Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia
Abstract
Objective:
The interpretability of results in psychiatric neuroimaging is significantly limited by an overreliance on correlational relationships. Purely correlational studies cannot alone determine whether behavior-imaging relationships are causal to illness, functionally compensatory processes, or purely epiphenomena. Negative symptoms (e.g., anhedonia, amotivation, and expressive deficits) are refractory to current medications and are among the foremost causes of disability in schizophrenia. The authors used a two-step approach in identifying and then empirically testing a brain network model of schizophrenia symptoms.
Methods:
In the first cohort (N=44), a data-driven resting-state functional connectivity analysis was used to identify a network with connectivity that corresponds to negative symptom severity. In the second cohort (N=11), this network connectivity was modulated with 5 days of twice-daily transcranial magnetic stimulation (TMS) to the cerebellar midline.
Results:
A breakdown of connectivity in a specific dorsolateral prefrontal cortex-to-cerebellum network directly corresponded to negative symptom severity. Restoration of network connectivity with TMS corresponded to amelioration of negative symptoms, showing a statistically significant strong relationship of negative symptom change in response to functional connectivity change.
Conclusions:
These results demonstrate that a connectivity breakdown between the cerebellum and the right dorsolateral prefrontal cortex is associated with negative symptom severity and that correction of this breakdown ameliorates negative symptom severity, supporting a novel network hypothesis for medication-refractory negative symptoms and suggesting that network manipulation may establish causal relationships between network markers and clinical phenomena.
Identification of brain network substrates of the symptoms of psychiatric illnesses has been a primary goal of psychiatric neuroimaging. Numerous factors have confounded the discovery of brain circuits and network dysfunction that cause symptoms and disability. Diagnostic heterogeneity, illness chronicity, technical limitations, and methodological differences have limited our ability to demonstrably identify the network basis of disabling psychiatric symptoms (1).
Psychotic disorders such as schizophrenia are disabling lifelong illnesses that afflict more than 3 million people in the United States. A robust literature has examined how psychotic symptoms predict functional outcomes such as employment, social functioning, and independent housing. These studies have demonstrated that the most readily recognizable positive symptoms, such as delusional thought and hallucinations, are not the best predictor of the functional status of individuals with psychosis. Rather, it is the severity of negative symptoms, such as amotivation, expressive deficits, and anhedonia, that best predicts functional outcomes and overall quality of life (2, 3). The development of new interventions for these negative symptoms is hampered by a critical shortfall in our understanding of their pathophysiology, and our ability to modify the functional impact of schizophrenia is further limited by the fact that psychotropic medications have limited efficacy in treating negative symptoms (4, 5).
Neuroimaging has the potential to illuminate the neuroanatomical basis of these deficits, but existing studies have not converged on a consensus circuit or network target for amelioration in the clinic. Potential explanations for this nonconvergence include methodological heterogeneity between studies (6), the effects of individual variance within a diagnosis (e.g., duration of illness [7]), and the possibility that multiple network pathology subtypes may present with similar clinical features (as suggested by Drysdale et al. [8] for depression). Even in scenarios in which there is agreement between studies, most imaging studies are inherently correlational and cannot establish a causal relationship between imaging signal and the phenotype of interest—that is, these findings may be causal to clinical manifestations of the illness, functionally compensatory processes, or purely epiphenomena. To address these concerns, there is a growing consensus that symptom-pathophysiology relationships may be best tested using a within-subjects design in which pathophysiology is experimentally manipulated to determine the impact on symptoms (9).
We therefore propose a strategy wherein a symptom-related circuit is empirically discovered, and then, in a separate cohort, this circuit is challenged in a targeted way to assess causal relations (10). Recent advances in noninvasive brain stimulation have demonstrated the feasibility of manipulating single-network connectivity (11) in a way that uniquely affects the targeted network (12) and concurrently affects behavior (13). This hypothesis predicts that if circuit pathology and psychiatric symptoms are causally linked, then it must follow that the manipulation of the circuit should be reflected in symptom modification.
In a cohort of participants with early-course schizophrenia or schizoaffective disorder, we sought to identify brain network correlates of negative symptoms. Methodological decisions included recruitment of participants with early-course illness to mitigate the effects of illness chronicity, imaging processing tailored to mitigate movement effects, and analysis using an unbiased, data-driven approach to locate symptom-imaging correlates at the individual voxel level. After identifying this network, we examined, in a separate experimental cohort, the effect of repetitive brain stimulation at the network node most strongly associated with symptom severity. We show that a frontal-cerebellar circuit abnormality is associated with negative symptoms and that noninvasive brain stimulation that modulates this circuit abnormality ameliorates negative symptoms, thus revealing a causal relation between the brain circuit abnormality and the clinical symptoms.
Methods
Participants
Network discovery.
Data on 44 participants with schizophrenia (N=35) or schizoaffective disorder (N=9) who were recruited for a clinical trial (NCT01561859) were included in the study. Before participation in the study, all participants provided written informed consent in accordance with the institutional review boards of the University of Pittsburgh and Beth Israel Deaconess Medical Center (Boston).
Network validation.
Data on 11 participants with schizophrenia recruited for a clinical trial (NCT01551979) were also included in the study. All participants provided written informed consent in accordance with the institutional review board of the Beth Israel Deaconess Medical Center.
MRI Acquisition
Imaging was conducted on Siemens 3.0-T MRI systems (Munich, Germany). Briefly, 1-mm3 T1-weighted anatomical scans were acquired, and multiple functional runs of approximately 6 minutes were acquired from all participants (124 time points, 3-second repetition time, 3-mm3 voxels).
MRI Data Processing
All analyses were preprocessed using the DPABI toolbox (Data Processing and Analysis for Brain Imaging) (14; http://rfmri.org/dpabi). As a quality control metric, scans that exceeded motion thresholds (>3 mm translation or >3° rotation) were discarded. Individual time points with framewise displacement >0.5 mm were discarded, and scans with >50% of volumes removed for framewise displacement were discarded. All data were preprocessed to remove motion (24-parameter), CSF signals, white matter signals, and an overall linear trend. A bandpass filter was applied (0.01–0.08 Hz). Data were normalized using the DARTEL toolbox (http://www.neurometrika.org/node/34) into Montreal Neurological Institute (MNI) space and smoothed with an 8-mm full-width half-maximum kernel. Analyses were conducted in a gray matter mask defined within the group.
Network identification was conducted with multivariate distance matrix regression. Time courses from regions identified with the network identification method were extracted using the DPABI toolbox for the network validation cohort and then correlated with z-transformed Pearson’s correlation coefficients. An additional analysis was conducted with SPM12 for voxel-wise maps.
Clinical Assessment
Participants were assessed with the Scale for Assessment of Negative Symptoms in the network discovery cohort and with the Positive and Negative Syndrome Scale (PANSS) for the network validation cohort.
Additional methodological details are presented in the online supplement.
Results
Network Discovery With Multivariate Distance Matrix Regression (MDMR)
MDMR reveals functional connectivity correlates of negative symptoms in schizophrenia.
We examined clinical, demographic, and resting-state functional MRI (fMRI) data on 44 participants diagnosed with schizophrenia or schizoaffective disorder (Table 1) (for further details on network discovery, see the online supplement). Negative symptom severity was quantified by trained raters who administered the Scale for the Assessment of Negative Symptoms (15). For preprocessing resting-state fMRI data, we used procedures optimized to control for motion-related effects (16). Individual fMRI scans were coregistered into a common space (MNI). After preprocessing, resting-state fMRI data were analyzed with MDMR (Figure 1A) (17). This method allows for an unbiased, data-driven approach to determining functional connectivity. In contrast to most other approaches, MDMR allows quantification of how a variable of interest (negative symptom severity here) is reflected in the connectivity of individual voxels to the whole brain (i.e., at the finest resolution possible) without parcellating the brain into regions defined a priori (Figure 1A) (for further details, see the online supplement). This approach has been used to examine the relationship between psychiatric pathology and connectivity (18–21). We modeled the effect of negative symptom severity on functional connectivity while covarying for effects of in-scanner motion, age, sex, study site, and prescribed medication dosage. This analysis identified the middle frontal gyrus (Brodmann’s area 9) in the dorsolateral prefrontal cortex bilaterally as the regions where functional connectivity covaried significantly with negative symptom severity. The right dorsolateral prefrontal cortex (peak voxel z=3.8; MNI coordinates: 36, 24, 30) demonstrated a more significant relationship between functional connectivity and negative symptom severity than the left dorsolateral prefrontal cortex (peak voxel z=2.95; MNI coordinates: −33, 30, 42) (Figure 1B).
| Cohort and Characteristic | ||
|---|---|---|
| Network discovery cohort | ||
| N | % | |
| Sex | ||
| Female | 15 | 34.1 |
| Male | 29 | 65.9 |
| Diagnosis | ||
| Schizoaffective disorder | 9 | 20.5 |
| Schizophrenia | 35 | 79.5 |
| Race/ethnicity | ||
| African American | 6 | 13.6 |
| Caucasian | 27 | 61.4 |
| Asian | 3 | 6.8 |
| Hispanic | 1 | 2.3 |
| More than one | 3 | 6.8 |
| Other | 4 | 9.1 |
| Mean | SD | |
| Age (years) | 24.16 | 4.50 |
| Brief Psychiatric Rating Scale | 41.75 | 9.47 |
| Montgomery-Åsberg Depression Rating Scale | 10.93 | 9.61 |
| Scale for the Assessment of Negative Symptoms | 33.73 | 15.48 |
| Scale for the Assessment of Positive Symptoms | 12.48 | 11.41 |
| Antipsychotic daily dose in chlorpromazine equivalents (mg) | 305.3 | 232.6 |
| Network validation cohort | ||
| N | % | |
| Sex | ||
| Female | 3 | 27.3 |
| Male | 8 | 72.7 |
| Race | ||
| African American | 2 | 18.2 |
| Caucasian | 8 | 72.7 |
| Hispanic-Caucasian | 1 | 9.1 |
| Mean | SD | |
| Age (years) | 35.55 | 10.50 |
| Calgary Depression Scale for Schizophrenia score | 5.18 | 4.92 |
| Positive and Negative Syndrome Scale | ||
| Positive subscale score | 17.09 | 5.82 |
| Negative subscale score | 23.00 | 10.65 |
| General subscale score | 35.82 | 11.70 |
| Antipsychotic daily dose in chlorpromazine equivalents (mg) | 614.2 | 606.5 |
TABLE 1. Demographic and clinical characteristics of participants in a study of functional connectivity in cerebellar-prefrontal network and negative symptoms in schizophrenia
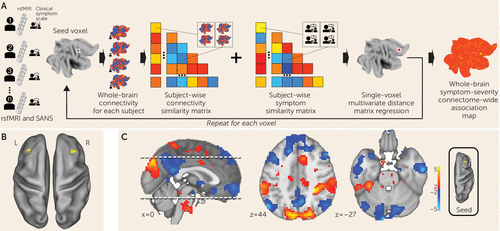
FIGURE 1. Multivariate distance matrix regression (MDMR) of negative symptoms in schizophreniaa
a Panel A illustrates the MDMR method (described by Shehzad et al. [17]). Symptom scales and resting-state functional MRI (rsfMRI) data were collected for each participant. For each voxel in the brain, the voxel was used as a seed region to create a connectivity map for each participant. These maps were compared with each other to create a subject-wise similarity matrix. The symptom scale scores for each participant were then combined with the connectivity similarity matrix to produce a pseudo-F statistic, which characterizes the symptom predictor’s ability to describe the similarity of the functional connectivity. A permutation test of the study subjects’ labels can be used to test the significance of this pseudo-F statistic. Each MDMR voxel-wise result was then combined to produce a map of the ability of the connectivity pattern to predict a symptom score in each voxel. Panel B shows the MDMR results for negative symptoms in the network discovery cohort (voxel-wise thresholded, p<0.005; cluster-corrected at p<0.05). Dorsolateral prefrontal cortex regions are identified bilaterally. Panel C shows the locations where the variability in connectivity from an MDMR-identified region covaries with symptom severity. In this post hoc analysis, a seed region was placed in the right dorsolateral prefrontal cortex in all study subjects, and this seed-based connectivity map then was correlated with symptom severity to identify locations where increased (red) or decreased (blue) connectivity to the dorsolateral prefrontal cortex corresponded to greater symptom severity. Thus, regions in blue correspond to locations where connectivity breakdown with the dorsolateral prefrontal cortex corresponded to greater symptom severity. The strongest dorsolateral prefrontal cortex connectivity breakdown to symptom severity correlation was observed in the midline cerebellum (Montreal Neurological Institute coordinates: −9, −96, −27). L=left; R=right; SANS=Scale for the Assessment of Negative Symptoms.
Negative symptom severity is inversely correlated with functional connectivity between the right dorsolateral prefrontal cortex and the default mode network.
MDMR identifies brain regions where connectivity shows a significant association with a phenotypic variable of interest. Post hoc testing is then necessary to determine the spatial distribution and the directionality of the connectivity. We calculated the subject-specific functional connectivity in the right dorsolateral prefrontal cortex and correlated these maps with negative symptom severity to identify the network that differentially connects to this region depending on symptom severity. We observed that negative symptom severity was inversely correlated with right dorsolateral prefrontal cortex connectivity to a distributed brain network that includes both cerebral and cerebellar nodes of the default network (22, 23) (Figure 1C). Notably, we found that a connectivity breakdown between the right dorsolateral prefrontal cortex and the midline cerebellar node in the default network (MNI coordinates: −9, –96, −27) was the most significant predictor of negative symptom severity.
In summary, we used a purely data-driven analysis to identify the most significant functional connectivity correlate of negative symptom severity in a sample of individuals with schizophrenia or schizoaffective disorder. If disconnectivity between the right dorsolateral prefrontal cortex and the cerebellum is causally related to negative symptom severity, then selectively reversing disconnectivity should be reflected in a reduction in negative symptom severity. We therefore sought to validate or refute a causal relationship between network disconnectivity and negative symptoms.
Network Validation With Transcranial Magnetic Stimulation (TMS)
Empirically testing the causal relationship between network connectivity and negative symptom severity.
We and other investigators have previously demonstrated that repetitive TMS (rTMS) can selectively modulate network functional connectivity in healthy individuals (11, 12, 24). We hypothesized that if breakdown in dorsolateral prefrontal cortex connectivity is causally linked to negative symptom severity, rTMS restoration of functional connectivity should be reflected in amelioration of negative symptom severity.
The most significant relationship between disconnectivity and symptom severity was between the right dorsolateral prefrontal cortex and the midline cerebellum. We therefore examined an independent, testing cohort of participants with schizophrenia who underwent an interventional, sham-controlled trial of rTMS that targeted the cerebellar vermis (midline) (N=11) (Figure 2A; for further details, see the online supplement). Participants underwent clinical characterization by a trained rater who was blind to the treatment condition. Negative symptom severity was quantified by using the negative symptom subscore from PANSS (25). Participants underwent a baseline resting-state fMRI scan and then were randomly assigned to either active or sham rTMS modulation of the cerebellum. Intermittent theta burst (10 bursts of three biphasic pulses at 50 Hz, repeated at 5 Hz, for 10 seconds for a total of 600 pulses) of 100% of active motor threshold was applied to the midline cerebellum. Sham electrodes were placed at the neckline on all study subjects. Blinding codes were used to determine which side of an active or passive Magpro coil (MagVenture Cool B65 A/P, MagVenture A/S, Farum, Denmark) was used for stimulation.
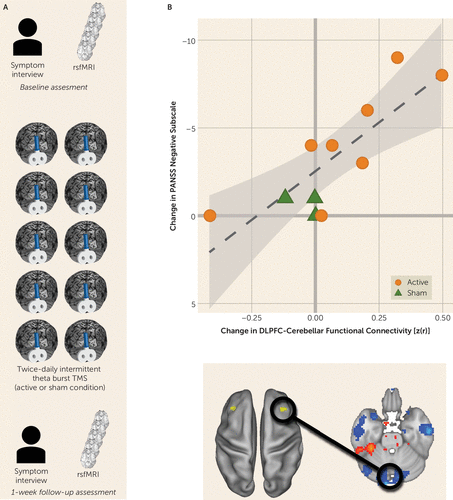
FIGURE 2. One week of twice-daily cerebellar intermittent theta burst modulating dorsolateral prefrontal cortex (DLPFC)-cerebellar functional connectivity in patients with schizophreniaa
a Panel A presents an overview of the transcranial magnetic stimulation (TMS) protocol: symptom scales and resting-state functional MRI (rsfMRI) data are collected at baseline and at the 1-week follow-up after TMS. TMS was delivered to the midline cerebellum (two times per day separated by 4 hours) in a randomized trial with active and sham arms. Panel B shows the change (from baseline to follow-up) in Positive and Negative Syndrome Scale (PANSS) negative symptoms correlated with change (from baseline to follow-up) in right DLPFC-cerebellar network functional connectivity.
Participants underwent two rTMS sessions per day separated by 4 hours for 5 days. This intensity, frequency, and duration of stimulation was chosen on the basis of demonstrated efficacy in ameliorating negative symptoms at the group level (26). After the week of repetitive stimulation, participants underwent clinical characterization and a follow-up resting-state fMRI scan (Figure 2A). We hypothesized that rTMS-induced change in functional connectivity and change in negative symptom severity would be correlated for all participants (i.e., regardless of whether they received sham or real stimulation). Specifically, an increase in cerebellar-dorsolateral prefrontal cortex connectivity should be reflected by a reduction in symptom severity.
Reversing cerebellar-dorsolateral prefrontal cortex disconnectivity ameliorates negative symptom severity.
We measured pretrial-to-posttrial within-subject change in negative symptom severity as well as change in functional connectivity between the right dorsolateral prefrontal cortex and the cerebellar node of the default network. As predicted, we observed a strong and significant relationship between increased connectivity and reduction in symptom severity (r=−0.809, p=0.003; 95% CI=−0.948, −0.405) (Figure 2B).
Testing distributed-network compared with cerebellar-specific effects on negative symptoms.
Consistent with our original hypothesis, we observed that selectively increasing functional connectivity between the cerebellar node of the default network and the right dorsolateral prefrontal cortex resulted in reduced negative symptom severity. We sought to determine whether this effect was mediated solely by changes in cerebellar-dorsolateral prefrontal cortex connectivity or was the result of cerebellar rTMS increasing functional connectivity broadly between the right dorsolateral prefrontal cortex and the rest of the default mode network—that is, both cerebellar and cerebral nodes. We tested these possibilities by generating whole-brain maps of change in functional connectivity (pre-rTMS compared with post-rTMS) to the right dorsolateral prefrontal cortex for each participant. We then regressed these maps against individual change in negative symptom scores to determine where connectivity change correlated with symptom change. This whole-brain analysis identified one area where connectivity change corresponded to symptomatic change: the cerebellum at the level of rTMS stimulation (p<0.001). In this region, the correlation between symptomatic improvement and connectivity change was particularly strong (r=−0.952, 95% CI=−0.987, −0.821). The cerebral nodes of the default network were not identified by this analysis even at a lower threshold (p<0.05) (Figure 3). This suggests that in our trial, symptomatic improvement across all participants was mediated by rescue of cerebellar-dorsolateral prefrontal cortex functional connectivity rather than a broader modulation of multiple cerebral networks, as would be expected if this network connectivity mediated negative symptoms.
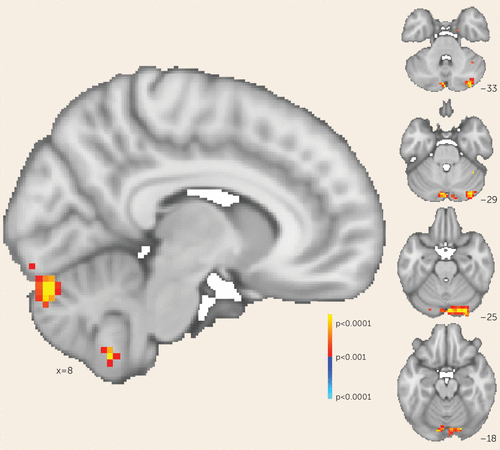
FIGURE 3. Voxel-wise analysis of symptom change and functional connectivity to the dorsolateral prefrontal cortex in patients with schizophreniaa
a Regions across the whole brain that either show increase (warm colors) or decrease (cool colors) in functional connectivity to the right dorsolateral prefrontal cortex correlating with a decrease in Positive and Negative Symptom Scale negative symptom subscale score. The map is voxel-wise thresholded at a p value <0.001.
We also found that the active stimulation condition was significantly more effective than the sham condition in increasing cerebellar-dorsolateral prefrontal cortex connectivity (t=2.938, df=8.96, p=0.017) and in reducing negative symptom severity (t=2.931, df=7.99, p=0.019).
In summary, we used a combination of resting-state fMRI and multivariate data analysis to discover a dorsolateral prefrontal cortex-default network connectivity breakdown associated with schizophrenia negative symptom severity. This connectivity breakdown was strongest between the dorsolateral prefrontal cortex and the midline cerebellum. When repeated rTMS stimulation was used to selectively rescue cerebellar-dorsolateral prefrontal cortex connectivity, negative symptom severity was ameliorated.
Discussion
The ability to use biology to differentiate phenotype and prognosis has improved greatly in recent years, but most studies to date have not been structured to answer questions about the causal relationship between biomarker and behavioral phenotype. This inability to differentiate the biology that mediates disease from compensatory processes or obligatory epiphenomena has led to a “causality gap in human psychiatric neuroscience” (27). In addition to limiting our understanding of the basic neuroscience of psychiatric illness, the absence of a biological target that mediates disease state means that even large trials that evaluate symptomatic response to an intervention cannot lead to a greater understanding of why some patients respond and others do not.
In this study, we combined two mature technologies for neuroscience discovery (resting-state fMRI and rTMS) and applied them to better understand disabling, medication-refractory symptoms in schizophrenia. Using recent developments in data-driven imaging data analysis, we identified a network biomarker of negative symptom severity in a sample of patients with schizophrenia or schizoaffective disorder. We tested the causal nature of network dysfunction through the selective modulation of this network in a trial designed to hold other factors constant (i.e., a within-subject design). We observed that changing cerebellar-dorsolateral prefrontal cortex connectivity appeared to reverse the experiential and expressive deficits referred to as negative symptoms. This finding provides empirical support for a causal relationship between dysfunctional connectivity and psychopathology.
Our findings suggest the existence of at least one network circuit linked directly to negative symptoms. Investigators using alternative approaches have identified different network-symptom relationships (28–30). We suspect that the network we identified is present within these previous data sets; however, it may be obfuscated by common confounders in psychiatric neuroimaging, such as diagnostic heterogeneity, duration of illness, and technical considerations, including motion in the scanner. Although our network discovery data set consisted of patients with early-course schizophrenia (<8 years since their diagnosis), we were able to validate this symptom-connectivity relationship even in participants with later-course illness. This suggests that there is a consistent network-symptom relationship that may be inconsistently observed in purely imaging studies.
There are some existing preliminary hypotheses regarding the emergence of negative symptoms from cerebellar-dorsolateral prefrontal cortex connectivity. Previous imaging studies have identified frontal abnormalities that may be associated with negative symptoms in schizophrenia patients (31, 32). What is the significance of identifying the dorsolateral prefrontal cortex as one node of a circuit that includes the cerebellum? Our findings may be seen as consistent with a dysmetria of thought theory (33–35) that hypothesizes that just as the cerebellum regulates the rate, rhythm, force, and accuracy of movements, so does it regulate the speed, consistency, capacity, and appropriateness of mental or cognitive processes, including those subserved by the prefrontal cortex. It has been hypothesized that cerebellar dysfunction may underlie the pathophysiology of schizophrenia (36–41), but this has not been demonstrated until now.
In sum, the precise localization of a prefrontal-cerebellar network is critical in identifying the underlying neural substrates of the disabling negative symptoms in schizophrenia. Our results provide experimental evidence in support of such a circuit, which, when directly modulated, rescues these deficits.
Our results are consistent with a potentially reproducible model of TMS-based rescue of a breakdown of connectivity. Two previous studies demonstrated a similar reduction in negative symptom severity at the group level after 10 sessions of intermittent theta-burst stimulation to the cerebellar vermis (26, 42). Unfortunately, although these studies produced potential therapeutic improvement, they provided no mechanistic explanation of how improvement occurred, nor did they demonstrate that improvement was a result of correction of existing circuit pathology. Taken together with the present data, these findings suggest an effect that is readily replicable. Furthermore, our network identification and TMS samples were collected at different facilities, which strengthens the generalizability of our results.
Our study has several strengths. First, it establishes a causal relationship between resting-state functional connectivity and disease expression in a psychiatric illness, moving the field away from purely correlational studies. Second, it establishes functional connectivity between the dorsolateral prefrontal cortex and the cerebellum as a quantifiable and engageable target that modulates disabling, medication-refractory negative symptoms in schizophrenia. Third, it is a model of how a precision-medicine approach (i.e., targeting disease-specific pathophysiology to change clinically observable symptoms) may be applied to psychiatric disorders. Fourth, by linking neuromodulation to a biological outcome (functional connectivity) rather than to symptomatic response alone, this model allows individual-level explanation of response and nonresponse to rTMS.
Limitations of this study include the small sample size of the network validation (TMS) experiment. In a traditional rTMS clinical trial with clinical response as the only readout of TMS engagement, our sample size would be inadequate to draw conclusions about the cerebellum’s role in negative symptoms. By combining clinical response with functional connectivity, our study was more than sufficiently powered to discover the strong relationships between connectivity and symptoms. However, with a larger sample size, we could have addressed questions about what factors mediate connectivity change (and therefore symptom change) in response to rTMS—for example, Do individual differences in duration of illness and network topography modulate connectivity change to a course of rTMS? Additionally, we did not assess participant and rater blinding, a procedure that may be helpful in the assessment of placebo response in future studies.
Finally, while our findings demonstrate a target biological substrate for the treatment of negative symptoms, we do not suggest that cerebellar-targeted TMS is the sole intervention that can do so. Our ability to measure cerebellar-dorsolateral prefrontal cortex circuit integrity by using fMRI suggests that functional connectivity may be a useful marker of efficacy for other therapeutic interventions in the treatment of negative symptoms, independent of rater assessment. Similarly, previous TMS studies did not systematically investigate stimulation parameters, and it is therefore plausible that our connectivity assessment may be useful as a direct measure of rapid protocol development.
1 : Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry 2012; 17:1174–1179Crossref, Medline, Google Scholar
2 : Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res 2012; 137:147–150Crossref, Medline, Google Scholar
3 : Social competence versus negative symptoms as predictors of real world social functioning in schizophrenia. Schizophr Res 2014; 160:136–141Crossref, Medline, Google Scholar
4 : Treating negative symptoms in schizophrenia: an update. Curr Treat Options Psychiatry 2016; 3:133–150Crossref, Medline, Google Scholar
5 : Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull 2015; 41:892–899Crossref, Medline, Google Scholar
6 : Rostral medial prefrontal dysfunctions and consummatory pleasure in schizophrenia: a meta-analysis of functional imaging studies. Psychiatry Res 2015; 231:187–196Crossref, Medline, Google Scholar
7 : Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull 2016Crossref, Google Scholar
8 : Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 2017; 23:28–38Crossref, Medline, Google Scholar
9 : Isolating biomarkers for symptomatic states: considering symptom-substrate chronometry. Mol Psychiatry 2016; 21:1180–1187Crossref, Medline, Google Scholar
10 : The NIMH Experimental Medicine Initiative. World Psychiatry 2015; 14:151–153Crossref, Medline, Google Scholar
11 : Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci USA 2011; 108:21229–21234Crossref, Medline, Google Scholar
12 : Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci 2014; 34:12049–12056Crossref, Medline, Google Scholar
13 : Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 2014; 345:1054–1057Crossref, Medline, Google Scholar
14 : DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016; 14:339–351Crossref, Medline, Google Scholar
15 : The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl 1989; 7:49–58Crossref, Medline, Google Scholar
16 : Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59:2142–2154Crossref, Medline, Google Scholar
17 : A multivariate distance-based analytic framework for connectome-wide association studies. Neuroimage 2014; 93:74–94Crossref, Medline, Google Scholar
18 : Common dimensional reward deficits across mood and psychotic disorders: a connectome-wide association study. Am J Psychiatry 2017; 174:657–666Link, Google Scholar
19 : Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am J Psychiatry 2016; 173:517–526Link, Google Scholar
20 : Connectome-wide network analysis of youth with psychosis-spectrum symptoms. Mol Psychiatry 2015; 20:1508–1515Crossref, Medline, Google Scholar
21 : Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivty. Mol Psychiatry 2016; 21:894–902Crossref, Medline, Google Scholar
22 : The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106:1125–1165Crossref, Medline, Google Scholar
23 : Functional network organization of the human brain. Neuron 2011; 72:665–678Crossref, Medline, Google Scholar
24 : Modulation of cognitive cerebello-cerebral functional connectivity by lateral cerebellar continuous theta burst stimulation. Neuroimage 2017; 158:48–57Crossref, Medline, Google Scholar
25 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
26 : Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res 2010; 124:91–100Crossref, Medline, Google Scholar
27 : Addressing the causality gap in human psychiatric neuroscience. JAMA Psychiatry 2018; 75:3–4Crossref, Medline, Google Scholar
28 : Evidence of a dissociation pattern in default mode subnetwork functional connectivity in schizophrenia. Sci Rep 2015; 5:14655Crossref, Medline, Google Scholar
29 : Insular dysfunction reflects altered between-network connectivity and severity of negative symptoms in schizophrenia during psychotic remission. Front Hum Neurosci 2013; 7:216Crossref, Medline, Google Scholar
30 : Differential patterns of dysconnectivity in mirror neuron and mentalizing networks in schizophrenia. Schizophr Bull 2016; 42:1135–1148Crossref, Medline, Google Scholar
31 : Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar
32 : Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional cerebral blood flow evidence. Arch Gen Psychiatry 1986; 43:114–124Crossref, Medline, Google Scholar
33 : An emerging concept: the cerebellar contribution to higher function. Arch Neurol 1991; 48:1178–1187Crossref, Medline, Google Scholar
34 : From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp 1996; 4:174–198Crossref, Medline, Google Scholar
35 : Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci 1998; 2:362–371Crossref, Medline, Google Scholar
36 : Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA 1996; 93:9985–9990Crossref, Medline, Google Scholar
37 : “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:203–218Crossref, Medline, Google Scholar
38 : The therapeutic potential of the cerebellum in schizophrenia. Front Syst Neurosci 2014; 8:163Crossref, Medline, Google Scholar
39 : Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry 2004; 55:1146–1153Crossref, Medline, Google Scholar
40 : Aberrant cerebellar connectivity in motor and association networks in schizophrenia. Front Hum Neurosci 2015; 9:134Crossref, Medline, Google Scholar
41 : Conditional deletion of cadherin 13 perturbs Golgi cells and disrupts social and cognitive behaviors. Genes Brain Behav 2018; 17:e12466Crossref, Medline, Google Scholar
42 : The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: a randomized rater blind-sham controlled study. Psychiatry Res 2016; 243:413–420Crossref, Medline, Google Scholar



