Expression of Interneuron Markers in the Dorsolateral Prefrontal Cortex of the Developing Human and in Schizophrenia
Abstract
Objective:
The onset of schizophrenia symptoms in late adolescence implies a neurodevelopmental trajectory for the disease. Indeed, the γ-aminobutyric acid (GABA) inhibitory system shows protracted development, and GABA-ergic deficits are widely replicated in postmortem schizophrenia studies. The authors examined expression of several interneuron markers across postnatal human development and in schizophrenia to assess whether protracted development of certain interneuron subpopulations may be associated with a particular vulnerability in schizophrenia.
Method:
RNA was extracted postmortem from dorsolateral prefrontal cortex of individuals from age 6 weeks to 49 years (N=68) and from a cohort of normal comparison subjects and schizophrenia patients (N=74, 37 pairs). Expression levels of parvalbumin, cholecystokinin, somatostatin, neuropeptide Y, calretinin, calbindin, and vasoactive intestinal peptide were measured by quantitative reverse transcription-polymerase chain reaction. Changes in calretinin protein levels were examined by Western blot.
Results:
Interneuron marker genes followed one of three general expression profiles: either increasing (parvalbumin, cholecystokinin) or decreasing (somatostatin, calretinin, neuropeptide Y) in expression over postnatal life, with the most dramatic changes seen in the first few years before reaching a plateau; or increasing to peak expression in the toddler years before decreasing (calbindin, vasoactive intestinal peptide). mRNA expression of all genes, with the exception of calbindin (which increased), showed a reduction (8%-31%) in schizophrenia. Somatostatin showed the most dramatic reduction (31%) in schizophrenia.
Conclusions:
It appears that a heterogeneous population of interneurons is implicated in schizophrenia. Further studies are needed to determine whether specific interneuron subpopulations are altered or whether common or distinct upstream pathways are responsible for interneuron deficits in schizophrenia.
Deficits in cortical γ-aminobutyric acid (GABA) neurotransmission are among the most consistent findings in schizophrenia research. These findings support the hypothesis that dysregulation of inhibitory interneurons and/or GABA neurotransmission may contribute to the pathology of this disorder. Postmortem studies demonstrate reduced expression of the 67-kDa isoform of glutamic acid decarboxylase (GAD67), the primary enzyme in GABA synthesis. While interneurons represent approximately 20% of cortical neurons, they are a heterogeneous population that varies in morphology, electrophysiological properties, laminar distribution, innervation of pyramidal neurons, and expression of biochemical markers (1, 2). In schizophrenia, GAD67 mRNA is down-regulated in only 25%-35% of GABA-ergic neurons in layers II-V of the dorsolateral prefrontal cortex. GAD67 expression in other GABA-ergic cells is maintained at normal levels (3, 4), which suggests that a subset of interneurons may be affected more severely. Another often replicated finding in schizophrenia is a reduction in parvalbumin (5), particularly in layers III and IV of the dorsolateral prefrontal cortex. In addition, a reduction in GABA transporter-1 and parvalbumin in cartridges on the axon initial segment of pyramidal neurons has implicated the chandelier subclass of interneurons in schizophrenia (6, 7). The GAD67 mRNA deficit is also present in layers II and V (8), however, which suggests that additional interneuron subtypes may also be involved in schizophrenia. Recently a deficit in somatostatin mRNA was identified in schizophrenia, particularly in layers II and III (9–11), and a reduction of neuropeptide Y mRNA in the dorsolateral prefrontal cortex (9) has implicated Martinotti and bitufted cells. In addition, cholecystokinin mRNA was reported to be deficient in schizophrenia (9, 12). These alterations may represent a failure to reach normal expression levels during development or a failure to maintain normal levels of expression during adulthood.
The onset of schizophrenia symptoms occurs in adolescence or early adulthood, and the development of GABA circuits is a prolonged process that continues until adolescence in rodents and primates (13). In this study, we aimed to determine the pattern of interneuron development by examining the expression of various inter-neuron markers across postnatal development in the human dorsolateral prefrontal cortex. The dorsolateral prefrontal cortex displays prominent interneuron pathology in schizophrenia and also contributes to the cognitive impairments observed in the disorder. We sought to address whether the interneuron subpopulations that are the most altered in schizophrenia also have the most delayed differentiation profiles or show normal maturational changes coincident with the age at onset of schizophrenia.
We measured the mRNA expression of seven calcium-binding proteins and neuropeptides expressed by GABA-ergic interneurons (parvalbumin, cholecystokinin, calbindin, vasoactive intestinal peptide, somatostatin, calretinin, and neuropeptide Y) in postmortem dorso-lateral prefrontal cortex tissue of individuals from age 6 weeks to 49 years as well as in an additional cohort of comparison subjects and patients with schizophrenia. We found that interneuron marker genes followed one of three general expression profiles: either increasing or decreasing in expression over postnatal life, with the most dramatic changes in the first few years before reaching a plateau; or a dynamic pattern increasing to peak expression in the toddler years. The development of interneuron markers is thus protracted in the human dorsolateral pre-frontal cortex, extending well into the toddler years and, for some markers, changing during the transition from the teen years to adulthood. Alterations in all seven markers in schizophrenia implicate multiple GABA-ergic interneuron subtypes in the pathology, and although altered markers do not necessarily share the same temporal pattern of maturation or the same spatial origin in development, interneurons implicated in schizophrenia may constitute a subgroup that is dependent on similar upstream growth factors for differentiation and/or survival.
Method
Postmortem Brain Samples
Human postmortem dorsolateral prefrontal cortex tissue was sampled from the middle third (rostro-caudally) of the middle frontal gyrus anterior to the premotor cortex. Samples were trimmed to include primarily gray matter. Developing tissue was obtained from the University of Maryland Brain Tissue Bank for Developmental Disorders (National Institute of Child Health and Human Development contract NO1-HD8-3283). Individuals in the developmental cohort (N=68) ranged in age from 6 weeks to 49 years and were categorized into seven developmental groups: neonates (N=11), infants (N=14), toddlers (N=9), school-age (N=9), teenagers (N=8), young adults (N=9), and adults (N=8) (see Table S1 in the data supplement that accompanies the online edition of this article). Dorsolateral pre-frontal cortex tissue from patients with schizophrenia or schizoaffective disorder (N=37) and matched normal comparison subjects (N=37) was obtained from the New South Wales Tissue Resource Centre (Sydney, Australia) with the approval of the Human Research Ethics Committee at the University of New South Wales. Groups in both cohorts (Table 1) were matched according to tissue pH, postmortem interval, RNA integrity number, and, in the schizophrenia cohort, age (14). Microarray analysis was performed on dorsolateral prefrontal cortex tissue from 45 individuals from the developmental cohort, as detailed previously (15).
| Characteristic | Comparison Group (N=37) | Schizophrenia Groupa (N=37) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Ageb (years) | 51.1 | 14.6 | 51.3 | 14.1 |
| Age at illness onset (years) | — | — | 23.7 | 0.10 |
| Duration of illness (years) | — | — | 27.6 | 2.3 |
| Postmortem interval (hours) | 24.8 | 10.97 | 28.8 | 14.07 |
| pH | 6.66 | 0.29 | 6.61 | 0.30 |
| RNA integrity number | 7.3 | 0.57 | 7.3 | 0.58 |
| N | % | N | % | |
| Male | 30 | 81 | 24 | 65 |
| Right hemisphere | 23 | 62 | 17 | 46 |
TABLE 1. Summary of Demographic and Clinical Characteristics for Comparison and Schizophrenia Groups
Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA was extracted from all subjects using TRIzol (Invitrogen, Carlsbad, Calif.). RNA quality was assessed using Agilent 2100 Bioanalyzer electrophoresis (Agilent Technologies, Santa Clara, Calif.) and cDNA synthesized using SuperScript First-Strand Synthesis kit and random hexamers (Invitrogen) from 3 μg of total RNA per sample, repeated 2–3 times and pooled. Transcript levels were measured by quantitative reverse transcription-polymerase chain reaction on an ABI Prism 7900HT Fast Real-Time PCR system with 384-well format and TaqMan Gene Expression Assays (Applied Biosystems, Carlsbad, Calif.) (parvalbumin, Hs00161045_ m1; cholecystokinin, Hs00174937_m1; calbindin, Hs01077191_g1; vasoactive intestinal peptide, Hs00929575_m1; somatostatin, Hs00356144_m1; calretinin, Hs00242372_m1; and neuropeptide Y, Hs00173470_m1; see Table S2 in the online data supplement). For each gene, all samples from the developmental cohort were run on the same 384-well plate, and all samples from the schizophrenia and comparison cohorts were run on an additional 384-well plate. A 7-point standard curve of pooled prefrontal cortex cDNA was run for each gene in both cohorts; thus all samples and standards to be compared within a cohort were run under identical amplification conditions. All measurements from each subject were performed in triplicate and relative quantities determined from the standard curve. If the variance of triplicates was greater than 30% of the mean, measurement outliers were removed and the mean recalculated on the basis of two values (<5% of measurements). Transcript quantities were normalized by the geometric mean of four housekeeping genes: β-glucuronidase, hydroxymethylbilane synthase, peptidylprolyl isomerase A (cyclophilin A), and ubiquitin C for the developmental series (the geometric mean was unchanged across developmental groups). Another set of housekeeping genes-ubiquitin C, actin β, glyceraldehyde-3-phosphate dehydrogenase, and TATA box binding protein–was used to calculate the geometric mean for the schizophrenia cohort as these genes did not vary according to diagnostic group and hence were appropriate housekeepers (14).
Western Blot Analysis
Pulverized frozen tissue (40 mg) was homogenized in 400 μL buffer (50 mM Tris pH 7.5, 50% glycerol, and 1:20 (by volume) protease inhibitor cocktail (Sigma, P8340) (final concentration: 2 mM aminoethylbenzenesulfonyl fluoride, 0.015 mM aprotinin, 0.038 mM leupeptin, 0.030 mM pepstatin A, 0.028 mM E-64, and 0.08 mM bestatin). Protein concentration was determined using Bradford (Sigma) and BCA (Thermo Scientific) assays. Western blot analyses were performed in duplicate for the developmental samples and in triplicate for the schizophrenia and comparison samples (separate cohorts were run in full on separate days and replicated in separate experiments), loading 2 μg total protein per lane (determined to be in a linear range of detection by a 7-point standard curve). Subjects were run in randomized order (across 6–8 gels). Rabbit polyclonal anti-calretinin primary antibody (1:2,000; Chemicon, Alta.5054) was applied overnight at 4°C in blocking buffer (1% [weight to volume] nonfat milk in 0.05% Triton-X in Tris-buffered saline). Goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000 in blocking buffer, Chemicon, AP132P) was applied for 1 hour at room temperature. Bands were visualized using chemiluminescent horseradish peroxidase substrate (ECL Western Blotting Detection Reagents, GE Healthcare; or Immobilon Western, Millipore) and quantified by densitometry using Image J. As a loading control, membranes were probed with mouse anti-actin (1:10,000; Chemicon, MAB1501), followed by goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5,000; Chemicon, AP308P). The average intensity for each sample was normalized with the respective actin average intensity. A separate lane of each gel contained an internal control (pooled protein), and samples from each gel were normalized to their internal control.
Statistical Analysis
Samples with RNA integrity numbers less than 5.8 were excluded from the developmental data (final N=57). Outliers within a developmental group (p<0.05, Grubbs' method) were removed. Microarray data were analyzed using the R program (www.r-project.org) to fit linear or quadratic regressions depending on the profile of gene expression changes with age. We report the schizophrenia compared with normal comparison group analyses with all cases included as well as with outliers (cases more than two standard deviations from their respective group mean) removed. The removal of outliers defined in this way is consistent with our previous publications. All data were normally distributed (p>0.20) and showed homogeneity of variance (p>0.38). Statistical tests were performed with Statistica, version 7.1 (StatSoft, Tulsa, Okla.), including one-way analysis of variance (ANOVA) with Fisher least-significant-difference post hoc tests to determine gene expression changes between developmental groups; Student's t tests; Pearson's correlations to analyze the impact of tissue factors (pH, postmortem interval, RNA integrity number, and age) on gene expression; and ANOVA or analysis of covariance (ANCOVA) to covary for sample variables that affect gene expression in the whole cohort (schizophrenia compared with normal comparison groups).
Results
Changes in Expression of Interneuron Markers During Development Identified by Microarray
As shown in Figure 1, interneuron marker mRNAs could be grouped into those that increased in expression over postnatal life (parvalbumin, cholecystokinin); those that initially up-regulated in the early postnatal years and then declined or plateaued around school age (calbindin, vaso-active intestinal peptide); and those that declined over postnatal life (somatostatin, calretinin, neuropeptide Y). Developmental profiles of mRNA expression determined by quantitative polymerase chain reaction replicated those demonstrated by microarray analysis, and thus two different measures of mRNA expression were used to show these developmental patterns.
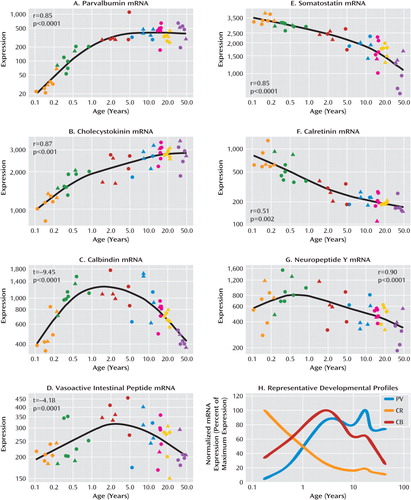
FIGURE 1. Microarray Expression of Seven Calcium-Binding Proteins and Neuropeptides in the Human Dorsolateral Prefrontal Cortex During Postnatal Developmenta
a Panels A-G show microarray expression (in arbitrary units in log scale) as determined in dorsolateral prefrontal cortex tissue from human brains of varying ages (x-axis, log years) by microarray analysis. Total RNA was hybridized to HG-U133 version 2.0 GeneChips (Affymetrix), and developmental profiles for each gene were generated using a linear or quadratic regression model. Circles=males; triangles=females; orange=neonates; green=infants; red=toddlers; blue=school age; magenta=teenage; yellow=young adults; purple=adults. In panel H, representative mRNA expression profiles illustrate three different developmental trajectories observed for interneuron development. Interneuron markers show trends to up-regulation (e.g., parvalbumin, PV) across development (x-axis, log years), decline across development (e.g., calretinin, CR), or a more dynamic pattern of expression with up-regulation early in postnatal life and then decline later in development (e.g., calbindin, CB).
Developmental Changes Verified by Quantitative Reverse Transcription-Polymerase Chain Reaction
Parvalbumin and cholecystokinin mRNA increases during postnatal development.
Both parvalbumin and cholecystokinin mRNAs demonstrated significant positive correlations with age (r=0.39, p=3.7×10−3 and r=0.45, p=5.0×10−4, respectively; Figure 2). Parvalbumin mRNA showed a dramatic up-regulation after infancy, demonstrating a significant increase between both the neonatal and infancy periods and the later developmental periods, increasing 20 times from the neonatal period to adulthood (F=9.15, df=6, 48, p<1.0×10−5). A similar change was observed in cholecystokinin mRNA, showing a significant increase across development (F=9.84, df=6, 48, p<1.0×10−5) until the teen years (doubling in expression compared to the neonatal period).
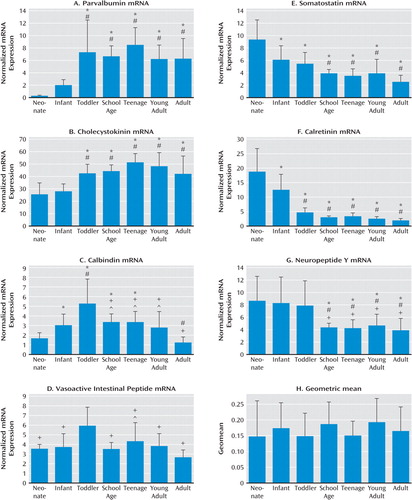
FIGURE 2. Expression of Interneuron Markers in the Human Dorsolateral Prefrontal Cortex During Postnatal Development Verified by Quantitative Reverse Transcription-Polymerase Chain Reactiona
a Expression of mRNA for the seven calcium-binding proteins and neuropeptides was analyzed and plotted by developmental group (panels A–G). Expression was normalized to the geometric mean of four housekeeping genes (panel H), showing that it was not significantly altered between developmental groups. Neonates, N=11; infants, N=14; toddlers, N=9; school-age, N=9; teenage, N=8; young adults, N=9; adults, N=8. Significant differences in mRNA expression between developmental groups by one-way analysis of variance with Fisher least-significant-difference post hoc analysis: *p<<0.05 compared with neonates; #p<0.05 compared with infants; +p<0.05 compared with toddlers; ∧p<0.05 compared with adults. Error bars indicate standard deviation.
Dynamic changes in calbindin and vasoactive intestinal peptide mRNA during development.
Calbindin mRNA expression increased in the early years of life from the neonatal period to peak in the toddler years (three times the expression of the neonatal period; Figure 2C). Calbindin mRNA then declined to school-age levels and plateaued until young adulthood. Another significant reduction in calbindin mRNA was observed in adulthood (overall F=13.22, df=6, 38, p=5.03×10−8; r=0.124). Expression of vasoactive intestinal peptide mRNA displayed a similar trend, with levels peaking during the toddler years then declining during the school-age years (Figure 2D). There was a significant decrease in vasoactive intestinal peptide levels in adulthood compared to the toddler and teenage periods (F=4.11, df=6, 49, p=0.0020; r=0.56).
Somatostatin, calretinin, and neuropeptide Y mRNA are down-regulated during development.
Somatostatin mRNA was negatively correlated with age (r=−0.54, p=1.4×10−5) and was down-regulated by about 70% from the neonatal period to adulthood (F=9.90, df=6, 50, p=1.0×10−5; Figure 2E). Across development, the pattern of calretinin mRNA expression was similar to that of somatostatin (r=−0.58, p=4.0×10−6; F=19.4, df=6, 48, p=1.0×10−5; Figure 2F). Calretinin mRNA was down-regulated in the first few years of postnatal life, with a significant decrease in expression between the neonatal period and infancy and between infancy and all later developmental periods (∼60% reduction from infancy to toddlerhood, all p values <0.001). Neuropeptide Y mRNA was also significantly reduced with increasing age (r=−0.44, p=5.9×10−4; F=3.94, df=6, 50, p=0.003; Figure 2G), with a 45% reduction between toddlerhood and the school-age period. No change in the normalizing factor (geometric mean of four housekeeping genes) was observed between developmental groups (Figure 2H).
Expression of interneuron marker mRNAs in schizophrenia.
In the schizophrenia cohort, a significant reduction was observed in the mRNA levels of 2/7 interneuron markers with a trend to reduction in another two mRNAs (Figure 3; see also Table S3 in the online data supplement). All mRNAs were altered when group outliers were excluded and ANCOVA analyses performed to covary for demographic variables that were correlated with gene expression for each analysis (see “Supplementary Results” and Tables S3 and S4 in the online data supplement). Mean cholecystokinin mRNA expression was reduced (10.1%, F=6.3, df=70, p=0.015; with outliers excluded, 8.49%, F=5.7, df=69, p=0.019); the most marked reduction was observed in somatostatin mRNA, reduced by 24.6% (F=12.2, df=69, p=0.001; with outliers excluded, 31.1%, F=22.7, df=66, p=1.1×10−5). Parvalbumin and vasoactive intestinal peptide were reduced when all cases were included, although the differences fell short of statistical significance (13.3%, F=3.6, df=71, p=0.061, and 15.0%, F=3.4, df=70, p=0.071, respectively) and were significantly reduced when the outliers were excluded (17.0%, F=9.2, df=65, p=0.004, and 21.3%, F=9.8, df=66, p=0.003, respectively). When all cases were included, calretinin and neuropeptide Y mRNA were not altered in schizophrenia, but when outliers were excluded, they were significantly reduced (14.1%, F=5.6, df=69, p=0.021, and 21.1%, F=5.3, df=67, p=0.024, respectively). In contrast to other inter-neuron mRNAs, there was a 14.9% increase in calbindin mRNA expression in schizophrenia (F=4.5, df=69, p=0.038; with outliers excluded, 12.0%, F=5.2, df=67, p=0.026).
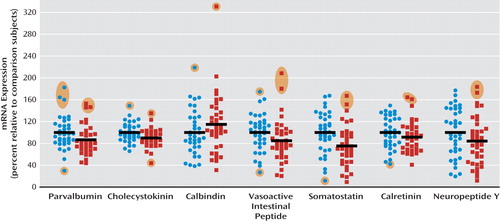
FIGURE 3. Change in Expression of Interneuron Marker mRNAs in the Dorsolateral Prefrontal Cortex of Patients With Schizophrenia (N=37) Relative to Comparison Subjects (N=37)a
a Quantitative reverse transcription-polymerase chain reaction was used to determine the relative expression of mRNA levels in the seven calcium-binding proteins and neuropeptides. Expression of each marker was normalized to the geometric mean of the expression of four housekeeping genes and expressed as a percentage of levels for comparison subjects. Blue circles=normal comparison subjects; red squares=schizophrenia patients. Group outliers more than two standard deviations from the mean are shaded in orange. Black horizontal bars indicate group means (including outliers).
Calretinin protein is reduced during development but unchanged in schizophrenia.
Western blot analysis was performed for calretinin protein in schizophrenia (N=37) and comparison (N=37) samples, resulting in a single distinct band at the expected 28-kDa size (Figure 4C). Normalized calretinin levels were not significantly altered in the schizophrenia relative to the comparison group (t=−0.20, df=68, p=0.84, Figure 4D). To confirm the validity of the assay, the expression of calretinin protein was also measured in the developmental cohort. We found a significant reduction in protein over the course of development mirroring that seen in mRNA (F=7.08, df=6, 36, p=5×10−15; Figure 4E).
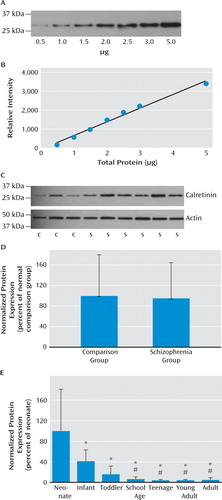
FIGURE 4. Measures of Calretinin at the Protein Level in Schizophrenia Over the Course of Prefrontal Cortex Developmenta
a Panel A, Western blot for calretinin was performed on increasing amounts of total protein from prefrontal cortex homogenates (0.5–5 μg). Panel B, the relative intensity of each band was measured, and a strong linear relationship was observed between all amounts of protein loaded and band intensity (r=0.994). Panel C, representative Western blots showing calretinin and actin immunoreactivity in comparison and schizophrenia prefrontal cortex homogenates. Panel D, no significant difference in calretinin immunoreactivity was observed in the schizophrenia (N=37) relative to the normal comparison group (N=37). Panel E, expression of calretinin immunoreactivity is reduced over the course of development. Neonates, N=7; infants, N=13; toddlers, N=8; school age, N=9; teens, N=8; young adults, N=8; adults, N=7. Significant differences between developmental groups: *p<0.005 compared with neonatal period; #p<0.05 compared with infancy. Error bars indicate standard deviations.
Discussion
A cortical GABA-ergic deficit is one of the most consistently reported findings in people with schizophrenia. We demonstrate here that six of seven biochemical marker mRNAs for GABA-ergic interneurons were reduced by 10%– 30% in the dorsolateral prefrontal cortex of patients with schizophrenia, with the most marked reduction observed in somatostatin mRNA. We also found that the development of interneuron markers is protracted in the human dorsolateral prefrontal cortex, extending well into the toddler years and, for some markers, extending beyond the teenage years. We considered three possible explanations for the alteration in interneuron markers in schizophrenia: that the interneurons that were most affected 1) share a common developmental origin and may be derived from the same aberrant precursor population; 2) demonstrate a common ontological course, whereby interneurons with protracted development would be most altered in the disease; or 3) may be dependent on a common growth factor that regulates their differentiation or survival.
Our first consideration is that altered expression of multiple interneuron marker mRNAs may indicate dys-regulation of a common precursor cell or of cells from a common developmental origin in schizophrenia. The main source of cortical interneurons in the rodent is the ganglionic eminence. It is thought that the majority of cortical interneurons arise from the medial and caudal ganglionic eminences. Parvalbumin (in axon and soma targeting chandelier and basket cells), somatostatin (primarily expressed in dendrite-targeting Martinotti cells), and calbindin (expressed in multiple subtypes) positive inter-neurons are produced in the medial ganglionic eminence (16). Neuropeptide Y and calretinin (expressed in several interneuron subtypes) interneurons primarily originate from the dorsal medial ganglionic eminence (17, 18), and vasoactive intestinal peptide (dendrite-targeting double bouquet and bipolar interneurons) and some calretinin positive neurons are derived from the caudal ganglionic eminence (19). In the primate cortex it has been suggested that interneurons, particularly calretinin-expressing inter-neurons, may also originate from the dorsal neuroepithelium of the lateral ventricle (20–22). Thus, the interneurons that are most altered in schizophrenia originate primarily from the medial ganglionic eminence, with some contribution from the caudal ganglionic eminence.
Given the developmental nature of schizophrenia, with the prodrome and disease-related symptoms often manifesting in adolescence or early adulthood (23), we next considered that late-maturing interneuron subtypes may be particularly vulnerable in schizophrenia. Our findings indicate that interneuron subtypes continue to differentiate and mature through the second decade of life and demonstrate peak mRNA expression of parvalbumin and cholecystokinin in the teenage years, consistent with previous reports (24, 25). Calbindin and vasoactive intestinal peptide mRNA show similar up-regulation during early development, with a peak in expression during the toddler years, and then decline from the teenage to the adulthood years. Indeed, mRNA expression of cholecystokinin, parv-albumin, and vasoactive intestinal peptide were reduced in schizophrenia, in accordance with reports of these reductions in the frontal cortex of people with schizophrenia (8–10, 12). Our finding of increased calbindin mRNA expression, however, is confounded by the expression of calbindin by pyramidal cells in layer III (26, 27) and is not widely replicated in schizophrenia; some studies have reported no change in calbindin immunopositive cell density (28) and others have reported reduced calbindin-positive cell density in layer II of the prefrontal cortex (25, 29, 30). The homogenate-based approach used here cannot distinguish whether an increase in calbindin expression may be attributed to interneurons and/or pyramidal neurons; in situ hybridization is required to determine this. One report, however, does support an increased density of calbindin-positive local circuit interneurons across all cortical layers, by 53% and 69% in Brodmann's areas 9 and 46, respectively, attributed particularly to changes in layers III and V/VI in the prefrontal cortex (31).
Consistent with our hypothesis, late-maturing interneuron markers displaying a delayed increase (parvalbumin and cholecystokinin) are altered in schizophrenia, as found in this study as well as others (9–11). Additionally, we found that interneuron marker mRNAs that decrease across post-natal life are reduced in schizophrenia, the most dramatic being somatostatin. Consistent with reports in developing primate frontal cortex, where high levels of somatostatin occur in the embryonic brain and decline to adult levels by postnatal day 60 (32), we found that somatostatin mRNA expression was gradually down-regulated over human postnatal development, although this reduction appears to occur over years. When outliers were excluded in our study, we observed a modest reduction in calretinin mRNA in schizophrenia, whereas prior studies have reported no alteration in calretinin mRNA in the dorsolateral pre-frontal cortex by polymerase chain reaction (10) or by in situ hybridization (8). This discrepancy may reflect the increased statistical power in our cohort (37 patients and 37 comparison subjects), which is more than double the size of other cohorts used in calretinin mRNA studies (12 pairs [8] or 15 pairs [10] of patients and comparison subjects) or may be due to differences in the cohorts studied. We did not observe a change in calretinin protein, which is consistent with other studies that found no change in the density of calretinin immunopositive cells (25, 28, 29). Significantly reduced expression of somatostatin (and of neuropep-tide Y and calretinin with the outliers excluded) mRNAs in schizophrenia suggest that not only markers indicative of late maturing interneuron subtypes but also interneuron mRNAs that are abundant during the early phases of life are reduced in schizophrenia. These results suggest that the interneuron deficit may be widespread and could indicate that the underlying mechanisms may be varied, such as a lack of early developmental induction in some individuals and/or a premature age-related decline in others.
Congruent with our third consideration, that aberrant expression of interneuron marker mRNAs may be triggered by alterations in a common upstream pathway, is the finding that expression of brain-derived growth factor (BDNF) and its receptor (TrkB), which are key in the differentiation and survival of interneurons, are reported to be altered in schizophrenia (33–35). BDNF and TrkB have been shown to up-regulate neuropeptide Y, somatostatin, and cholecystokinin expression in cortical neurons in vitro and in vivo (36–40). We observed reductions in somatostatin, neuropeptide Y, and cholecystokinin, which would be consistent with an impairment in BDNF signaling. Additionally, mice hypomorphic for TrkB demonstrate a reduction in GAD67 and parvalbumin expression (33) and have fewer somatostatin-positive cells (11), which suggests that BDNF-TrkB signaling may represent a common pathway for the differentiation of multiple interneuron subtypes, providing a feasible link to deficient interneuron differentiation or maintenance in the disease state, as has been suggested previously (33). Our results implicating multiple interneuron subtypes in the cortical pathology of schizophrenia suggest that inhibitory regulation of pyramidal cells may be affected at multiple subcellular levels. For example, parvalbumin-expressing chandelier cells target the axon of pyramidal cells, also implicated by reductions in GABA transporter-1 cartridges on axons and increased GABAA receptor α2 subunit expression in the axon initial segment (5). In contrast, somatostatin, calretinin, and neuropeptide Y positive cells generally target the dendrites of pyramidal cells. Our recent finding (41) showing a significant reduction in the mRNA encoding GABAA receptor α5 subunits, enriched in the dendrites, suggests that GABA-ergic abnormalities may occur pre- and postsynaptically on the dendrites of glutamatergic neurons. The reduction of six interneuron markers and an increase in calbindin mRNA levels in schizophrenia suggest that no one interneuron subtype or developmental profile appears to be particularly vulnerable in schizophrenia, although it appears that calretinin interneurons, but not necessarily vasoactive intestinal peptide interneurons, arising from the caudal ganglionic eminence are the least affected in schizophrenia, as a reduction in calretinin is most inconsistently changed across studies and not confirmed by protein expression in our study. These findings suggest that deficits in GABA-ergic interneurons may be commonly linked through upstream pathways that regulate their differentiation, such as BDNF-TrkB signaling, although further studies are needed to support this hypothesis.
1. : Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 2008; 9:557–568Crossref, Medline, Google Scholar
2. : Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 2004; 5:793–807Crossref, Medline, Google Scholar
3. : GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry 2001; 158:256–265Link, Google Scholar
4. : Decreased glutamic acid decarboxylase 67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry 2000; 57:237–245Crossref, Medline, Google Scholar
5. : Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology 2008; 33:141–165Crossref, Medline, Google Scholar
6. : Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry 1999; 156:1709–1719Abstract, Google Scholar
7. : A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA 1998; 95:5341–5346Crossref, Medline, Google Scholar
8. : Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 2003; 23:6315–6326Crossref, Medline, Google Scholar
9. : Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 2008; 13:147–161Crossref, Medline, Google Scholar
10. : Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry 2008; 165:479–489Link, Google Scholar
11. : Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex 2008; 18:1575–1587Crossref, Medline, Google Scholar
12. : Cholecystokinin messenger RNA deficit in frontal and temporal cerebral cortex in schizophrenia. Biol Psychiatry 1995; 37:694–701Crossref, Medline, Google Scholar
13. : Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin Genet 2007; 72:1–8Crossref, Medline, Google Scholar
14. : Selection of reference gene expression in a schizophrenia brain cohort. Aust NZ J Psychiatry 2010; 44:59–70Crossref, Medline, Google Scholar
15. : Transcriptome analysis of male-female differences in prefrontal cortical development. Mol Psychiatry 2009; 14:558–561Crossref, Medline, Google Scholar
16. : The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol 2009; 87:81–118Crossref, Medline, Google Scholar
17. : Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci 2007; 27:10935–10946Crossref, Medline, Google Scholar
18. : Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol 2008; 506:16–29Crossref, Medline, Google Scholar
19. : Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci 2010; 30:1582–1594Crossref, Medline, Google Scholar
20. : Origin of GABAergic neurons in the human neocortex. Nature 2002; 417:645–649Crossref, Medline, Google Scholar
21. : Origins of cortical GABAergic neurons in the cynomolgus monkey. Cereb Cortex 2009; 19:249–262Crossref, Medline, Google Scholar
22. : Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb Cortex 2009; 19:2196–2207Crossref, Medline, Google Scholar
23. : The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry 2001; 50:884–897Crossref, Medline, Google Scholar
24. : Regional distribution of cholecystokinin messenger RNA in rat brain during development: quantitation and correlation with cholecystokinin immunoreactivity. Neuropeptides 1990; 15:201–212Crossref, Medline, Google Scholar
25. : GABAergic neuronal subtypes in the human frontal cortex-development and deficits in schizophrenia. J Chem Neuroanat 2001; 22:95–100Crossref, Medline, Google Scholar
26. : Neocortical neuronal subpopulations labeled by a monoclonal antibody to calbindin exhibit differential vulnerability in Alzheimer's disease. Exp Neurol 1991; 111:293–301Crossref, Medline, Google Scholar
27. : Nonphosphorylated neurofilament protein and calbindin immunoreactivity in layer III pyramidal neurons of human neocortex. Cereb Cortex 1992; 2:56–67Crossref, Medline, Google Scholar
28. : Neurons expressing calcium-binding proteins in the prefrontal cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28:273–278Crossref, Medline, Google Scholar
29. : Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry 2002; 52:708–715Crossref, Medline, Google Scholar
30. : Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology 2008; 28:143–150Crossref, Medline, Google Scholar
31. : Local circuit neurons of the prefrontal cortex in schizophrenia: selective increase in the density of calbindinimmunoreactive neurons. Psychiatry Res 1995; 59:81–96Crossref, Medline, Google Scholar
32. : Ontogeny of somatostatin in cerebral cortex of macaque monkey: an immunohistochemical study. Brain Res Dev Brain Res 1989; 45:103–111Crossref, Medline, Google Scholar
33. : Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci 2005; 25:372–383Crossref, Medline, Google Scholar
34. : Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry 2003; 8:592–610Crossref, Medline, Google Scholar
35. : Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry 2005; 10:637–650Crossref, Medline, Google Scholar
36. : Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry 2006; 11:633–648Crossref, Medline, Google Scholar
37. : Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry 2008; 65:1006–1014Crossref, Google Scholar
38. : Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol 1994; 165:243–256Crossref, Medline, Google Scholar
39. : Regulation of neuropeptide expression in cultured cerebral cortical neurons by brain-derived neurotrophic factor. J Neurochem 1993; 60:772–775Crossref, Medline, Google Scholar
40. : Intraventricular administration of BDNF increases neuropeptide expression in newborn rat brain. J Neurosci 1994; 14:3751–3765Crossref, Medline, Google Scholar
41. : Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res 2010; 44:673–681Crossref, Medline, Google Scholar



