analysis
Screening for Cardiovascular Disease
Meta-analyses on CVD screening disparities are reported in Table 2. Regarding screening for any CVD, disparities emerged for any, mood, schizophrenia spectrum, and other mental disorders (odds ratios, 0.757, 0.877, 0.611, and 0.812, respectively; all p values <0.05). Similar results emerged for CAD in any, mood, schizophrenia spectrum, and other mental disorders (odds ratios, 0.718, 0.830, 0.552, and 0.856, respectively; all p values <0.05). For CBVD, a significant gap in CVD screening was confirmed in any mental disorder and schizophrenia spectrum disorders (odds ratios, 0.683 and 0.591, respectively; both p values <0.05), and only one study was included for mood and mixed mental disorders. For mixed CVDs, patients with any, schizophrenia spectrum, and mixed mental disorders had lower rates of CVD screening (odds ratios, 0.786, 0.775, and 0.730, respectively; all p values <0.05), while no difference emerged among those with mood disorders.
| Cardiovascular Disease and Mental Disorder | Publications and Samples (k/k) | Odds Ratio | 95% CI | p | I2 | Egger’s Test p | Fail-Safe N | Effect Size After Trim and Filla | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Any cardiovascular disease | |||||||||
| Any | 23/38 | 0.757 | 0.719, 0.797 | <0.001 | 92.900 | 0.43 | 5,391 | 0.813 | 0.769, 0.859 |
| Mood disorders | 8/9 | 0.877 | 0.792, 0.973 | 0.013 | 84.358 | 0.80 | 241 | ||
| Schizophrenia spectrum disorders | 15/16 | 0.611 | 0.554, 0.673 | <0.001 | 91.055 | 0.005 | 2,709 | 0.728 | 0.660, 0.803 |
| Mixedb | 10/13 | 0.812 | 0.733, 0.899 | <0.001 | 92,982 | 0.695 | 603 | ||
| Acute myocardial infarction, ischemic heart disease | |||||||||
| Any | 16/29 | 0.718 | 0.670, 0.770 | <0.001 | 94.461 | 0.048 | 3,302 | 0.800 | 0.745, 0.860 |
| Mood disorders | 6/7 | 0.830 | 0.732, 0.941 | <0.001 | 82.248 | 0.697 | 240 | ||
| Schizophrenia spectrum disorders | 12/13 | 0.552 | 0.490, 0.622 | <0.001 | 88.030 | 0.06 | 1,861 | 0.673 | 0.597, 0.757 |
| Mixedb | 6/9 | 0.856 | 0.748, 0.980 | <0.001 | 93.625 | 0.993 | 277 | ||
| Cerebrovascular disease, stroke, transient ischemic attack | |||||||||
| Any | 3/4 | 0.683 | 0.533, 0.87 | 0.003 | 0 | 0.078 | 5 | Unchanged | |
| Mood disorders | 1 | ||||||||
| Schizophrenia spectrum disorders | 2/2 | 0.591 | 0.388, 0.899 | <0.001 | 0 | NPc | |||
| Mixedb | 1 | ||||||||
| Mixed cardiovascular disease | |||||||||
| Any | 7/10 | 0.786 | 0.712, 0.868 | <0.001 | 88.739 | 0.397 | 326 | ||
| Mood disorders | 3/3 | 1.011 | 0.935, 1.094 | 0.784 | 0 | 0.610 | NPc | ||
| Schizophrenia spectrum disorders | 4/4 | 0.775 | 0.719, 0.835 | <0.001 | 46.769 | 0.017 | 149 | 0.797 | 0.733, 0.867 |
| Mixedb | 3/3 | 0.730 | 0.552, 0.966 | <0.001 | 95.934 | 0.514 | 48 | ||
TABLE 2. Disparities in screening for any and specific cardiovascular diseases across mental disorders in a systematic review and meta-analysis
Treatment of Cardiovascular Disease
Meta-analytic results on CVD treatment disparities are summarized in Table 3. Regarding treatment for CVD, across any, mood, schizophrenia spectrum, and mixed mental disorders, disparities emerged for any CVD (odds ratios, 0.765, 0.816, 0.597, and 0.843; all p values <0.05), for CAD (odds ratios, 0.728, 0.830, 0.556, and 0.816, respectively; all p values <0.05), and for CBVD (odds ratios, 0.811, 0.813, 0.719, and 0.849; all p values <0.05). For mixed CVDs, treatment was significantly less likely to be administered only for the any mental disorder category (odds ratio=0.837, p<0.05) and schizophrenia spectrum disorders (odds ratio=0.683, p<0.05), and not for mood disorders or other mental disorders.
| Cardiovascular Disease and Mental Disorder | Publications and Samples (k/k) | Odds Ratio | 95% CI | p | I2 | Egger’s Test p | Fail-Safe N | Effect Size After Trim and Filla | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Any cardiovascular disease | |||||||||
| Any | 45/86 | 0.765 | 0.730, 0.801 | <0.001 | 94.613 | 0.276 | 6,062 | ||
| Mood disorders | 17/21 | 0.816 | 0.755, 0.882 | <0.001 | 94.829 | 0.858 | 1,361 | ||
| Schizophrenia spectrum disorders | 27/28 | 0.597 | 0.538, 0.662 | <0.001 | 93.97 | 0.046 | 6,723 | 0.609 | 0.549, 0.674 |
| Mixedb | 25/37 | 0.843 | 0.792, 0.898 | <0.001 | 91.792 | 0.310 | 2,483 | ||
| Acute myocardial infarction, ischemic heart disease | |||||||||
| Any | 33/61 | 0.728 | 0.681, 0.778 | <0.001 | 95.661 | 0.115 | 8,254 | ||
| Mood disorders | 12/14 | 0.830 | 0.755, 0.912 | <0.001 | 91.470 | 0.251 | 255 | ||
| Schizophrenia spectrum disorders | 20/21 | 0.556 | 0.489, 0.633 | <0.001 | 93.470 | 0.140 | 4,444 | ||
| Mixedb | 18/26 | 0.816 | 0.754, 0.884 | <0.001 | 92.637 | 0.511 | 1,726 | ||
| Cerebrovascular disease, stroke, transient ischemic attack | |||||||||
| Any | 7/17 | 0.811 | 0.779, 0.844 | <0.001 | 74.451 | 0.511 | 985 | ||
| Mood disorders | 3/5 | 0.813 | 0.766, 0.863 | <0.001 | 71.612 | 0.239 | 349 | ||
| Schizophrenia spectrum disorders | 4/4 | 0.719 | 0.619, 0.835 | <0.001 | 56.059 | 0.266 | 132 | ||
| Mixedb | 4/8 | 0.849 | 0.807, 0.894 | <0.001 | 31.973 | 0.901 | 54 | ||
| Mixed cardiovascular disease | |||||||||
| Any | 9/12 | 0.837 | 0.723, 0.969 | 0.017 | 84.441 | 0.113 | 46 | ||
| Mood disorders | 2/2 | 0.667 | 0.447 | 0.995 | 0.047 | NPc | |||
| Schizophrenia spectrum disorders | 4/4 | 0.683 | 0.492, 0.947 | 0.022 | 81.197 | 0.101 | 22 | ||
| Mixedb | 6/6 | 0.940 | 0.805, 1.097 | 0.433 | 78.478 | 0.452 | NPc | ||
TABLE 3. Disparities in treatment of any and specific cardiovascular diseases across mental disorders in a systematic review and meta-analysis
Specific Procedures and Medications for CVDs
Disparities in specific procedures for CVDs across mental disorders are reported in Figure 2. For catheterization in CAD, disparities emerged for any, mood, and schizophrenia spectrum disorders (odds ratios, 0.736, 0.771, and 0.505, respectively; all p values <0.05), but not for mixed mental disorders. Similarly, in CAD, lower rates of revascularization emerged across any, mood, schizophrenia spectrum, and mixed mental disorders (odds ratios, 0.763, 0.818, 0.559, and 0.806; all p values <0.05), as well as lower rates of coronary artery bypass grafting (odds ratios, 0.694, 0.682, 0.504, and 0.798, respectively; all p values <0.05). A significantly lower frequency of intravenous thrombolysis in stroke emerged for any, mood, schizophrenia spectrum, and mixed mental disorders (odds ratios, 0.845, 0.813, 0.716, and 0.849, respectively; all p values <0.05). A gap in treatment with CVD-targeting medications emerged for any and schizophrenia spectrum disorders (odds ratios, 0.840 and 0.701, respectively; both p values <0.05), but not for mood or mixed mental disorders.
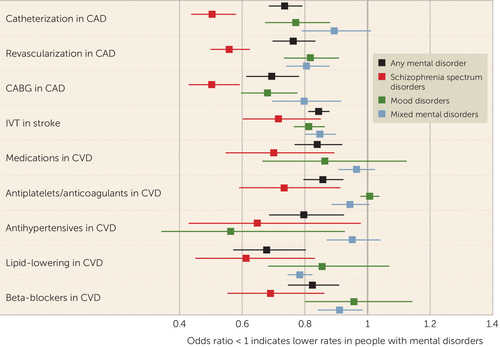
FIGURE 2. Frequency of specific screening, treatment procedures, and medications for cardiovascular disease in patients with and without mental disordersa
a CABG=coronary artery bypass grafting; CAD=coronary artery disease; CVD=cardiovascular disease; IVT=intravenous thrombolysis.
Notably, there was a significant gap for antiplatelet or anticoagulant treatment in any and schizophrenia spectrum disorders (odds ratios, 0.858 and 0.735, respectively; all p values <0.05), but not in mood and mixed mental disorders. Antihypertensives were less frequently used in patients with any and mood disorders (odds ratios, 0.797 and 0.649, respectively; all p values <0.05), but not in those with schizophrenia spectrum and mixed mental disorders. In any, schizophrenia spectrum, and mixed mental disorders, there was also less prescribing of lipid-lowering agents (odds ratios, 0.679, 0.613, and 0.784, respectively; all p values <0.05) and beta-blockers (odds ratios, 0.824, 0.691, and 0.911, respectively; all p values <0.05), but this was not the case in mood disorders.
Subgroup Analyses
Subgroup analyses (see Tables S6 and S7 in the online supplement) showed significant differences across countries regarding disparities in screening or treatment for any CVD (largest in Taiwan [odds ratio=0.384, 95% CI=0.289, 0.510], nonsignificant in France [odds ratio=1.163, 95% CI=0.979, 1.381]; subgroup comparison, p<0.001), for CAD (largest in Taiwan [odds ratio=0.384, 95% CI=0.289, 0.510], smallest in Finland [odds ratio=0.893, 95% CI=0.868, 0.919]; subgroup comparison, p<0.001), and for mixed CVDs (largest in the United Kingdom [odds ratio=0.289, 95% CI=0.150, 0.559], absent in France [odds ratio=1.163, 95% CI=0.979, 1.381]; subgroup comparison, p<0.001), but not for CBVD (p=0.505).
Meta-analytic estimates from studies vulnerable to confounding by indication failed to identify disparities in screening or treatment for any CVD, CAD, CBVD, and mixed CVD disorders. Subgroup differences emerged in screening or treatment for any CVD (p<0.05) and for CAD (p<0.05).
Meta-Regression
Higher study quality (beta=−0.018, standard error=0.009, p=0.046) and percentage female (beta=−0.003, standard error=0.001, p=0.004) moderated larger disparities. Age and sample size were not significant moderators (p values, 0.232 and 0.950, respectively).
Publication Bias
In main analyses on the primary outcome, publication bias was present in one association, and among sensitivity analyses in six associations. All associations remained significant after trim-and-fill analysis. The median fail-safe number was 364 in the main analysis (interquartile range, 163–3,666) and 476 in the sensitivity analysis (interquartile range, 171–2,652).
Discussion
This systematic review and meta-analysis investigated any CVD and specific CVD screening and treatment procedure disparities in more than 1.25 million people with mental disorders compared with more than 23 million people without mental disorders from four continents. The results indicate that people with mental disorders suffer from significantly lower screening for or treatment of any CVD, CAD, CBVD, and other CVD, including heart failure, and that these disadvantages extend across different mental disorders, being highest in people with schizophrenia spectrum disorders.
These findings extend previous narrative reviews and confirm the hypothesis that people with mental disorders undergo fewer screening and treatment procedures for CVDs than the general population, although people with mental illness have a greater likelihood of having and dying from CVDs (1, 9). The results are consistent with previous work from Mitchell and colleagues, who showed that people treated with antipsychotics (as a proxy indicator of having mental disorders) receive less metabolic screening than mandated by guidelines (39) and that people with mental disorders receive lower metabolic quality of care for CVD in general (40), less frequent revascularization in coronary artery disease (25), and fewer prescriptions of medications for physical disorders (23). However, our data expand these previous findings by including any mental disorder, any screening and treatment procedures, and for any CVD, in one quantitative evidence synthesis and by having a more than tenfold larger sample size. The results show that disparities in physical health care of people with mental disorders clearly and concerningly extend beyond cancer screening (21), diabetes care (41, 42), and treatment of metabolic syndrome (43–45).
There are many possible reasons for these disparities in the management of CVD in people with mental disorders. First, mental health professionals reportedly undertake physical examinations in less than 50% of people with mental disorders (46). Second, mental health professionals often do not feel confident in prescribing physical health medications and leave the task to physicians in primary care, internal medicine, or specific medical specialties (23). Third, family physicians spend less time with persons with mental disorders than with other patients (40, 47), possibly because they have to deal with frequently overly busy schedules and the competing demands of other patients (48, 49). Similarly, low mental health literacy among non–mental health professionals may result in stigma and barriers to offering treatment (50). Indeed, overshadowing of mental disorders, limiting health care for physical disorders, frequently occurs. Fourth, symptoms and impairment in social functioning and in cognition, reduced illness insight, nonadherence to treatments, and financial or insurance problems may compromise health care access and utilization, especially when people with mental illness live in poverty or have no fixed address (21). In this context, social withdrawal, avoidance behavior, and depressed mood, among other symptoms, could contribute to the patient’s reduced interest in self-care, including medical care (21). Core symptomatology could be a determining factor in schizophrenia, as patients with schizophrenia reportedly have fewer medical visits and fewer documented medical problems compared with people with depression (51). This factor could explain the fact that disparities in CVD screening and treatment are largest in schizophrenia. Patients with schizophrenia frequently also do not accept proposed treatments, after acute myocardial infarction, for instance (52). Fifth, physicians may disregard physical complaints by patients with mental illness, as a result of diagnostic overshadowing (the assumption that the complaint results from the mental condition) (53, 54). Sixth, physical and mental health care providers often deliver care in “silos,” limiting comprehensive care for both physical and mental illness (55). Seventh, people with mental disorders tend to receive CVD care too late, when the disease is already well established, rather than receiving preventive care when risk factors, such as diabetes and hypertension, first appear. This problem has been shown in comparison with the general population according to data collected from Medicaid and Medicare in the United States (55). Indeed, people with mental illness are less likely to receive blood glucose and lipid tests, hospital care, and medications for diabetes (56).
Importantly, the present results suggest that disparities in CVD status between people with and without mental disorders are actually larger than previously reported (9). If those with mental disorders are infrequently screened for CVD, many may die without their CVD ever being diagnosed or treated. Such a hypothesis is supported by evidence from a study reporting on 72,451 people who died from CVD, showing that individuals with schizophrenia and women with bipolar disorder were, respectively, 66% and 38% less likely to be diagnosed with CVD before their CVD-related death (57). Conversely, timely pharmacological treatment of CVDs could substantially reduce the high premature mortality rates typically observed for people with mental disorders (58).
Specific interventions should be tested in randomized clinical trials. A possible approach to avoid delaying CVD diagnosis and treatment could be to routinely offer a CVD risk assessment to patients accessing mental health services and to establish a close, efficient collaboration with cardiology services, where patients with elevated CVD risk can be referred (59). Mental health professionals could then follow up with patients regarding cardiologists’ prescriptions, to promote treatment adherence. The effectiveness and cost-effectiveness of this pathway could be tested in cluster-randomized clinical trials, with centers randomly assigned to either CVD screening plus dedicated cardiologist referral (experimental arm) or treatment as usual (control arm). The experimental arm should be tailored to specific health care organizations across different countries, as well as to the magnitude of disparities in each country. Any rehabilitative treatment should aim to promote autonomy and recovery, ultimately leading patients with mental disorders to autonomously use health services, as the general population does. The real-world evidence included in the present work shows that this autonomy remains to be achieved and that specific adjustments to facilitate CVD screening and treatment are needed to counter both lack of prescription of and adherence to CVD screening and care in people with mental disorders.
Importantly, the need to improve screening and treatment for physical conditions in people with mental disorders extends to acute-setting health care service utilization (hospitalization, emergency department) for physical health (60), suggesting that disparities in screening and treatment of physical disorders might go beyond CVD, despite effective options being available (61). Finally, our findings are also important from a methodological standpoint. The results clearly show that studies biased by confounding by indication either underestimate the gap in CVD screening and treatment as a result of undetected CVDs in people with mental illness or overestimate screening and treatment as a result of higher frequencies of CVDs in people with mental illness compared with the general population, failing to find existing disparities in CVD screening and treatment for people with mental disorders. Additionally, studies with relatively lower quality reported relatively smaller disparities.
This work has several strengths. It is the largest and most comprehensive evidence synthesis on CVD screening and treatment disparities in people with mental disorders to date. It includes high-quality observational studies. It sheds light on mechanisms underlying poor CVD status, with considerable clinical and service organization implications. And it supports the research and evidence-synthesis recommendations from the Lancet Psychiatry Commission’s “blueprint for protecting physical health in people with mental illness” (1).
The study is also not without limitations. First, virtually all analyses showed high heterogeneity. However, we identified moderators, which could be responsible for heterogeneous estimates, such as country, confounding by indication, and study quality. Second, observational studies are affected by several types of bias, which even high-quality meta-analytic methodology can only partially address (28). Finally, scant evidence was available from low- and middle-income countries.
Conclusions
People with mental disorders receive CVD screening and treatment procedures significantly less frequently than the general population. Such disparities are not confined to a particular mental disorder, although they are most pronounced for people with schizophrenia spectrum disorders. Such gaps in screening and treatment for CVDs may contribute to increased mortality and lower life expectancy in men and women with mental disorders. With nearly a twofold elevation in risk for cardiovascular mortality, this situation represents a dramatic but modifiable health disparity. Targeted efforts to address this public health disparity are urgent. Given the persistent mortality gap, seen over several decades, affecting people with mental disorders compared with the general population, and largely attributed to CVDs, a collaborative model of care involving preventive screening and collaboration among primary care clinicians, medical specialists, and mental health care providers should be tested and implemented widely after efficacy is demonstrated.
1. : The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019; 6:675–712Crossref, Medline, Google Scholar
2. : Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol 2021; 18:136–145Crossref, Medline, Google Scholar
3. : Diabetes mellitus in people with schizophrenia, bipolar disorder, and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry 2016; 15:166–174Crossref, Medline, Google Scholar
4. : Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder, and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 2015; 14:339–347Crossref, Medline, Google Scholar
5. : Diet as a hot topic in psychiatry: a population-scale study of nutritional intake and inflammatory potential in severe mental illness. World Psychiatry 2018; 17:365–367Crossref, Medline, Google Scholar
6. : Dietary intake of people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry 2019; 214:251–259Crossref, Medline, Google Scholar
7. : Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder, and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry 2017; 16:308–315Crossref, Medline, Google Scholar
8. : Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag 2017; 13:757–777Crossref, Medline, Google Scholar
9. : Prevalence, incidence, and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017; 16:163–180Crossref, Medline, Google Scholar
10. : The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin Neurosci 2018; 20:31–40Crossref, Medline, Google Scholar
11. : Depression and the risk of myocardial infarction and coronary death: a meta-analysis of prospective cohort studies. Medicine 2016; 95:e2815Crossref, Medline, Google Scholar
12. : Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015; 72:334–341Crossref, Medline, Google Scholar
13. : Outcomes of Nordic mental health systems: life expectancy of patients with mental disorders. Br J Psychiatry 2011; 199:453–458Crossref, Medline, Google Scholar
14. : Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry 2017; 4:295–301Crossref, Medline, Google Scholar
15. : Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry 2013; 70:931–939Crossref, Medline, Google Scholar
16. : Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry 2012; 25:83–88Crossref, Medline, Google Scholar
17. : A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet 2019; 394:1827–1835Crossref, Medline, Google Scholar
18. : Excess cancer mortality in psychiatric patients. Can J Psychiatry 2008; 53:753–761Crossref, Medline, Google Scholar
19. : Excess cancer mortality in Western Australian psychiatric patients due to higher case fatality rates. Acta Psychiatr Scand 2000; 101:382–388Crossref, Medline, Google Scholar
20. : Cancer mortality in patients with schizophrenia: systematic review and meta-analysis. Br J Psychiatry 2017; 211:7–13Crossref, Medline, Google Scholar
21. : Disparities in cancer screening in people with mental illness across the world versus the general population: prevalence and comparative meta-analysis including 4 717 839 people. Lancet Psychiatry 2020; 7:52–63Crossref, Medline, Google Scholar
22. : Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry 2010; 32:519–543Crossref, Medline, Google Scholar
23. : Differences in the prescribing of medication for physical disorders in individuals with v without mental illness: meta-analysis. Br J Psychiatry 2012; 201:435–443Crossref, Medline, Google Scholar
24. : Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry 2009; 194:491–499Crossref, Medline, Google Scholar
25. : Revascularisation and mortality rates following acute coronary syndromes in people with severe mental illness: comparative meta-analysis. Br J Psychiatry 2011; 198:434–441Crossref, Medline, Google Scholar
26. : Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry 2013; 170:324–333Link, Google Scholar
27. : Comorbid psychiatric disease is associated with lower rates of thrombolysis in ischemic stroke. Stroke 2018; 49:738–740Crossref, Medline, Google Scholar
28. : Consideration of confounding was suboptimal in the reporting of observational studies in psychiatry: a meta-epidemiological study. J Clin Epidemiol 2020; 119:75–84Crossref, Medline, Google Scholar
29. : Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatry 2019; 76:1241–1255Crossref, Medline, Google Scholar
30. : The role of meta-analyses and umbrella reviews in assessing the harms of psychotropic medications: beyond qualitative synthesis. Epidemiol Psychiatr Sci 2018; 27:537–542Crossref, Medline, Google Scholar
31. : Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4:1Crossref, Medline, Google Scholar
32. : Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-Analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012Crossref, Medline, Google Scholar
33. : The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2012 (http//www.ohri.ca/programs/clinical_epidemiology/oxford.asp)Google Scholar
34. : Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188Crossref, Medline, Google Scholar
35. : Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28:105–114Crossref, Medline, Google Scholar
36. : Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560Crossref, Medline, Google Scholar
37. : Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634Crossref, Medline, Google Scholar
38. : Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463Crossref, Medline, Google Scholar
39. : Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 2012; 42:125–147Crossref, Medline, Google Scholar
40. : Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol 2010; 24(4 suppl):69–80Crossref, Medline, Google Scholar
41. : Mental disorders and quality of diabetes care in the Veterans Health Administration. Am J Psychiatry 2002; 159:1584–1590Link, Google Scholar
42. : Disparities in diabetes care: impact of mental illness. Arch Intern Med 2005; 165:2631–2638Crossref, Medline, Google Scholar
43. : Metabolic syndrome in people with schizophrenia: a review. World Psychiatry 2009; 8:15–22Crossref, Medline, Google Scholar
44. : Cardio-metabolic risk in individuals prescribed long-acting injectable antipsychotic medication. Psychiatry Res 2019; 281:112606Crossref, Medline, Google Scholar
45. : Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders: a systematic review and meta-analysis. Schizophr Bull 2013; 39:306–318Crossref, Medline, Google Scholar
46. : Change in psychiatrists’ attitudes towards the physical health care of patients with schizophrenia coinciding with the dissemination of the consensus on physical health in patients with schizophrenia. Eur Psychiatry 2011; 26:305–312Crossref, Medline, Google Scholar
47. : Association between perceived health status and satisfaction with quality of care: evidence from users of primary health care in Oman. Fam Pract 2004; 21:519–527Crossref, Medline, Google Scholar
48. : Better practices in collaborative mental health care: an analysis of the evidence base. Can J Psychiatry 2006; 51(suppl 1):7S–72SMedline, Google Scholar
49. : Variables associated with general practitioners taking on serious mental disorder patients. BMC Fam Pract 2009; 10:41Crossref, Medline, Google Scholar
50. : Stigmatisation of those with mental health conditions in the acute general hospital setting: a qualitative framework synthesis. Soc Sci Med 2020; 255:112974Crossref, Medline, Google Scholar
51. : Medical comorbidity and receipt of medical care by older homeless people with schizophrenia or depression. Psychiatr Serv 2002; 53:1456–1460Link, Google Scholar
52. : Treatment following myocardial infarction in patients with schizophrenia. PLoS One 2017;12:e0189289Crossref, Medline, Google Scholar
53. : Emergency department staff views and experiences on diagnostic overshadowing related to people with mental illness. Epidemiol Psychiatr Sci 2013; 22:255–262Crossref, Medline, Google Scholar
54. : Diagnostic overshadowing and other challenges involved in the diagnostic process of patients with mental illness who present in emergency departments with physical symptoms: a qualitative study. PLoS One 2014; 9:e111682Crossref, Medline, Google Scholar
55. : Disparities in diabetes and hypertension care for individuals with serious mental illness. Am J Manag Care 2017; 23:304–308Medline, Google Scholar
56. : Quality of care for cardiovascular disease and diabetes amongst individuals with serious mental illness and those using antipsychotic medications. J Healthc Qual 2012; 34:15–21Crossref, Medline, Google Scholar
57. : Undiagnosed cardiovascular disease prior to cardiovascular death in individuals with severe mental illness. Acta Psychiatr Scand 2019; 139:558–571Crossref, Medline, Google Scholar
58. : Association of secondary preventive cardiovascular treatment after myocardial infarction with mortality among patients with schizophrenia. JAMA Psychiatry 2018; 75:1234–1240Crossref, Medline, Google Scholar
59. : Access to primary and specialized somatic health care for persons with severe mental illness: a qualitative study of perceived barriers and facilitators in Swedish health care. BMC Fam Pract 2018; 19:12Crossref, Medline, Google Scholar
60. : Severe mental illness and health service utilisation for nonpsychiatric medical disorders: a systematic review and meta-analysis. PLoS Med 2020; 17:e1003284Crossref, Medline, Google Scholar
61. : The impact of pharmacological and non-pharmacological interventions to improve physical health outcomes in people with schizophrenia: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019; 18:53–66Crossref, Medline, Google Scholar



