Examining and Modulating Neural Circuits in Psychiatric Disorders With Transcranial Magnetic Stimulation and Electroencephalography: Present Practices and Future Developments
Abstract
Transcranial magnetic stimulation (TMS) is a noninvasive brain stimulation technique uniquely equipped to both examine and modulate neural systems and related cognitive and behavioral functions in humans. As an examination tool, TMS can be used in combination with EEG (TMS-EEG) to elucidate directly, objectively, and noninvasively the intrinsic properties of a specific cortical region, including excitation, inhibition, reactivity, and oscillatory activity, irrespective of the individual’s conscious effort. Additionally, when applied in repetitive patterns, TMS has been shown to modulate brain networks in healthy individuals, as well as ameliorate symptoms in individuals with psychiatric disorders. The key role of TMS in assessing and modulating neural dysfunctions and associated clinical and cognitive deficits in psychiatric populations is therefore becoming increasingly evident. In this article, the authors review TMS-EEG studies in schizophrenia and mood disorders, as most TMS-EEG studies to date have focused on individuals with these disorders. The authors present the evidence on the efficacy of repetitive TMS (rTMS) and theta burst stimulation (TBS), when targeting specific cortical areas, in modulating neural circuits and ameliorating symptoms and abnormal behaviors in individuals with psychiatric disorders, especially when informed by resting-state and task-related neuroimaging measures. Examples of how the combination of TMS-EEG assessments and rTMS and TBS paradigms can be utilized to both characterize and modulate neural circuit alterations in individuals with psychiatric disorders are also provided. This approach, along with the evaluation of the behavioral effects of TMS-related neuromodulation, has the potential to lead to the development of more effective and personalized interventions for individuals with psychiatric disorders.
Noninvasive brain stimulation is a set of techniques that can be used to target brain circuits in vivo transcranially (1). Among noninvasive brain stimulation techniques, transcranial magnetic stimulation (TMS) is uniquely equipped to both examine and modulate neural systems and related cognitive and behavioral functions in humans (2).
As a probe, TMS can be used in combination with EEG and functional MRI (fMRI). TMS with concurrent fMRI presents several challenges, including the synchronization of TMS and fMRI signals, the effects of the magnetic field of the MR scanner on the TMS coil and the TMS-generated magnetic field, the difficulty of TMS coil positioning and brain targeting inside the scanner, and the need to have access to an MR scanner and fMRI-compatible TMS coils, which makes TMS with concurrent fMRI feasible only in a few specialized research centers (3, 4). In contrast, the availability of TMS with simultaneous EEG (TMS-EEG) has grown over the past several years. TMS-EEG offers the opportunity for investigating the activity and connectivity of neuronal circuits across various behavioral and pathophysiological states (5). TMS-EEG also provides certain advantages compared with traditional electrophysiological studies. First, EEG recordings collected using peripheral stimuli reach the cortical areas contributing to the scalp-recorded signal after several synaptic relays, and EEG measured during a task can be affected by participant motivation and level of cognitive engagement. TMS-EEG, however, can be used to elucidate directly, objectively, and noninvasively the intrinsic properties of a specific cortical region: excitation, inhibition, reactivity, and oscillatory activity, including its power, synchronization, and main oscillatory frequency, or natural frequency, irrespective of the participant’s conscious effort (6). This can help determine the neurophysiological properties of a given cortical area in healthy individuals, as well as characterize how these properties may differ across different psychiatric disorders (7). Second, TMS-EEG can elucidate causal relationships between neural regions—that is, the effect of one cortical area on the rest of the brain on a temporal scale that approximates neuronal activity (8). Hence, TMS-evoked EEG responses can be used to identify biological markers of brain health and disease, as well as to examine the functional integrity of neural circuits.
In addition to being utilized in combination with EEG as a probe, TMS can be applied in repetitive patterns to modulate brain networks in healthy individuals (9), as well as to ameliorate symptoms in individuals affected by psychiatric disorders, especially major depressive disorder (10, 11). TMS can thus be used to induce acute changes in neural circuits while assessing, with EEG and cognitive tasks, the impact of these changes on neuronal and behavioral measures (12). This approach can in turn elucidate understanding of neural circuit-behavior relationships related to learning and memory (13, 14). A parallel approach, which pertains to psychiatric disorders, is to investigate more sustained effects of TMS on symptoms, which likely relies on the modulation of underlying neural circuits (15). The key role of TMS in assessing and modulating neural dysfunctions and associated clinical and cognitive deficits in psychiatric populations is therefore becoming increasingly evident (16).
In this review, we provide an overview of the different uses that TMS has in the examination of neural circuit dysfunction and neural circuit-behavioral relationships in psychiatric disorders. After providing a brief description of the origin of TMS, we review TMS-EEG studies in schizophrenia and mood disorders, as most TMS-EEG studies to date have focused on individuals with these disorders. We present the evidence on the efficacy of stimulation paradigms, including repetitive TMS (rTMS) and theta burst stimulation (TBS), when targeting specific cortical areas, in modulating neural circuits and in ameliorating symptoms and abnormal behaviors in individuals with psychiatric disorders, especially when informed by resting-state and task-related neuroimaging measures. We also provide some examples of how the combination of TMS-based assessments (i.e., TMS-EEG) and rTMS and TBS paradigms can be utilized to both characterize and modulate neural circuit alterations in individuals with psychiatric disorders. This approach, along with the evaluation of the behavioral effects of TMS-related neuromodulation, has the potential to lead to the development of more effective interventions for individuals with psychiatric disorders.
THE ORIGIN OF TMS
TMS involves delivering brief, time-varying currents through insulated wires in an induction coil resting over the scalp. The resulting time-varying magnetic field, according to Faraday’s law, produces a secondary electrical current in underlying cortical neurons but does not usually reach deep brain structures. Although the exact neuronal substrate (i.e., axonal fibers versus neuronal cell body or dendrites) has yet to be fully established, the TMS-induced electric field enhances neuronal cortical excitability and, if powerful enough, leads to neuronal discharge. Indeed, TMS was introduced by Anthony T. Baker (17) in 1985 as a tool to noninvasively investigate the functional properties of the motor corticospinal pathways in humans. Specifically, applying TMS to the motor cortex (e.g., hand motor area) can produce action potentials in a peripheral muscle (e.g., abductor pollicis brevis), which is described as motor-evoked potentials (MEPs). MEP amplitude, which depends on cortical, corticospinal, and spinal-muscular excitability, is a straightforward measure of corticospinal excitability, and over the past three decades, various TMS and MEP paradigms have been developed to assess excitation, inhibition, and plasticity of the motor cortex in both healthy individuals and individuals with psychiatric disorders (7, 18). For example, the resting motor threshold (RMT), which is considered a measure of motor corticospinal excitability, is the minimum TMS intensity needed to produce an MEP amplitude ≥50 µV in five out of 10 trials in a peripheral hand muscle at rest (18). Regarding motor cortical inhibition, short-interval intracortical inhibition (SICI) compares the MEP amplitude of a single, suprathreshold TMS test stimulus to a paired-pulse condition with a subthreshold conditioning stimulus followed by a suprathreshold test stimulus after 2-ms to 5-ms intervals, whereas long-interval cortical inhibition (LICI) compares a suprathreshold test stimulus with a paired-pulse suprathreshold conditioning stimulus and test stimulus at 50-ms to 200-ms intervals (19). Another measure of motor cortical inhibition, the silent period, involves measuring the duration of absent muscle activity following a single, suprathreshold test stimulus given during a muscle contraction. Intracortical facilitation involves comparing a suprathreshold test stimulus with a paired-pulse subthreshold conditioning stimulus and suprathreshold test stimulus at 7-ms to 30-ms intervals (20).
Although TMS-MEP paradigms have been extremely helpful in characterizing the neurophysiological properties of the motor cortex in healthy individuals, as well as in identifying motor cortical abnormalities in psychiatric populations (as reviewed elsewhere; see 7, 19), these protocols could not provide direct information about other cortical areas.
TMS-EEG: THE TECHNIQUE
The first demonstration of the feasibility of combining TMS with simultaneous EEG was provided by Cracco and colleagues (21) in 1989 by delivering TMS to the frontal cortex and measuring the EEG response in the contralateral homologous site. In that study, TMS evoked a contralateral positive EEG component with an onset latency of 8.8–12.2 ms, a duration of 7–15 ms, and an amplitude that reached up to 20 µV, thus providing initial evidence of human transcallosal responses to TMS. A few years later, Ilmoniemi and colleagues (22) showed that TMS-EEG could be utilized to measure the local and long-distance cortical responses evoked by single-pulse TMS of either motor or occipital areas. Building on this pioneering work, several TMS-EEG systems have been developed more recently to overcome the saturation of the EEG amplifiers caused by the large TMS-induced voltage that exceeds the 5-mV limit of conventional amplifiers. A sample-and-hold circuit, which involves blocking the input of the EEG amplifier from 50 µs prior to 2.5 ms after the TMS pulse while maintaining the voltage constant during this interval, is an effective way to prevent the EEG amplifier saturation (23). A direct current amplifier, which combines a high sampling rate (e.g., >5 KHz) with a wide operational range (e.g., >5 mV), is also adequate because the amplifiers can absorb the TMS stimulus without being saturated. In addition, given the short duration of the TMS pulse (<1 ms), a preamplifier that limits the rate of voltage change can be utilized to couple TMS with simultaneous EEG without amplifier saturation (24). TMS-EEG can be applied to virtually any cortical area. As a result, concurrent TMS-EEG is a powerful tool to investigate the neuronal properties of neural regions and circuits beyond the motor cortex.
When performing TMS-EEG experiments, 60–200 TMS stimuli should be delivered for each session to obtain a good signal-to-noise ratio and to ensure test-retest reliability of EEG responses (25). The intensity of stimulation can be determined as a percentage of the resting motor threshold (%RMT). While %RMT is usually used in TMS-EEG protocols, another approach involves using a TMS neuronavigational system and adjusting in real time the intensity of stimulation on the basis of the estimated electric field (expressed as V/m) generated by the TMS in the brain areas of interest. For example, an electric field corresponding to a %RMT can be determined in the motor cortex, and the same intensity can be applied to a nonmotor cortical area. A neuronavigational system can also be utilized to more precisely identify and target a given cortical area, which otherwise can be indirectly inferred from EEG scalp electrode placement. (For a more detailed description of the TMS-EEG methodology, see reference 5.)
TMS-EEG TO EXAMINE NEURAL CIRCUITS IN HEALTHY INDIVIDUALS
TMS-evoked EEG potentials (TEPs) consist of several peaks and troughs at specific latencies that last several hundred milliseconds after the TMS pulse (Figure 1). In healthy individuals, TEPs can be utilized for several purposes, including measuring cortical inhibition and excitation, assessing cortical oscillatory activity, and examining cortico-cortical connectivity.
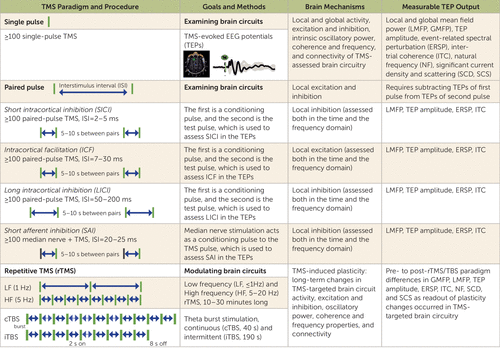
FIGURE 1. Transcranial magnetic stimulation (TMS) as a tool to examine and modulate brain circuitsa
a The chart summarizes TMS paradigms (single-pulse, paired-pulse, and repetitive TMS [rTMS]) and procedures (number of stimuli, interstimulus interval), goals (probing or modulating), and methods (collecting TMS-evoked EEG potentials [TEPs]); brain mechanisms (local and global activity, excitation, inhibition, oscillatory activity, and connectivity); and measurable TEP output.
TMS-Assessed Cortical Excitation, Inhibition, and Oscillatory Properties
The amplitude of TEP peaks and troughs obtained with single-pulse TMS protocols can be measured to assess cortical excitation and inhibition. For example, the peak-to-peak amplitude of the N15 and P30 TEP components of the motor cortex was correlated with the amplitude of the MEPs, thus representing a measure of motor cortical excitability (26). On the other hand, TMS-EEG experiments conducted during pharmacological manipulation have shown that N45 and N100 reflect cortical inhibition, representing GABAA and GABAB inhibitory activity, respectively (27). Additionally, measuring the area under the curve of the rectified TEP signal can provide information about the overall TMS-evoked mean field power, which can be calculated locally (local mean field power), which is where the TMS is applied, or globally (global field mean power). Local mean field power, which is also called cortical-evoked activity, and global field mean power are less affected by variability in the width and amplitude of TEPs in providing a quantification of overall brain circuit activity, but they ignore the polarity of the signals, which complicates the discrimination between excitatory and inhibitory effects. Building on previous single-pulse and paired-pulse TMS and MEP paradigms to investigate motor cortex excitation and inhibition, several TMS-EEG protocols—namely, LICI, SICI, intracortical facilitation, and short-latency afferent inhibition—have been developed to assess inhibition and excitation in motor and nonmotor cortical areas (Figure 1). LICI occurs when two suprathreshold TMS stimuli are applied between 50 ms and 200 ms, is characterized by a reduction in the response to the second stimulus 50–150 ms after TMS, and is thought to reflect GABAB neurotransmission (19). A suppression of several TEP components following LICI has been observed in both motor and prefrontal cortical areas in healthy individuals (27, 28). In SICI, a first lower-intensity stimulus inhibits a second higher-intensity TMS pulse at an interstimulus interval of 2–5 ms, whereas longer interstimulus intervals (7–30 ms) result in intracortical facilitation. SICI is associated with GABAA activity, while intracortical facilitation relies on both GABAA and N-methyl-d-aspartate (NMDA) neurotransmission (29). TMS-EEG studies have shown that early TEP components are decreased after SICI and increased following intracortical facilitation in both motor and prefrontal cortical areas (30, 31). A TMS paradigm of short-latency afferent inhibition involves the combination of median nerve stimulation with TMS delivered at an interstimulus interval of 20–25 ms and is mainly related to cholinergic and GABAergic activity (29). TMS-EEG studies of short-latency afferent inhibition have shown a modulation of early components of TEPs in both motor and prefrontal areas in healthy individuals (32). Altogether, findings from these TMS-EEG studies have begun to reveal the neuronal and molecular mechanisms regulating the balance between excitation and inhibition within human cortical areas.
TMS-EEG has also been utilized to assess modulations of cortical excitability and cortical plasticity. Specifically, one study measured the TMS-evoked EEG responses before and after a single dose of levodopa, a compound that is used as a dopamine replacement agent in the treatment of Parkinson’s disease. After levodopa intake, an increase was found in cortical excitability in the supplementary motor areas, but not in the superior parietal lobule, that was greater on the more affected side of the brain (33). In another study, in which TMS-EEG recordings were performed before and after anodal or sham transcranial direct current stimulation (tDCS) coupled with a verbal fluency task in healthy individuals, an increase in TMS-evoked EEG responses occurred only after anodal tDCS. This increase was observed in areas involved in language production and was associated with the degree of cognitive enhancement (34).
TMS-EEG can also be used to assess the oscillatory properties, including power and synchronization, of different cortical areas. Neuronal oscillations are phylogenetically preserved and reflect the physical architecture of neuronal networks, including the number of excitatory and inhibitory interneurons, and their functional characteristics (35). Increasing evidence indicates that neuronal oscillations are critically implicated in human cognition and behavior (36) and that aberrant rhythmic oscillatory activity is commonly observed in psychiatric patients (35, 37). Cortical oscillations tend to occur in specific frequency bands, and their activity and level of synchronization may vary across behavioral states and cognitive processes. Therefore, while TMS-related measures of cortical excitation and inhibition are mostly collected in the time domain as an increase or decrease in the amplitude of TEP components, investigations of cortical oscillations are primarily performed in the frequency domain. The event-related spectral perturbation is the parameter most commonly computed to measure the TMS-related activity in a given frequency band, whereas the intertrail coherence provides an assessment of the synchronization of the TMS-evoked EEG response across trials (see Figure S1 in the online supplement). Additionally, by directly probing the cortical surface, TMS-evoked EEG responses can help determine the main oscillatory frequency, or natural frequency, of cortical circuits. TMS-EEG studies conducted by our group and other groups have characterized the oscillatory properties of various cortical areas and demonstrated that each of these areas oscillates at a preferred natural frequency, specifically the alpha band (8–12 Hz) for the occipital cortex (38), the low beta range (15–19 Hz) for the parietal cortex (38, 39), and the fast beta and gamma bands for the frontal cortical regions, including the motor (20–24 Hz) (39), premotor (25–29 Hz) (38, 39), and prefrontal (≥30 Hz) (39) cortices.
TMS as a Probe of Cortico-Cortical Effective Connectivity
TMS-EEG also allows the study of neural regional connectivity with enhanced temporal resolution. Neuroimaging techniques, including fMRI and positron emission tomography, rely on changes in blood flow that can be measured every few seconds, whereas TMS-evoked responses can be characterized at the millisecond scale, the timing of neuronal activity. Additionally, while neuroimaging signals are based on temporal correlations of vascular (blood-oxygen-level-dependent signal, regional cerebral blood flow) activities across different neural regions, therefore providing a measure of functional connectivity, TMS-evoked EEG responses can measure effective connectivity, which is the ability of a cortical area to influence the activity of other brain regions, thereby assessing the putative directionality and causality of changes in activation. Effective connectivity can be measured through the spatio-temporal dissemination of TEPs or TMS-evoked oscillatory activity (40). Cortical areas involved in TMS-assessed effective connectivity can be identified using EEG source localization. Source localization refers to the attempt to identify the neuronal sources underlying scalp-recorded EEG signals, which is also described as solving the inverse problem of EEG. Because the possible neuronal sources are far more than the EEG voltages recorded, the inverse problem does not have a unique solution and therefore represents the best approximation based on the available data. In addition, neighboring scalp electrodes tend to record similar time series as a result of volume conduction through nonexcitable tissue between depolarizing neurons and recording electrodes, thus creating artificial common sources. Also, the same sensor can record activity from multiple neuronal sources, such that two instantaneously interacting (i.e., zero-phase lag) sources are difficult to distinguish from a single source whose activity is recorded by the same scalp electrode (41). These challenges can be mitigated by performing high-density (>60 channels) EEG recordings, utilizing individual MRI, and employing data analysis tools, such as debiased weighted phase lag index (42). Source-modeling measures can therefore be used to identify the brain regions underlying scalp-recorded EEG signals, and these measures have been introduced to quantify the distribution of TMS-evoked cortical currents (significant current density), as well as the propagation of cortical currents (significant current scattering) (43).
rTMS and TBS as Neuromodulatory Paradigms for Neural Circuits in Healthy Individuals
TMS can be employed to modulate the activity of neural circuits. This is usually achieved through repeated stimulation paradigms, which include rTMS and TBS.
rTMS- and TBS-Induced Changes in Neural Circuits Assessed With EEG and Behavioral Measures
rTMS can induce modifications of synaptic efficacy that outlast the period of stimulation, which in turn determines changes in local cortical excitability. Evidence from pharmacological and animal studies indicates that rTMS affects the neural processes involved in the initiation and maintenance of synaptic plasticity, especially the long-term potentiation and long-term depression of excitatory synaptic transmission (44, 45). The molecular mechanisms associated with TMS-induced changes likely involve NMDA receptors located on the postsynaptic membrane (46, 47). Long-term-potentiation-like effects are thought to occur through a rapid postsynaptic increase in Ca+ influx followed by increased gene and protein expression, whereas a small and slow flow of calcium ions induces long-term-depression-like effects by reducing postsynaptic neuronal activity (47). When applied over the motor cortex, low-frequency rTMS (≤1 Hz) usually decreases, whereas high-frequency rTMS (≥5 Hz) increases motor responsiveness, as assessed with MEPs (18) and motor performance in both healthy individuals (48) and neuropsychiatric patients (49). The inhibitory effects of low-frequency rTMS have recently been confirmed by a TMS-EEG study of healthy individuals. Specifically, the amplitude of GABA-regulated TMS-evoked early-negative EEG components was increased, and the amplitude of the MEPs was decreased, after 1-Hz rTMS of the motor cortex, thus suggesting motor cortical inhibition (50). It is important, however, to take into account that there is a significant across-day variability of rTMS effects, as shown by a study examining the modulation of corticospinal excitability at various frequencies (1, 10, and 20 Hz) and at different time points in healthy subjects (51). The modulatory effects of rTMS can also be observed in neural regions anatomically connected to the target area, as shown by another TMS-EEG study establishing that 5-Hz rTMS applied to the motor cortex increased the amplitudes of TEPs in premotor cortices bilaterally (52). Several animal and imaging studies have also shown that long-distance rTMS effects are mediated through white matter connectivity and that rTMS can be utilized to modulate local oscillations and interregional synchrony, as reported in an elegant review (53).
TBS is a repetitive TMS protocol that employs short bursts (three pulses at 50 Hz) delivered at a frequency of 5 Hz that can both increase and decrease the excitability of cortical neurons (54). Such theta burst leads to a short-latency facilitation, likely related to the (fast) rate of postsynaptic calcium inflow, with a longer latency and weaker inhibition due to the overall amount of calcium entry (55). TBS can be applied intermittently (intermittent TBS, or iTBS), which consists of 20 2-second trains interleaved with periods of silence (approximately 8 seconds), for a total duration of approximately 190 seconds, or continuously (continuous TBS, or cTBS), for a total of 40 seconds, both of which are significantly shorter than rTMS paradigms, thus allowing for TBS to induce more rapid effects on neural activity than conventional rTMS (56). When applied to the motor cortex, iTBS leads to an increase, whereas cTBS determines a decrease, in corticospinal excitability as reflected in changes in MEP amplitude (57). A reduction in the TMS-evoked EEG responses, assessed both in the time domain (local mean field power) and the frequency domain (event-related spectral perturbation, intertrail coherence), has been observed in the theta frequency range following cTBS of the motor cortex (58). In contrast, iTBS of the dorsolateral prefrontal cortex (DLPFC) increased both the amplitude of early TEPs (i.e., N120) and the power of TMS-evoked theta oscillations, assessed with single-pulse TMS, as well as the LICI of theta oscillations, evaluated with paired-pulse TMS, in healthy individuals (59). Two recent studies showed that cTBS to the somatosensory cortex interfered with normal sensory function, and it blocked motor memory consolidation but not the ability to retrieve a consolidated motor memory (60), and that cTBS of the visual cortex, but not of a control region (vertex), applied immediately after the offset of a visual task training, interfered with the consolidation of visual perceptual learning (61). Together, these findings suggest that this approach can elucidate understanding of neural circuit-behavior relationships. It is important, however, to point out that the excitatory effects of high-frequency rTMS and iTBS and the inhibitory effects of low-frequency rTMS and cTBS have been observed primarily in the motor cortex with group-level analyses, and therefore their ultimate effects on individual subjects and nonmotor cortical areas still need to be fully established. Furthermore, one limitation of this approach is that the TMS frequency refers to the repetition rate of a given stimulation pulse that in turn has a specific pulse width; thus, TMS does not represent an ideal method to entrain a given oscillation.
TMS AS AN EXAMINATION AND A NEUROMODULATORY TOOL IN PSYCHIATRY
TMS has been utilized to establish dysfunctions of the excitatory and inhibitory properties, the oscillatory activity, and the connectivity of cortical areas in individuals with psychiatric disorders (Box 1). As a result of their ability to induce changes in the activity and connectivity of neural circuits that outlast the duration of stimulation, rTMS and, more recently, TBS have been increasingly utilized in psychiatric populations as a potential treatment (11). In addition, because ECT is arguably the most effective intervention for several treatment-resistant psychiatric disorders, including schizophrenia and mood disorders, noninvasive brain stimulation techniques, such as rTMS and TBS, have emerged as promising treatment options that require less intense stimulation than ECT while also providing more focal interventions and greater specificity of the neural targets being stimulated, although prospective, properly randomized clinical trials comparing the efficacy of these interventions are needed for psychiatric patients (Box 1). Here we focus on TMS findings in schizophrenia and mood disorders, the psychiatric disorders most studied using this technique.
BOX 1. Transcranial magnetic stimulation (TMS): current practices in psychiatry
TMS as a Probe
TMS-assessed EEG abnormalities of cortical excitation (intracortical facilitation), inhibition (short-interval intracortical inhibition, long-interval cortical inhibition, and short-latency afferent inhibition), and oscillatory activity (event-related spectral perturbation, intertrail coherence, and natural frequency) of different cortical areas (parietal, motor, premotor, and prefrontal) in psychiatric patients
TMS-related EEG measures of altered cortico-cortical connectivity (significant current scattering and significant current density) in psychiatric populations
TMS as a Treatment Tool
Repetitive TMS (rTMS) (low frequency and high frequency) and theta-burst stimulation (TBS) (continuous TBS and intermittent TBS), sham-controlled paradigms applied in different combinations (ipsilateral, bilateral simultaneous, bilateral sequential, and priming) to ameliorate clinical symptoms in psychiatric patients
Symptom improvement is the target and the main outcome measure, although baseline resting-state functional MRI functional connectivity patterns are being increasingly used both to guide treatment and to assess treatment response.
TMS Findings in Schizophrenia
Schizophrenia is characterized by positive (i.e., hallucinations, delusions), negative (i.e., emotional and social withdrawal), and cognitive symptoms, with a lifetime prevalence of approximately 1% (62). Although many brain regions and molecular mechanisms are implicated in the pathophysiology of this disorder, including abnormalities in the mesocortical dopaminergic pathway (63) and in hippocampal (64) and thalamocortical (64) glutamatergic activity, converging postmortem and electrophysiological evidence points toward alterations in frontal-prefrontal cortical areas and in GABAergic neurotransmission (65, 66). Specifically, several human postmortem studies of individuals with schizophrenia have consistently reported molecular abnormalities in GABAergic cortical inhibitory neurons (67). Additionally, aberrant fast, beta, and gamma oscillations, which are thought to be generated and modulated by GABAergic neurotransmission (68), have been reported in numerous studies of patients with schizophrenia (37). Building on these findings, TMS-EEG has been increasingly employed to directly assess the intrinsic properties of local cortical neurons, as well as cortico-cortical connectivity, while rTMS and TBS paradigms have been utilized to modulate these cortical properties in schizophrenia, as described below.
TMS-Assessed Abnormalities of Cortical Excitation, Inhibition, and Oscillatory Activity in Schizophrenia
Among TMS and MEP findings, RMT and MEP amplitudes are the most reported measures of motor cortical excitability. In a meta-analysis of 21 studies, RMT did not differ between patients with schizophrenia (N=500) and healthy individuals (N=617) across studies (69). Similarly, MEP amplitude did not differ between patients with schizophrenia and healthy control subjects across eight studies (7). In addition, no differences were found in intracortical facilitation in individuals at risk of developing schizophrenia, in individuals with first-episode psychosis, and in both medicated and unmedicated chronic schizophrenia groups compared with healthy control groups (7, 19). TMS and MEP studies have also investigated motor cortical inhibition, and reduced SICI has been reported in patients at various stages of illness, including in individuals at risk of developing schizophrenia, patients with first-episode psychosis, patients with recent-onset psychosis, and patients with chronic illness (19).
Several TMS-EEG studies have characterized cortical alterations in patients with schizophrenia. For example, in an initial TMS-EEG study, we showed a reduction in the global field mean power of early TEPs, as well as TMS-assessed oscillatory properties, including amplitude, assessed with event-related spectral perturbation, and in synchronization, assessed with intertrail coherence, of the premotor cortex in individuals with schizophrenia compared with healthy individuals (70). In a follow-up study in which we investigated the oscillatory properties of four cortical areas (parietal, motor, premotor, and prefrontal cortices), individuals with schizophrenia had a reduction in TMS-related amplitude (event-related spectral perturbation) and synchronization (intertrail coherence) of beta and gamma oscillations in frontal and prefrontal regions, but not in parietal regions, compared with healthy individuals (39). Additionally, individuals with schizophrenia showed a slowing in the natural frequency of frontal and prefrontal regions compared with healthy individuals (from a mean 2-Hz decrease for the motor area to an almost 10-Hz decrease for the prefrontal cortex), to the extent that the prefrontal natural frequency of individuals with schizophrenia was slower than that of any healthy individual. These findings point toward intrinsic abnormalities in frontal and prefrontal cortical neurons in schizophrenia, which are likely mediated by alterations in GABAergic activity. Specifically, modeling and in vivo electrophysiological animal studies have demonstrated that inhibiting fast-spiking GABAergic interneurons suppressed power and synchronization of gamma oscillations, whereas driving these interneurons was sufficient to generate gamma band rhythmicity (71, 72). Abnormalities in prefrontal cortical inhibition have been reported in other TMS-EEG studies, which have shown a reduced inhibition of gamma oscillations induced by LICI in the DLPFC, but not in the motor cortex, of individuals with schizophrenia and their first-degree relatives compared with healthy individuals (73).
TMS as a Probe of Altered Effective Connectivity in Schizophrenia
TMS-evoked EEG responses have also been utilized to study alterations in cortico-cortical connectivity in schizophrenia. One TMS-EEG study examining the motor cortex showed an aberrant, widespread pattern of propagation in individuals with schizophrenia, which was observed for several hundred milliseconds after the TMS pulse (i.e., between 400 ms and 700 ms post-TMS) and was characterized by an increase in global TMS-evoked voltage activity and enhanced fast oscillations in fronto-parietal regions, compared with healthy individuals (74). By targeting the premotor cortex in both schizophrenia patients and control subjects, and by performing source-modeling analysis of the TMS-evoked EEG responses, we previously demonstrated that in healthy individuals, TMS-evoked cortical activity propagated from the premotor to other functional and anatomically connected cortical areas (right sensorimotor areas and left premotor and sensorimotor regions), whereas in individuals with schizophrenia, the evoked activity was mostly localized to the stimulated area (70). In addition, by utilizing source-based measures of TMS-evoked cortical activity (significant current density) and connectivity (significant current scattering), there were reductions in significant current density and significant current scattering in premotor and prefrontal areas, which were associated with impaired cognitive function in individuals with schizophrenia compared with healthy individuals (75).
rTMS and TBS as Interventions in Schizophrenia
Low-frequency 1-Hz rTMS of the left temporo-parietal cortex has been the most consistently used paradigm to treat auditory hallucinations in schizophrenia. Auditory hallucinations, a core symptom of schizophrenia, are thought to emerge from hyperactivation in temporo-parietal regions involved in auditory and speech processing, as shown in electrophysiological and neuroimaging studies (76). A recent meta-analysis comparing active and sham stimulation reported a medium effect size (effect size=0.51) and found that younger age, female gender, higher antipsychotic doses, brief trial duration (<3 week), and shorter scalp-to-temporal cortex distance predicted a better response to treatment (77). However, the improvement of auditory hallucinations was relatively short-lasting (4–6 weeks) and did not extend to other psychotic symptoms. Additionally, other rTMS protocols, including cTBS, have failed to show a consistent improvement of auditory hallucinations or related psychotic symptoms (15).
Negative symptoms, another core feature of schizophrenia, are often resistant to treatment with antipsychotics. Evidence regarding the efficacy of rTMS for negative symptoms is mixed. Two meta-analyses showed that active rTMS (≥5 Hz) was more effective than sham rTMS, corresponding to small to medium effect sizes (range, 0.49–0.64) (77, 78), whereas a third meta-analysis (79) found no differences between these two interventions. In a recent randomized clinical trial in which schizophrenia patients received 20 sessions of active or sham rTMS of the left prefrontal cortex at 20 Hz over 4 weeks, the improvement in negative symptoms (anhedonia, alogia, avolition, and attention impairment) in the active treatment group was statistically significant compared with the sham group (80). Notably, most of these studies targeted the prefrontal cortex, given that several neuroimaging studies have shown hypometabolism and hypoperfusion of prefrontal regions in patients experiencing negative symptoms (81). One promising alternative approach, based on the observation that the functional connectivity of the cerebellum with the right prefrontal cortex was inversely associated with negative symptom severity, has been to target prefrontal activity by modulating the cerebellum. In a recent study in patients with schizophrenia, iTBS of the cerebellum resulted in improvement of negative symptoms and reversal of cerebellar-prefrontal functional dysconnectivity (82).
Cognitive deficits represent one of the most persistent treatment-resistant features of schizophrenia. Most neuromodulation studies have employed high-frequency rTMS (10–20 Hz) to the left or both the left and right DLPFC, given the critical role of this region in the cognitive dysfunctions of schizophrenia (83). Pilot data from a randomized clinical trial showed that 20-Hz active rTMS over 4 weeks was associated with significant improvement in working memory compared with sham rTMS in individuals with schizophrenia (84). Another pilot study found that individuals with schizophrenia receiving bilateral 20-Hz active rTMS for 2 weeks, compared with sham stimulation, showed an improvement on a standardized cognitive battery both immediately following treatment and 2 weeks after treatment (85). In contrast, a large multicenter study found no benefits of 3-week left-prefrontal 10-Hz stimulation, when compared with sham stimulation, in individuals with schizophrenia (86). A recent meta-analysis of cognitive benefits with rTMS in schizophrenia showed significant efficacy of high-frequency rTMS on working memory when compared with sham stimulation, corresponding to an effect size of 0.34, and the effect persisted into the 1-month follow-up assessment (87). Overall, despite some positive findings, the relatively small degree of improvement, restricted to specific cognitive domains, shows the need to develop novel neuromodulation protocols, including TBS paradigms, to effectively enhance cognitive function in individuals with schizophrenia.
TMS FINDINGS IN BIPOLAR DISORDER AND MAJOR DEPRESSIVE DISORDER
Bipolar disorder comprises a group of brain disorders characterized by a history of manic or hypomanic episodes, periods of elevated or irritable mood, and energized behaviors (88). With a lifetime prevalence >2%, bipolar disorder represents one of the leading causes of disability worldwide (89). In a critical review of neuroimaging findings, we reported that fMRI studies suggest a dysfunction in a ventrolateral prefrontal-hippocampal-amygdala circuit bilaterally, combined with hyperactive left-sided ventral striatal-ventrolateral and orbitofrontal cortical reward-processing circuits, which lead to emotion dysregulation and heightened reward sensitivity, respectively (90). Also, we found that structural imaging findings point toward gray matter volume decreases in the prefrontal and temporal cortices, the amygdala, and the hippocampus, as well as fractional anisotropy decreases in white matter tracts connecting prefrontal and subcortical regions (90).
Major depressive disorder is the most common mood disorder, and it is characterized by depressed mood or loss of interest and pleasure in daily activities, along with several vegetative and psychological symptoms (88). Regarding the pathophysiology of major depressive disorder, the traditional monoamine hypothesis postulates that depression is caused by disrupted dopaminergic, noradrenergic, and serotonergic neurotransmission, as suggested by the antidepressant effects of monoaminergic agents (91). Although this hypothesis remains highly influential, more recent theories have focused on altered neuronal interaction and disturbed glutamatergic and GABAergic neurotransmission as critically implicated in the neurobiology of depression. For example, the neuroplasticity theory of depression hypothesizes that impaired neuroplasticity is the cellular basis of depressed mood and contributes to the cognitive bias and impairments, which are often present in depressed patients (92), while the “synaptogenic hypothesis of depression” postulates that dysfunctional synaptic transmission, involving primarily glutamatergic and GABAergic neurotransmission, is a fundamental element of the pathophysiology of major depressive disorder (93). Clinical studies have shown altered glutamate levels in the serum, CSF, and CNS of depressed patients, along with altered glutamatergic NMDA receptor activity in postmortem analyses (94), and altered GABA concentrations have been reported in the prefrontal cortex of depressed patients using MR spectroscopy (95) and in postmortem studies showing reduction of cortical GABAergic neurons (96).
Because of its ability to serve as both an examining tool and a neuromodulatory tool, TMS can be employed to characterize and possibly ameliorate these neuronal and molecular dysfunctions in patients with bipolar disorder and major depressive disorder (as outlined below).
TMS-Assessed Abnormalities of Cortical Excitation, Inhibition, and Oscillatory Activity in Mood Disorders
Among TMS and MEP findings, in a meta-analysis of excitability measures in patients with major depressive disorder and healthy subjects, no group differences were found in RMT (major depressive disorder patients, N=176; healthy control subjects, N=188) or intracortical facilitation (major depressive disorder patients, N=115; healthy control subjects, N=130) (69). Three of these studies also measured MEP amplitude and reported no differences between patients with major depressive disorder and healthy control subjects (69). In the only TMS-EEG study to our knowledge that involved both individuals with bipolar disorder and those with major depressive disorder, in which the main natural oscillatory frequency of a frontal area (the premotor cortex) was the main outcome measure, both patient populations, along with a group of patients with schizophrenia, showed a reduction in the premotor natural frequency compared with healthy individuals (97). Another study found that individuals with major depressive disorder had a higher cortical reactivity, assessed with the global field mean power, and a stronger inhibitory response, reflected by larger negative peaks, in the DLPFC compared with healthy individuals. Also, these TMS-evoked EEG measures were positively correlated with each other in healthy individuals but not in individuals with major depressive disorder, suggesting an imbalance between excitation and inhibition in major depressive disorder (98). However, it is important to point out that this study did not account for the TMS click auditory response, and a sham condition was not included; thus, its main findings should be replicated in future work employing these TMS control conditions.
TMS as a Probe of Altered Effective Connectivity in Mood Disorders
An increase in DLPFC cortical reactivity, but not in motor or parietal cortical areas, along with increased cortico-cortical connectivity, was also recently reported in youths with major depressive disorder compared with healthy youths, and the increase in cortical reactivity correlated with anhedonia severity in the former (99).
rTMS and TBS as Interventions in Mood Disorders
Among psychiatric disorders, rTMS and TBS paradigms have shown the strongest evidence of efficacy in major depressive disorder. The U.S. Food and Drug Administration approved rTMS in 2008 and TBS in 2018 for the treatment of major depressive disorder. In addition, according to the Canadian Network for Mood and Anxiety Treatments, rTMS is a first-line treatment for individuals who have failed to benefit adequately from at least one antidepressant trial. Major depressive disorder is characterized by metabolic and neuronal activity asymmetry in the two prefrontal areas, which consists of enhanced glucose and oxygen consumption, along with higher EEG activity on the right side and reduced activation on the left side (100). Thus, the most commonly employed stimulation paradigms in major depressive disorder have been high-frequency rTMS (≥10 Hz) targeting the left DLPFC and low-frequency rTMS (≤1 Hz) targeting the right DLPFC. Other TMS treatment paradigms have involved the combination of high-frequency and low-frequency protocols, which can be administered as “bilateral” rTMS, with high frequency and low frequency being applied simultaneously or, more commonly, sequentially to contralateral cortical areas, and “priming”’ rTMS, where an rTMS protocol is applied to the same neural region to boost the effects of a second paradigm (e.g., high frequency before low frequency to augment low-frequency effects) (11).
A network meta-analysis that evaluated the efficacy and tolerability of different TMS protocols with each active treatment compared with sham stimulation (101) found that the treatments that were more effective than sham included high-frequency, low-frequency, bilateral, priming rTMS, and TBS. A meta-analysis specifically assessing the clinical efficacy and safety of rTMS in bipolar depression reported better responses to low-frequency rTMS over the right DLPFC compared with high-frequency rTMS over the left DLPFC when compared with sham stimulation, although the studies showed considerable methodological heterogeneity and included relatively small sample sizes (102). Randomized clinical trials have shown that rTMS paradigms have comparable efficacy to antidepressant treatments, including for individuals with moderate to high degrees of refractoriness (103). Together, these findings indicate that both rTMS and TBS are effective treatment interventions for major depressive disorder, although more evidence is needed to demonstrate their efficacy in individuals with treatment-resistant depression or with bipolar disorder.
CHALLENGES AND OPEN QUESTIONS
To reach its full potential, the combination of TMS-EEG with rTMS and TBS will require addressing the challenges and current limitations of each of these techniques. Regarding TMS-EEG, one challenge is the influence of medications on TMS-evoked activity, given that most individuals with psychiatric disorders are medicated at the time of the assessment. In addition to recruiting unmedicated patients, a way to address this issue involves testing patients in the early stages of illness, when they are still medication naive or minimally treated. In recent work, we showed the feasibility of this approach in individuals with first-episode psychosis (104). Other challenges include ensuring that the TMS coil is properly placed and maintained over the cortical area of interest throughout the TMS-EEG session. The use of TMS neuronavigational devices has been shown to be effective in minimizing this possible confounder. Other common pitfalls associated with the TMS-EEG procedure are somatosensory evoked potentials related to superficial scalp activation from the TMS pulse and auditory evoked potentials to the TMS discharge sound, which can contaminate the TMS-evoked EEG responses to direct cortical activation. Previous work from our research group and other groups has shown that these potential contaminants can be substantially mitigated through the placement of a foam layer underneath the coil and with auditory noise masking (38, 39). Nonetheless, concerns remain about the challenge of disentangling genuine cortical responses to TMS from those resulting from concomitant sensory activation (105), which warrants the development of standard procedures in TMS-EEG studies (106, 107). Similarly, although TMS-EEG data analyses have developed significantly since the technique was first introduced, and some open-source TMS-EEG analysis software programs are currently available, there is still relatively large interindividual variability of the TMS-related measures (108, 109), which highlights the need to develop standardized preprocessing and postprocessing analysis pipelines (16) and has brought about some important initiatives, such as the Big TMS Data Collaboration. Regarding the rTMS and TBS paradigms, in addition to identifying the ideal cortical area to maximize treatment response, which may require combining clinical, neuroimaging, and neurophysiological information, it will be important to optimize the patterns and frequencies of stimulation. This is especially relevant for TBS, which can induce opposite effects on the motor cortex by modifying the pattern, but not the frequency, of stimulation. Future work should therefore establish these effects in nonmotor areas and develop the TBS paradigms that best modulate the activity of these areas in the desired direction. Other factors to consider are the TMS dose to apply (given that an accelerated high-dose iTBS protocol delivered in an unblinded study was found to be safe and induced remission in 19 of 22 individuals with treatment-resistant depression [110]), controlling the cognitive state (e.g., level of vigilance, rest versus performing a task while inducing symptoms) of individuals at the time of the rTMS or TBS procedure, and exploring the combination of neuromodulation with other treatment interventions, including pharmacotherapy and psychotherapy. Nonetheless, evidence accumulated to date indicates that TMS is uniquely suited to both examine and modulate neural circuit function. Thus, there is an enormous potential to develop a TMS-based personalized precision-medicine approach to assess, as well as treat, individuals with psychiatric disorders, as outlined below.
CONCLUSIONS AND FUTURE DIRECTIONS
Building on this growing body of evidence, we suggest three areas of study for future TMS work in psychiatric disorders. These areas of study can ultimately contribute to the development of better neuromodulation-based interventions for individuals with these disorders: employing TMS-EEG to characterize the local and long-range cortical abnormalities that are to be used as neural targets in TMS target-engagement studies; acutely modulating neural circuits with rTMS and TBS paradigms to determine the impact of such neuromodulation on related biological and clinical parameters, to better inform subsequent neuromodulation-based treatment interventions; and combining neuroimaging, neurophysiological, and clinical measures related to TMS-targeted neural circuits to better predict and track clinical outcomes in TMS clinical trial studies (Box 2).
BOX 2. TMS: future developments in psychiatry
Employing TMS-related EEG measures to elucidate neural circuit dysfunctions, to provide more accurate neural targets for TMS-based interventions in psychiatric disorders
Acutely modulating neural circuits with rTMS and TBS paradigms and examining their impact on related biological and clinical parameters, to better inform subsequent neuromodulation-based treatment interventions
Combining neuroimaging, neurophysiological, and clinical measures related to TMS-targeted neural circuitry to better predict and track clinical outcomes in TMS clinical trials
Addressing challenges and limitations of each of the current approaches (TMS-EEG, rTMS, and TBS)
Employing TMS-Related EEG Measures to Elucidate Neural Circuit Dysfunctions in Order to Provide More Accurate Neural Targets for TMS-Based Interventions in Psychiatric Disorders
While TMS-EEG has been utilized to characterize the neurophysiological abnormalities of major psychiatric disorders, it could also be employed in combination with rTMS and TBS paradigms to identify predictive, prognostic, and pathophysiological biomarkers of noninvasive brain stimulation-based treatment interventions. For example, one study showed that TMS-assessed cortical inhibition of the DLPFC predicted the response to a course of magnetic seizure therapy, an rTMS paradigm recently developed as an alternative to ECT in treatment-resistant depression (111). Specifically, a greater decrease in suicidal ideation was associated with larger pre-magnetic seizure therapy DLPFC LICI values, suggesting that higher cortical inhibition at baseline is an indicator of remission of suicidal ideation (i.e., a predictive biomarker). Another TMS-EEG study of treatment-resistant depression showed an increase in the immediate power and slope of TEPs after several ECT sessions and that this increase was associated with reduced depression severity (112). Although these findings need to be replicated in larger samples, as well as in studies that include rTMS and TBS paradigms, some of these TMS-evoked EEG measures are potential predictive biomarkers of neuromodulation interventions in mood disorders. Additionally, TMS-EEG neurophysiological measures that are altered in schizophrenia (e.g., LICI [73], generation and modulation of gamma oscillatory activity [16], and natural frequency [39] of the DLPFC) could be utilized to develop rTMS and TBS protocols targeting the DLPFC or other interconnected neural regions to delay, halt, or even reverse pathophysiological processes and related clinical and functional impairments in individuals with schizophrenia, thereby serving as target engagement biomarkers for noninvasive brain stimulation-based treatment interventions.
Acutely Modulating Neural Circuits With rTMS and TBS Paradigms and Examining Their Impact on Related Biological and Clinical Parameters to Better Inform Subsequent Neuromodulation-Based Treatment Interventions
Increasing evidence indicates that rTMS and TBS paradigms can acutely modulate the activity of neural circuits and related behavioral parameters in healthy individuals, as reported above. In contrast, little is known about the acute effects of these paradigms on psychiatric disorders. Traditionally, TMS-based interventions target a given cortical area (e.g., the DLPFC) and measure the effect of these interventions on clinical parameters, such as improvement in depression. More recently, functional neuroimaging approaches, especially those including measures of functional connectivity, have been used to prospectively identify TMS targets for future treatment interventions. One fMRI study of individuals with major depression showed that compared with responders, nonresponders had higher anhedonia and lower connectivity in a neural circuit classically associated with reward, comprising the ventral tegmental area, the striatum, and part of the ventromedial prefrontal cortex (113). Another fMRI study, using a large multisite sample, showed that individuals with major depression can be subdivided into four neurophysiological subtypes (“biotypes”) defined by distinct patterns of dysfunctional connectivity in limbic and fronto-striatal networks (114). These biotypes were associated with differing symptom profiles and predicted responsiveness to TMS therapy. Specifically, individuals in biotype 1 were approximately three times more likely to benefit from rTMS over the dorsomedial prefrontal cortex than those in biotypes 2 and 4. However, these biotypes have been hard to replicate (115). One of the challenges of relying on these functional biotypes is establishing a causal relationship between brain activity and symptoms. By acutely modulating altered neural circuits with rTMS and TBS paradigms and examining their immediate impact on related biological and clinical parameters, this challenge can begin to be addressed. Specifically, neuroimaging or neurophysiological assessments, along with clinical evaluations before and after acute rTMS and TBS protocols, can elucidate the causal role of a given neural circuit in the development of specific symptoms or behaviors.
Combining Neuroimaging, Neurophysiological, and Clinical Measures Related to TMS-Targeted Neural Circuits to Better Predict and Track Clinical Outcomes in TMS Clinical Trial Studies
Combining TMS-related EEG parameters with rTMS and TBS paradigms has the potential to significantly improve clinical outcomes in psychiatric disorders. TEPs can characterize the neurophysiological properties of the cortical area to be targeted by rTMS, which in turn may help monitor treatment response. Consistent with this approach, a randomized sham-controlled clinical trial of rTMS in major depression combining fMRI and TMS-EEG reported that baseline DLPFC fMRI global connectivity predicted clinical outcome, and local and distributed changes in TMS-EEG potentials tracked clinical outcome (116). Moreover, TMS-evoked EEG responses provide information about the effective connectivity of the TMS-targeted cortical area with the whole brain. This information, combined with the functional connectivity maps obtained by fMRI, can better characterize the effect of rTMS on the target neural region and the neural circuit of interest, which in turn should lead to better prediction and monitoring of response to treatment in individuals with psychiatric disorders.
1 : Noninvasive brain stimulation: challenges and opportunities for a new clinical specialty . J Neuropsychiatry Clin Neurosci 2018 ; 30 : 173 – 179 Crossref, Medline, Google Scholar
2 : Non-invasive brain stimulation: probing intracortical circuits and improving cognition in the aging brain . Front Aging Neurosci 2018 ; 10 : 177 Crossref, Medline, Google Scholar
3 : EPI distortion correction for concurrent human brain stimulation and imaging at 3T . J Neurosci Methods 2019 ; 327 :
4 : Design of TMS coils with reduced Lorentz forces: application to concurrent TMS-fMRI . J Neural Eng 2020 ; 17 :
5 : Characterizing and modulating brain circuitry through transcranial magnetic stimulation combined with electroencephalography . Front Neural Circuits 2016 ; 10 : 73 Crossref, Medline, Google Scholar
6 : TMS-EEG: a window into the neurophysiological effects of transcranial electrical stimulation in non-motor brain regions . Neurosci Biobehav Rev 2016 ; 64 : 175 – 184 Crossref, Medline, Google Scholar
7 : Investigating the neurobiology of schizophrenia and other major psychiatric disorders with transcranial magnetic stimulation . Schizophr Res 2018 ; 192 : 30 – 38 Crossref, Medline, Google Scholar
8 : Functional and effective connectivity: a review . Brain Connect 2011 ; 1 : 13 – 36 Crossref, Medline, Google Scholar
9 : Transcranial magnetic stimulation: Neurophysiological and clinical applications . Handb Clin Neurol 2019 ; 163 : 73 – 92 Crossref, Medline, Google Scholar
10 : Whither TMS: a one-trick pony or the beginning of a neuroscientific revolution? Am J Psychiatry 2019 ; 176 : 904 – 910 Link, Google Scholar
11 : Noninvasive brain stimulation in psychiatric disorders: a primer . Br J Psychiatry 2019 ; 41 : 70 – 81 Crossref, Google Scholar
12 : A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience . Brain Topogr 2010 ; 22 : 219 – 232 Crossref, Medline, Google Scholar
13 : Effects of transcranial magnetic stimulation on the cognitive event-related potential p300: a literature review . Clin EEG Neurosci 2012 ; 43 : 285 – 290 Crossref, Medline, Google Scholar
14 : Combined neurostimulation and neuroimaging in cognitive neuroscience: past, present, and future . Ann N Y Acad Sci 2013 ; 1296 : 11 – 30 Crossref, Medline, Google Scholar
15 : Investigational and therapeutic applications of transcranial magnetic stimulation in schizophrenia . Curr Psychiatry Rep 2019 ; 21 : 89 Crossref, Medline, Google Scholar
16 : Clinical utility and prospective of TMS-EEG . Clin Neurophysiol 2019 ; 130 : 802 – 844 Crossref, Medline, Google Scholar
17 : Non-invasive magnetic stimulation of human motor cortex . Lancet 1985 ; 1 : 1106 – 1107 Crossref, Medline, Google Scholar
18 : Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application: an updated report from an IFCN Committee . Clin Neurophysiol 2015 ; 126 : 1071 – 1107 Crossref, Medline, Google Scholar
19 : Cortical inhibition, excitation, and connectivity in schizophrenia: a review of insights from transcranial magnetic stimulation . Schizophr Bull 2014 ; 40 : 685 – 696 Crossref, Medline, Google Scholar
20 : Transcranial magnetic stimulation in neurology . Lancet Neurol 2003 ; 2 : 145 – 156 Crossref, Medline, Google Scholar
21 : Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation . Electroencephalogr Clin Neurophysiol 1989 ; 74 : 417 – 424 Crossref, Medline, Google Scholar
22 : Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity . Neuroreport 1997 ; 8 : 3537 – 3540 Crossref, Medline, Google Scholar
23 : Instrumentation for the measurement of electric brain responses to transcranial magnetic stimulation . Med Biol Eng Comput 1999 ; 37 : 322 – 326 Crossref, Medline, Google Scholar
24 : Electroencephalographic recording during transcranial magnetic stimulation in humans and animals . Clin Neurophysiol 2006 ; 117 : 1870 – 1875 Crossref, Medline, Google Scholar
25 : Test-retest reliability of transcranial magnetic stimulation EEG evoked potentials . Brain Stimul 2018 ; 11 : 536 – 544 Crossref, Medline, Google Scholar
26 : The relationship between peripheral and early cortical activation induced by transcranial magnetic stimulation . Neurosci Lett 2010 ; 478 : 24 – 28 Crossref, Medline, Google Scholar
27 : Characterization of GABAB-receptor mediated neurotransmission in the human cortex by paired-pulse TMS-EEG . Neuroimage 2014 ; 103 : 152 – 162 Crossref, Medline, Google Scholar
28 : Cortical inhibition of distinct mechanisms in the dorsolateral prefrontal cortex is related to working memory performance: a TMS-EEG study . Cortex 2015 ; 64 : 68 – 77 Crossref, Medline, Google Scholar
29 : TMS and drugs revisited 2014 . Clin Neurophysiol 2015 ; 126 : 1847 – 1868 Crossref, Medline, Google Scholar
30 : Human brain connectivity during single and paired pulse transcranial magnetic stimulation . Neuroimage 2011 ; 54 : 90 – 102 Crossref, Medline, Google Scholar
31 : Characterization of glutamatergic and GABAA-mediated neurotransmission in motor and dorsolateral prefrontal cortex using paired-pulse TMS-EEG . Neuropsychopharmacology 2017 ; 42 : 502 – 511 Crossref, Medline, Google Scholar
32 : A combined TMS-EEG study of short-latency afferent inhibition in the motor and dorsolateral prefrontal cortex . J Neurophysiol 2016 ; 116 : 938 – 948 Crossref, Medline, Google Scholar
33 : Excitability of the supplementary motor area in Parkinson’s disease depends on subcortical damage . Brain Stimul 2019 ; 12 : 152 – 160 Crossref, Medline, Google Scholar
34 : Cognitive enhancement induced by anodal tDCS drives circuit-specific cortical plasticity . Cereb Cortex 2018 ; 28 : 1132 – 1140 Crossref, Medline, Google Scholar
35 : Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease . Dialogues Clin Neurosci 2012 ; 14 : 345 – 367 Crossref, Medline, Google Scholar
36 : Experiments and models of cortical oscillations as a target for noninvasive brain stimulation . Prog Brain Res 2015 ; 222 : 41 – 73 Crossref, Medline, Google Scholar
37 : Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities . Biol Psychiatry 2015 ; 77 : 1001 – 1009 Crossref, Medline, Google Scholar
38 : Natural frequencies of human corticothalamic circuits . J Neurosci 2009 ; 29 : 7679 – 7685 Crossref, Medline, Google Scholar
39 : Reduced natural oscillatory frequency of frontal thalamocortical circuits in schizophrenia . Arch Gen Psychiatry 2012 ; 69 : 766 – 774 Crossref, Medline, Google Scholar
40 : The contribution of TMS-EEG coregistration in the exploration of the human cortical connectome . Neurosci Biobehav Rev 2015 ; 49 : 114 – 124 Crossref, Medline, Google Scholar
41 : Ghost interactions in MEG/EEG source space: a note of caution on inter-areal coupling measures . Neuroimage 2018 ; 173 : 632 – 643 Crossref, Medline, Google Scholar
42 : Network-targeted, multi-site direct cortical stimulation enhances working memory by modulating phase lag of low-frequency oscillations . Cell Rep 2019 ; 29 : 2590 – 2598e4 Crossref, Medline, Google Scholar
43 : General indices to characterize the electrical response of the cerebral cortex to TMS . Neuroimage 2010 ; 49 : 1459 – 1468 Crossref, Medline, Google Scholar
44 : Neurophysiology of cortical stimulation . Int Rev Neurobiol 2012 ; 107 : 57 – 85 Crossref, Medline, Google Scholar
45 : Transcranial magnetic stimulation: from neurophysiology to pharmacology, molecular biology and genomics . Neuroscientist 2010 ; 16 : 210 – 221 Crossref, Medline, Google Scholar
46 : Plasticity in the human central nervous system . Brain 2006 ; 129 : 1659 – 1673 Crossref, Medline, Google Scholar
47 : Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation . Front Hum Neurosci 2015 ; 9 : 303 Crossref, Medline, Google Scholar
48 : Brain topological correlates of motor performance changes after repetitive transcranial magnetic stimulation . Brain Connect 2014 ; 4 : 265 – 272 Crossref, Medline, Google Scholar
49 : Akinesia in Parkinson’s disease. II: effects of subthreshold repetitive transcranial motor cortex stimulation . Neurology 1994 ; 44 : 892 – 898 Crossref, Medline, Google Scholar
50 : Low-frequency rTMS inhibitory effects in the primary motor cortex: insights from TMS-evoked potentials . Neuroimage 2014 ; 98 : 225 – 232 Crossref, Medline, Google Scholar
51 : Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation . Clin Neurophysiol 2000 ; 111 : 800 – 805 Crossref, Medline, Google Scholar
52 : A direct demonstration of cortical LTP in humans: a combined TMS/EEG study . Brain Res Bull 2006 ; 69 : 86 – 94 Crossref, Medline, Google Scholar
53 : Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights . Neurosci Biobehav Rev 2017 ; 83 : 381 – 404 Crossref, Medline, Google Scholar
54 : Theta burst stimulation of the human motor cortex . Neuron 2005 ; 45 : 201 – 206 Crossref, Medline, Google Scholar
55 : The theoretical model of theta burst form of repetitive transcranial magnetic stimulation . Clin Neurophysiol 2011 ; 122 : 1011 – 1018 Crossref, Medline, Google Scholar
56 : Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial . Lancet 2018 ; 391 : 1683 – 1692 Crossref, Medline, Google Scholar
57 : Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects . Brain Stimul 2016 ; 9 : 323 – 335 Crossref, Medline, Google Scholar
58 : Variability and predictors of response to continuous theta burst stimulation: a TMS-EEG study . Front Neurosci 2018 ; 12 : 400 Crossref, Medline, Google Scholar
59 : Demonstration of short-term plasticity in the dorsolateral prefrontal cortex with theta burst stimulation: A TMS-EEG study . Clin Neurophysiol 2017 ; 128 : 1117 – 1126 Crossref, Medline, Google Scholar
60 : Somatosensory cortex participates in the consolidation of human motor memory . PLoS Biol 2019 ; 17 :
61 : Post-training TMS abolishes performance improvement and releases future learning from interference . Commun Biol 2019 ; 2 : 320 Crossref, Medline, Google Scholar
62 : Prevalence of psychotic disorders and its association with methodological issues: a systematic review and meta-analyses . PLoS One 2018 ; 13 :
63 : Schizophrenia, dopamine and the striatum: from biology to symptoms . Trends Neurosci 2019 ; 42 : 205 – 220 Crossref, Medline, Google Scholar
64 : Cross-sectional study of glutamate in the anterior cingulate and hippocampus in schizophrenia . Schizophr Bull 2016 ; 42 : 425 – 433 Crossref, Medline, Google Scholar
65 : Cortical inhibitory neurons and schizophrenia . Nat Rev Neurosci 2005 ; 6 : 312 – 324 Crossref, Medline, Google Scholar
66 : High-frequency oscillations and the neurobiology of schizophrenia . Dialogues Clin Neurosci 2013 ; 15 : 301 – 313 Crossref, Medline, Google Scholar
67 : Mapping pathologic circuitry in schizophrenia . Handb Clin Neurol 2018 ; 150 : 389 – 417 Crossref, Medline, Google Scholar
68 : Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities . Curr Opin Psychiatry 2016 ; 29 : 202 – 210 Crossref, Medline, Google Scholar
69 : A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders . Clin Neurophysiol 2013 ; 124 : 1309 – 1320 Crossref, Medline, Google Scholar
70 : Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study . Am J Psychiatry 2008 ; 165 : 996 – 1005 Link, Google Scholar
71 : The functional consequences of cortical circuit abnormalities on gamma oscillations in schizophrenia: insights from computational modeling . Front Hum Neurosci 2009 ; 3 : 33 Crossref, Medline, Google Scholar
72 : Parvalbumin neurons and gamma rhythms enhance cortical circuit performance . Nature 2009 ; 459 : 698 – 702 Crossref, Medline, Google Scholar
73 : Investigating cortical inhibition in first-degree relatives and probands in schizophrenia . Sci Rep 2017 ; 7 : 43629 Crossref, Medline, Google Scholar
74 : Disrupted cortical conductivity in schizophrenia: TMS-EEG study . Cereb Cortex 2014 ; 24 : 211 – 221 Crossref, Medline, Google Scholar
75 : Altered prefrontal activity and connectivity predict different cognitive deficits in schizophrenia . Hum Brain Mapp 2015 ; 36 : 4539 – 4552 Crossref, Medline, Google Scholar
76 : Auditory verbal hallucinations: imaging, analysis, and intervention . Eur Arch Psychiatry Clin Neurosci 2012 ; 262 ( Suppl 2 ): S91 – S95 Crossref, Medline, Google Scholar
77 : Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials . Eur Psychiatry 2018 ; 49 : 69 – 77 Crossref, Medline, Google Scholar
78 : Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: meta-analysis of controlled trials . Neurosci Biobehav Rev 2018 ; 89 : 111 – 118 Crossref, Medline, Google Scholar
79 : Repetitive transcranial magnetic stimulation for treating the symptoms of schizophrenia: a PRISMA compliant meta-analysis . Clin Neurophysiol 2017 ; 128 : 716 – 724 Crossref, Medline, Google Scholar
80 : A randomized, double blind, sham-controlled trial of repetitive transcranial magnetic stimulation (rTMS) in the treatment of negative symptoms in schizophrenia . Brain Stimul 2020 ; 13 : 840 – 849 Crossref, Medline, Google Scholar
81 : Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies . Acta Psychiatr Scand 2004 ; 110 : 243 – 256 Crossref, Medline, Google Scholar
82 : Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia . Am J Psychiatry 2019 ; 176 : 512 – 520 Link, Google Scholar
83 : Memory and cognition in schizophrenia . Mol Psychiatry 2019 ; 24 : 633 – 642 Crossref, Medline, Google Scholar
84 : Can repetitive magnetic stimulation improve cognition in schizophrenia? pilot data from a randomized controlled trial . Biol Psychiatry 2013 ; 73 : 510 – 517 Crossref, Medline, Google Scholar
85 : Cognitive effects of bilateral high frequency repetitive transcranial magnetic stimulation in early phase psychosis: a pilot study . Brain Imaging Behav 2019 ; 13 : 852 – 861 Crossref, Medline, Google Scholar
86 : Cognitive effects of high-frequency rTMS in schizophrenia patients with predominant negative symptoms: results from a multicenter randomized sham-controlled trial . Schizophr Bull 2016 ; 42 : 608 – 618 Crossref, Medline, Google Scholar
87 : Effects of high-frequency transcranial magnetic stimulation for cognitive deficit in schizophrenia: a meta-analysis . Front Psychiatry 2019 ; 10 : 135 Crossref, Medline, Google Scholar
88 : Diagnostic and Statistical Manual of Mental Disorders (DSM) . CoDAS 2013 ; 25 : 191 – 192 Medline, Google Scholar
89 : Grand challenges in global mental health . Nature 2011 ; 475 : 27 – 30 Crossref, Medline, Google Scholar
90 : A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research . Am J Psychiatry 2014 ; 171 : 829 – 843 Link, Google Scholar
91 : Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders . Depress Anxiety 2000 ; 12 ( Suppl 1 ): 2 – 19 Crossref, Medline, Google Scholar
92 : Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model . Mol Psychiatry 2020 ; 25 : 530 – 543 Crossref, Medline, Google Scholar
93 : Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments . Neuron 2019 ; 102 : 75 – 90 Crossref, Medline, Google Scholar
94 : Targeting glutamate signalling in depression: progress and prospects . Nat Rev Drug Discov 2017 ; 16 : 472 – 486 Crossref, Medline, Google Scholar
95 : Glutamatergic and GABA-ergic abnormalities in first-episode depression: a 1-year follow-up 1H-MR spectroscopic study . J Affect Disord 2020 ; 266 : 572 – 577 Crossref, Medline, Google Scholar
96 : Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies . Biol Psychiatry 2010 ; 67 : 465 – 470 Crossref, Medline, Google Scholar
97 : Shared reduction of oscillatory natural frequencies in bipolar disorder, major depressive disorder and schizophrenia . J Affect Disord 2015 ; 184 : 111 – 115 Crossref, Medline, Google Scholar
98 : Altered transcranial magnetic stimulation-electroencephalographic markers of inhibition and excitation in the dorsolateral prefrontal cortex in major depressive disorder . Biol Psychiatry 2019 ; 85 : 477 – 486 Crossref, Medline, Google Scholar
99 : Prefrontal cortical reactivity and connectivity markers distinguish youth depression from healthy youth . Cereb Cortex 2020 ; 30 : 3884 – 3894 Crossref, Medline, Google Scholar
100 : Changes in regional cerebral blood flow on recovery from depression . Psychol Med 1995 ; 25 : 247 – 261 Crossref, Medline, Google Scholar
101 : Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis . JAMA Psychiatry 2017 ; 74 : 143 – 152 Crossref, Medline, Google Scholar
102 : Clinical efficacy and safety of repetitive transcranial magnetic stimulation in acute bipolar depression . World Psychiatry 2016 ; 15 : 85 – 86 Crossref, Medline, Google Scholar
103 : The efficacy and safety of low frequency repetitive transcranial magnetic stimulation for treatment-resistant depression: the results from a large multicenter French RCT . Brain Stimul 2014 ; 7 : 855 – 863 Crossref, Medline, Google Scholar
104 : Abnormalities in the evoked frontal oscillatory activity of first-episode psychosis: a TMS/EEG study . Schizophr Res 2019 ; 206 : 436 – 439 Crossref, Medline, Google Scholar
105 : The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies . Neuroimage 2019 ; 185 : 300 – 312 Crossref, Medline, Google Scholar
106 : Reproducibility in TMS-EEG studies: a call for data sharing, standard procedures and effective experimental control . Brain Stimul 2019 ; 12 : 787 – 790 Crossref, Medline, Google Scholar
107 : Distilling the essence of TMS-evoked EEG potentials (TEPs): a call for securing mechanistic specificity and experimental rigor . Brain Stimul 2019 ; 12 : 1051 – 1054 Crossref, Medline, Google Scholar
108 : Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) . Clin Neurophysiol 2002 ; 113 : 376 – 382 Crossref, Medline, Google Scholar
109 : Transcranial magnetic stimulation: studying motor neurophysiology of psychiatric disorders . Psychopharmacology (Berl) 2003 ; 168 : 359 – 376 Crossref, Medline, Google Scholar
110 : Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry . 2020 ; 177 : 716 – 726 Link, Google Scholar
111 : Indicators for remission of suicidal ideation following magnetic seizure therapy in patients with treatment-resistant depression . JAMA Psychiatry 2016 ; 73 : 337 – 345 Crossref, Medline, Google Scholar
112 : Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography . Brain Topogr 2013 ; 26 : 326 – 337 Crossref, Medline, Google Scholar
113 : Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression . Biol Psychiatry 2014 ; 76 : 176 – 185 Crossref, Medline, Google Scholar
114 : Resting-state connectivity biomarkers define neurophysiological subtypes of depression . Nat Med 2017 ; 23 : 28 – 38 Crossref, Medline, Google Scholar
115 : Evaluating the evidence for biotypes of depression: ethodological replication and extension of . Neuroimage Clin 2019 ; 22 :
116 : Global connectivity and local excitability changes underlie antidepressant effects of repetitive transcranial magnetic stimulation . Neuropsychopharmacology 2020 ; 45 : 1018 – 1025 Crossref, Medline, Google Scholar



