The Nature of Prefrontal Cortical GABA Neuron Alterations in Schizophrenia: Markedly Lower Somatostatin and Parvalbumin Gene Expression Without Missing Neurons
Abstract
Objective:
In schizophrenia, somatostatin (SST) and parvalbumin (PV) mRNA levels are lower in the dorsolateral prefrontal cortex (DLPFC), but it remains unclear whether these findings reflect lower transcript levels per neuron, fewer neurons, or both. Distinguishing among these alternatives has implications for understanding the pathogenesis of, and developing new treatments for, DLPFC dysfunction in schizophrenia.
Methods:
To identify SST and PV neurons in postmortem human DLPFC, the authors used fluorescent in situ hybridization to label cells expressing two transcripts not altered in schizophrenia: vesicular GABA transporter (VGAT; a marker of all GABA neurons) and SOX6 (a marker of only SST and PV neurons). In cortical layers 2 and 4, where SST and PV neurons, respectively, are differentially enriched, levels of SST and PV mRNA per neuron and the relative densities of SST-, PV-, and VGAT/SOX6–positive neurons were quantified.
Results:
In individuals with schizophrenia, mRNA levels per positive neuron were markedly and significantly lower for SST in both layers (effect sizes >1.48) and for PV only in layer 4 (effect size=1.14) relative to matched unaffected individuals. In contrast, the relative densities of all SST-, PV-, or VGAT/SOX6–positive neurons were unaltered in schizophrenia.
Conclusions:
Novel multiplex fluorescent in situ hybridization techniques permit definitive distinction between cellular levels of transcripts and the presence of neurons expressing those transcripts. In schizophrenia, pronounced SST and PV mRNA deficits are attributable to lower levels of each transcript per neuron, not fewer neurons, arguing against death or abnormal migration of these neurons. Instead, these neurons appear to be functionally altered and thus amenable to therapeutic interventions.
Alterations in markers of cortical gamma-aminobutyric acid (GABA) signaling are among the most widely replicated findings in postmortem brain studies of schizophrenia (1, 2). In the dorsolateral prefrontal cortex (DLPFC), consistent findings of lower levels of gene products related to GABA neuron signaling converge on the notion that inhibitory neurotransmission signaling is weaker in people with schizophrenia (1). Lower tissue levels of GABA-related transcripts could be a consequence of lower levels of these transcripts per neuron, fewer neurons that contain these transcripts, or both. Discriminating among these possibilities has major implications both for understanding the pathogenesis of GABA neuron dysfunction in schizophrenia and for developing novel treatment strategies. For example, the possibility of missing neurons in the DLPFC in schizophrenia, due to either incomplete neural migration or excessive neuronal death (3–5), has motivated interest in transplantation of embryonic GABA neurons as a therapeutic strategy (6, 7). In contrast, the alternative view that cortical GABA neurons are present but functionally altered in schizophrenia has motivated efforts to develop therapeutics that augment the function of the affected GABA neuron subtypes (8, 9). Moreover, this distinction has important implications for the development of animal models useful for the study of schizophrenia, as these model systems should recapitulate the nature of GABA neuron alterations observed in the postmortem brains of people with schizophrenia (10–12).
All published studies to date, whether using markers of all GABA neurons or of specific subsets of GABA neurons, have not been able to definitively determine whether lower levels of GABA-related transcripts reflect deficits in expression levels per neuron or in neuron number. Indeed, the first study of the mRNA encoding the 67-kDa isoform of glutamic acid decarboxylase (GAD67) mRNA, a principal GABA-synthesizing enzyme in the cortex, illustrates this challenge (13). The authors found markedly fewer neurons that were detectable by single-label in situ hybridization for GAD67 mRNA in the DLPFC of people with schizophrenia, findings interpreted as lower expression per neuron given that the density of all Nissl-stained neurons was not altered in the same individuals (13). However, other investigators reported that the density of small Nissl-stained neurons, presumably GABA neurons, was lower in the DLPFC of people with schizophrenia (14).
Studies of the subset of GABA neurons that contain the calcium-binding protein parvalbumin (PV) have produced similar interpretive challenges. Although lower levels of PV mRNA in schizophrenia have been confirmed in multiple studies of DLPFC tissue homogenates (15–20), the nature of this deficit remains a matter of debate (1, 5). Studies that measured PV mRNA at a cellular level of resolution reported lower levels of PV mRNA per neuron without a deficit in PV mRNA-positive neuron density (21, 22), whereas some (23–25), but not all (26–29), studies of PV protein reported a lower density of PV-immunoreactive neurons in schizophrenia. Interpretive uncertainty also exists for studies of somatostatin (SST), a neuropeptide present in a different subpopulation of cortical GABA neurons. SST mRNA levels are markedly lower in DLPFC tissue homogenates from people with schizophrenia (15, 17, 18, 30), and the only single-label in situ study reported both fewer SST mRNA-positive neurons and lower SST mRNA levels per positive neuron in schizophrenia (20).
One possible explanation for these apparently disparate findings is that the levels of the identifying gene products, SST and PV, are lower in many of these GABA neurons in schizophrenia, with levels in some neurons falling below the detectability threshold for the methods employed (31). In particular, using SST or PV—the levels of which are markedly lower in schizophrenia—as the sole label to identify the neurons of interest, and then quantifying the relative densities of neurons containing each label, conflates the independent and dependent variables and clouds interpretive clarity. Indeed, all previous studies that used single-label methods could not dissociate the presence of SST or PV neurons from the detectability of the identifying transcripts. Thus, counting neurons based on the detectability of a transcript in the presence of lower tissue levels of that transcript means that some neurons could be “missed” even if they are not “missing.”
In this study, we sought to address the challenge of determining whether lower tissue levels of SST and PV mRNAs in schizophrenia are due to lower levels of these transcripts per neuron, fewer neurons that contain these transcripts, or both. To do so, we used a novel multiplex fluorescent in situ hybridization approach to label four mRNA targets simultaneously with cellular resolution. This approach, which has not previously been used in postmortem human brain studies, permits the independent determination of transcript levels per neuron and of neuron density without conflating the two measures. Specifically, proxy markers (i.e., transcripts that are expressed in SST and PV neurons but whose expression in the DLPFC is not altered in schizophrenia [32–34]) were used to independently identify SST and PV neurons, and then levels of SST and PV in those neurons were quantified. We focused our analysis on layers 2 and 4 of the human DLPFC, as these layers exhibit the highest density of GABA neurons (13, 35, 36) and are differentially enriched for SST and PV neurons, respectively (20, 21, 37). Because the goal of the study was to identify the basis for the SST and PV mRNA deficits, we selected a subset of subjects with schizophrenia known to have pronounced deficits of both transcripts relative to matched unaffected comparison subjects. Given that the only published stereological analysis of total neuron number found a normal complement of neurons in the frontal lobe of people with schizophrenia (38), we predicted that levels of SST and PV mRNAs per neuron would be lower without a difference in neuron density in schizophrenia.
Methods
Human Postmortem Brain Tissue Specimens
Brain specimens (N=60) were obtained during routine autopsies conducted at the Allegheny County Office of the Medical Examiner (Pittsburgh; N=57) or the Davidson County Medical Examiner’s Office (Nashville; N=3) after consent was obtained from next of kin. An independent team of clinicians made consensus lifetime DSM-IV diagnoses for each subject using the results of an extensive psychological autopsy, including structured interviews with family members and review of medical records as well as toxicology and neuropathology reports (39). Unaffected comparison individuals had no known history of psychiatric or neurological disorders except for psychiatric diagnoses in remission in one subject (see the footnotes in Table S1 in the online supplement). The listed race and biological sex of the decedents (Table 1; see also Table S1) reflect the concurrence of information available in the autopsy report and next of kin interviews. All procedures were approved by the University of Pittsburgh Committee for Oversight of Research and Clinical Training Involving Decedents and Institutional Review Board for Biomedical Research.
| Characteristic | Unaffected Comparison Subjects (N=30) | Schizophrenia Subjects (N=30) | Analysis | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | χ2 | p | ||
| Sex | 1 | ||||||
| Male | 22 | 73.3 | 22 | 73.3 | |||
| Female | 8 | 26.7 | 8 | 26.7 | |||
| Race | 0.42 | 0.52 | |||||
| White | 25 | 23 | |||||
| Black | 5 | 7 | |||||
| Mean | SD | Mean | SD | t | df | p | |
| Age (years) | 51.5 | 14.3 | 50.1 | 13.8 | 0.40 | 1,58 | 0.70 |
| Body mass index | 28.6 | 6.1 | 28.3 | 8.2 | 0.15 | 1,56 | 0.88 |
| Postmortem interval (hours) | 19.0 | 5.7 | 19.0 | 8.6 | 0.04 | 1,58 | 0.97 |
| Brain pH | 6.7 | 0.2 | 6.5 | 0.3 | 2.60 | 1,58 | 0.01 |
| RNA integrity number | 8.2 | 0.6 | 8.0 | 0.7 | 1.20 | 1,58 | 0.22 |
| Tissue storage time (months) | 202.9 | 47.0 | 206.7 | 56.1 | 0.29 | 1,58 | 0.78 |
TABLE 1. Demographic and tissue characteristics of individuals with schizophrenia and unaffected comparison subjects
As discussed above, our goal in this study was to investigate the nature of DLPFC SST and PV mRNA deficits in schizophrenia. We therefore constructed a biased cohort of individuals with schizophrenia who had documented deficits in both SST and PV mRNAs. In a previous study (15), SST and PV mRNA levels were measured using quantitative polymerase chain reaction (qPCR) in DLPFC total gray matter homogenates from 62 subject pairs of individuals with schizophrenia and matched unaffected comparison individuals. From that study, we selected a subset of 30 subject pairs in which the individuals with schizophrenia had the most pronounced deficits in both transcripts. In this cohort, the mean deficit in the schizophrenia group was 50.1% for SST mRNA levels and 36.7% for PV mRNA levels in the DLPFC. The paired design, in which each schizophrenia sample was matched to one unaffected comparison sample for sex and as closely as possible for age (see Table S1 in the online supplement), was employed to reduce biological variance between groups and to control for experimental variance at every stage of the experiment. Mean age, postmortem interval, RNA integrity number, storage time at −80°C, or the distribution of race did not differ significantly between groups (Table 1). Although brain pH significantly differed between groups, the mean difference was 0.2 pH units and is of uncertain biological significance.
Fluorescent In Situ Hybridization Tissue Labeling
For each subject, fresh-frozen coronal tissue blocks containing right DLPFC area 9 were mounted in a cryostat, and sections 20 μm in thickness were cut, thaw-mounted onto SuperFrost slides (ThermoFisher Scientific, Waltham, Mass.), and stored at −80°C until tissue labeling. mRNA probes were designed by Advanced Cell Diagnostics, Inc. (Hayward, Calif.) to detect mRNAs encoding SST (gene symbol SST, HGNC ID:11329), PV (gene symbol PVALB, HGNC ID:9704), SRY-box transcription factor 6 (SOX6; HGNC ID:16421), and the vesicular GABA transporter (VGAT; gene symbol SLC32A1, HGNC ID:11018) (see Table S2 in the online supplement). VGAT was used as a GABA neuron marker because it is expressed by all GABA neurons but no other cell types, and levels of VGAT mRNA are unaltered or only modestly lower in the DLPFC of people with schizophrenia (33, 34). SOX6 (40, 41), which is selectively expressed in SST and PV neurons as a result of their shared embryonic origin in the medial ganglionic eminence and which continues to be robustly expressed in the adult primate neocortex (42), was used to distinguish SST and PV neurons from other GABA neurons. For details on probe fluorophore assignments and mRNA labeling protocols, see the Methods section in the online supplement.
Sampling
Images were collected on a custom widefield Olympus IX83 inverted microscope (Olympus, Center Valley, Pa.) equipped with a six-line (350-, 405-, 488-, 568-, 647-, 750-nm) Spectra III light engine (Lumencor, Beaverton, Ore.). The present study focused sampling on layers 2 and 4 for imaging, as these layers are known to be differentially enriched for SST and PV neurons, respectively, in the primate neocortex (20, 21, 37). Each imaging site was collected as a three-dimensional image stack (two-dimensional images successively captured at intervals separated by 0.25 μm in the z-dimension). Autofluorescence from lipofuscin was imaged in a separate channel, as previously described (43, 44). See the Methods section in the online supplement for details on imaging parameters.
Image Processing
For segmenting fluorescent grains, a Gaussian filter was applied for each channel (except the DAPI channel) by calculating a difference of Gaussians using sigma values of 0.7 and 2 in MATLAB, and an average z-projection algorithm was used to generate a two-dimensional representation of the three-dimensional image stack. Lipofuscin was subtracted from the resulting two-dimensional image (see the Methods section in the online supplement for details). The average lipofuscin levels in both subject groups for VGAT-positive and SOX6-positive neurons in layers 2 and 4 are shown in Figure S1 in the online supplement. DAPI-labeled nuclei were segmented using a deep learning algorithm, and fluorescent grains were counted within a cellular region of interest constituting the segmented nucleus and a 2-µm perimeter around the nucleus using CellProfiler, version 4.2.0 (45). Cells were classified based on the specific expression of a given transcript above background levels, and relative transcript levels per cell were quantified as the average grain density per cell (grains/μm2). See the Methods section in the online supplement for details on cell segmentation and classification.
Statistical Analysis
Linear mixed models (46, 47) were used to evaluate diagnostic differences between two dependent measures, the relative density of positive cells, and grain density per cell, for each cell type. These models included main effects of diagnosis, layer, and diagnosis-by-layer interaction. Covariates included age, sex, postmortem interval, pH, and RNA integrity number, with human subject included as a random factor. Tissue storage time at −80°C was not included as a covariate because it was previously shown not to be associated with SST or PV mRNA levels (15, 33). Within each layer, post hoc testing was conducted using estimated marginal means derived from the mixed model (48). We report an overall F test for the main effects of diagnosis, layer, and an interaction for each measure, and we report post hoc t tests for within-layer diagnosis effects derived from the main models. Statistical significance was set at an alpha of 0.05. Group differences in grain density per neuron were compared using the average grain density per neuron (grains/μm2) in each layer per subject. Likewise, analyses of neuron density were conducted on values of neurons/mm2 in each layer per subject. All statistical analyses and figure generation were conducted in R (46–51).
Effect sizes were computed for each dependent measure within a layer using the strategy described by Cohen (52). Here, a negative effect size indicates a lower value in the schizophrenia group relative to the unaffected comparison group, with effect sizes of 0 to −0.2 defined as nominal, −0.2 to −0.4 defined as small, −0.4 to −0.6 defined as medium, and −0.6 and greater defined as large. Bayes factors were used to evaluate the strength of the evidence in favor of either the alternative or the null hypothesis (53) and are reported relative to the alternative hypothesis notation, designated as BF10. We use Jeffrey’s interpretation of Bayes factor values (54): for evidence in favor of the alternative hypothesis, BF10 values of 1–3 are considered weak, values of 3–10 are considered strong, values of 10–100 are considered very strong, and values >100 are considered decisive. For evidence in favor of the null hypothesis, BF10 values of 0.34–0.99 are considered weak, values of 0.10–0.33 are considered strong, values of 0.01–0.10 are considered very strong, and values <0.01 are considered decisive. See the Methods section and tables in the online supplement for details on Bayes factor analyses, selection of priors, analyses of comorbid factors in individuals with schizophrenia, and complete statistical information from the analysis.
Results
Laminar Distribution of DLPFC GABA Neurons in Unaffected Individuals
Fluorescent in situ hybridization revealed robust labeling of all four mRNA targets. Most neurons labeled for both VGAT and SOX6 mRNAs (referred to as VGAT+/SOX6+) were either SST or PV mRNA–positive (referred to as SST+ or PV+, respectively) (Figure 1; see also Figure S2 in the online supplement). Conversely, most SST+ and PV+ neurons were VGAT+/SOX6+, and, as expected, VGAT+/SOX6− neurons expressed neither SST nor PV (Figure 1A). For the schizophrenia individual in pair 2 (see Table S1 in the online supplement), labeling of all four target mRNAs was poor; data from this pair were removed from the analysis.
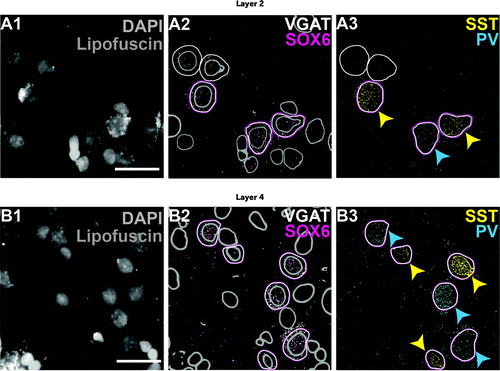
FIGURE 1. Representative high-magnification (60× objective) images of multiplex fluorescent in situ hybridization for four mRNA targets in layer 2 and layer 4 from an unaffected comparison individuala
aImages are from the comparison individual in pair 24 (case 1792; see Table S1 in the online supplement). Each row illustrates a single multichannel fluorescent image, and within each row, colors represent separate fluorophores. In panels A1 and B1, cellular nuclei counterstained by DAPI are shown in gray, and lipofuscin granules (clusters of light gray signal) are evident over cellular nuclei and surrounding cytoplasm (not labeled). In panels A2 and B2, segmented nuclei are indicated by gray outlines. Nuclei expressing both VGAT and SOX6 mRNAs are highlighted with magenta and white boundaries that demarcate the perinuclear area in which fluorescent mRNA grains were counted. Nuclei with only a white perinuclear boundary are VGAT+/SOX6−. Panels A3 and B3 show VGAT+/SOX6+ nuclei that contain either SST mRNA (yellow arrowheads) or PV mRNA (blue arrowheads). Scale bars equal 30 μm and apply to all panels in the same row. DAPI=4',6-diamidino-2-phenylindole; PV=parvalbumin; SOX6=SRY-box transcription factor 6; SST=somatostatin; VGAT=vesicular GABA transporter.
In the unaffected comparison individuals, the density of VGAT+/SOX6− neurons was greater in layer 2 (mean=56.6 neurons/mm2, SD=13.9; 9.6% of all layer 2 nuclei) than in layer 4 (mean=28.0 neurons/mm2, SD=6.8; 4.3% of all layer 4 nuclei), consistent with previous findings that caudal ganglionic eminence–derived interneurons (e.g., those containing calretinin and/or vasoactive intestinal polypeptide [55]) are enriched in the superficial layers of the primate neocortex (56). In contrast, the density of VGAT+/SOX6+ neurons was similar in layers 2 (mean=61.9 neurons/mm2, SD=15.5; 10.5% of all layer 2 nuclei) and 4 (mean=59.9 neurons/mm2, SD=9.9; 9.2% of all layer 4 nuclei). Among VGAT+/SOX6+ neurons, the density of SST+ neurons was greater in layer 2 (mean=36.0 neurons/mm2, SD=11.5) than in layer 4 (mean=22.2 neurons/mm2, SD=5.7), whereas the density of PV+ neurons was greater in layer 4 (mean=31.7 neurons/mm2, SD=7.0) than in layer 2 (mean=13.4 neurons/mm2, SD=6.1), findings consistent with previous studies of the differential laminar distribution of SST and PV neurons (20, 21, 37).
Markedly Lower Levels of SST and PV mRNA per Neuron Without a Deficit in Neuron Density in Schizophrenia
In SST+ neurons, levels of SST mRNA per neuron were lower in individuals with schizophrenia relative to unaffected individuals (F=40.7, df=1,51, p<0.0001) in both layers 2 and 4 (t ratio>5.3, p<0.0001), with large effect sizes (layer 2 effect size=−1.48; layer 4 effect size=−1.64) (Figure 2A). SST mRNA levels per neuron were lower in the individual with schizophrenia in 24 and 26 pairs in layers 2 and 4, respectively (Figure 2B). Across all SST+ neurons, the distribution of SST levels per neuron in both layers was left-shifted in schizophrenia (see Figure S3A in the online supplement). In contrast, the relative density of SST neurons did not differ significantly between subject groups (F=0.001, df=1,51, p=0.97) in either layer 2 or layer 4 (Figure 2C,D). Bayes factor values strongly supported the evidence of there being no group difference in SST neuron density in either layer (both BF10 values <0.27). In addition, SST levels per neuron averaged across both layers were positively correlated (r=0.86, p<0.0001) (see Figure S4A in the online supplement) with SST transcript levels measured previously by qPCR in total tissue homogenates in these same subjects (15), whereas the density of SST neurons was not (r=0.23, p=0.09) (see Figure S4B in the online supplement); these comparisons further support the hypothesis that lower tissue levels of SST mRNA in schizophrenia solely reflect lower SST mRNA levels per neuron.
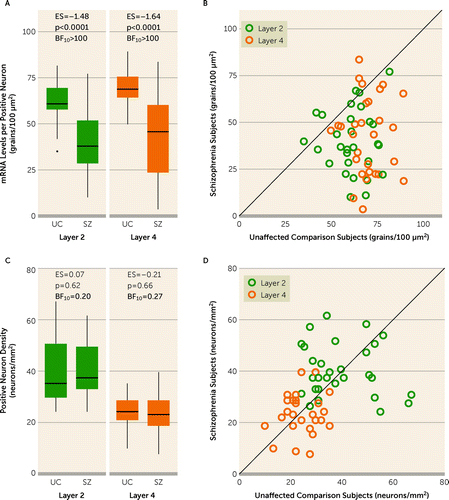
FIGURE 2. SST mRNA levels per SST+ neuron and relative density of SST+ neuronsa
aSST+ neurons were defined as any nucleus that contained SST, VGAT, and SOX6 mRNAs. Panel A presents box plots of the median, quartile, and 95% range of unaffected comparison (UC) and schizophrenia (SZ) groups for SST mRNA levels per SST+ neuron. Subjects outside the 95% range are shown as dots in the plot for layer 2. Within the box plots, values for the effect size (ES), the post hoc between-group comparison p value, and the Bayes factor (BF10) in favor of the alternative hypothesis are shown; bolded effect sizes and p values indicate large and statistically significant effects, respectively. Bolded BF10 values indicate decisive evidence for the alternative hypothesis. In panel B, SST levels per SST+ neuron are shown as individual subject data in unity plots. Individual points represent a subject pair; the x-axis shows the value for the unaffected comparison individual, and the y-axis shows the value for the individual with schizophrenia. Points below the line indicate a lower value in the individual with schizophrenia relative to their matched unaffected individual. SST mRNA levels per neuron were lower in the individual with schizophrenia in 24 of 29 and 26 of 29 subject pairs in layers 2 and 4, respectively. Panel C presents box plots of the median, quartile, and 95% range of unaffected comparison and schizophrenia groups for the relative density of SST neurons in each layer. The bolded BF10 values indicate strong evidence in favor of the null hypothesis. Panel D is a unity plot of the individual subject data for the relative density of SST neurons in each layer. In all panels, data are shown for 29 subject pairs. SOX6=SRY-box transcription factor 6; SST=somatostatin; VGAT=vesicular GABA transporter.
In PV+ neurons, levels of PV mRNA were markedly lower in schizophrenia (F=14.0, df=1,51, p=0.0005). The diagnosis-by-layer interaction was also significant (F=18.7, df=1,56, p<0.0001), reflecting the fact that PV levels were lower in layer 4, with a large effect size (effect size=−1.1), but not in layer 2 (effect size=−0.23), in schizophrenia relative to unaffected individuals (Figure 3A). In layer 4, levels of PV mRNA per neuron were lower in schizophrenia in 24 of the pairs (Figure 3B), and the distribution of PV levels per neuron appeared to be left-shifted in schizophrenia (see Figure S3B in the online supplement). The relative density of PV neurons did not differ significantly between subject groups (F=0.05, df=1,51, p=0.83) in either layer 2 or 4 (Figure 3C, 3D), and Bayes factor values strongly supported the evidence of no group difference in both layers (both BF10 values <0.27). In addition, PV levels per neuron in layer 4 were highly correlated (r=0.55, p<0.0001; see Figure S4C in the online supplement) with PV transcript levels measured previously by qPCR in total tissue homogenates in these same subjects (15), whereas the density of PV neurons was not (r<0.01, p>0.99) (see Figure S4D in the online supplement). These comparisons further support the hypothesis that lower tissue levels of PV mRNA in schizophrenia solely reflect lower PV mRNA levels per neuron in layer 4.
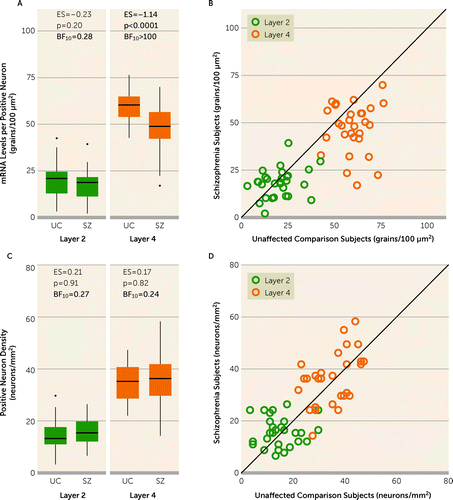
FIGURE 3. PV mRNA levels per PV+ neuron and relative density of PV+ neuronsa
aPV+ neurons were defined as any nucleus that contained PV, VGAT, and SOX6 mRNAs. Panel A presents box plots of the median, quartile, and 95% range of unaffected comparison (UC) and schizophrenia (SZ) groups for PV mRNA levels per PV+ neuron. Subjects outside the 95% range are shown as dots in the plots. Within the box plots, values for the effect size (ES), the post hoc between-group comparison p value, and the Bayes factor (BF10) in favor of the alternative hypothesis are shown; bolded values indicate large and statistically significant effects for PV levels per PV+ neuron in layer 4, and the bolded BF10 value indicates strong evidence for the null hypothesis for PV levels per PV+ neuron in layer 2 or decisive evidence for the alternative hypothesis for PV levels per PV+ neuron in layer 4. In panel B, PV levels per PV+ neuron are shown as individual subject data in unity plots. In these plots, individual points represent a subject pair; the x-axis shows the value for the unaffected comparison individual, and the y-axis shows the value for the individual with schizophrenia. Points below the line indicate a lower value in the individual with schizophrenia relative to their matched unaffected individual. For PV mRNA levels per neuron, PV mRNA levels were lower in the individual with schizophrenia in 24 of 29 subject pairs in layer 4. Panel C presents box plots of the median, quartile, and 95% range of unaffected and schizophrenia subjects for the relative density of PV neurons in each layer. Subjects outside the 95% range are shown as dots in the plot for layer 2. The bolded BF10 values indicate strong evidence in favor of the null hypothesis. Panel D is a unity plot of the individual subject data for the relative density of PV neurons in each layer. In all panels, data are shown for 29 subject pairs in each layer. PV=parvalbumin; SOX6=SRY-box transcription factor 6; VGAT=vesicular GABA transporter.
Unaltered Density of VGAT+/SOX6+ Neurons in Schizophrenia
To assess the robustness of the finding that the densities of SST and PV neurons were not altered in schizophrenia, we also quantified the density of VGAT+/SOX6+ in both layers, independent of the presence or absence of SST or PV mRNAs. The density of VGAT+/SOX6+ neurons did not differ between diagnostic groups (F=3.1, df=1,51, p=0.08) in either layer 2 (t ratio=−1.8, p=0.08) or layer 4 (t ratio=−1.0, p=0.32) (Figure 4A,B). In fact, although not significant, the effect size of the group difference in the density of VGAT+/SOX6+ neurons was small but positive in both layers 2 and 4 (effect sizes, 0.37 and 0.23, respectively).
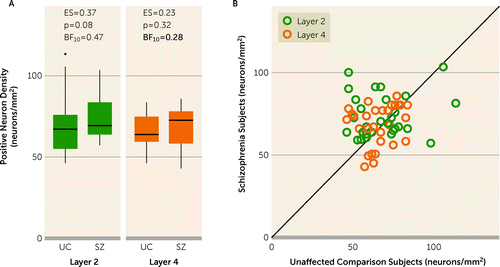
FIGURE 4. Relative densities of all VGAT+/SOX6+ neurons independent of the presence or absence of SST or PV mRNAsa
aPanel A presents box plots of the median, quartile, and 95% range of unaffected comparison (UC) and schizophrenia (SZ) groups for the relative density of VGAT+/SOX6+ neurons. Subjects outside the 95% range are shown as dots in the plot for layer 2. Within the box plots, values for effect size (ES), the post hoc between-group comparison p value, and the Bayes factor (BF10) in favor of the alternative hypothesis are shown. The bolded BF10 value indicates strong evidence for the null hypothesis. In panel B, the relative density of VGAT+/SOX6+ neurons is shown as individual subject data in unity plots. In these plots, individual points represent a subject pair; the x-axis shows the value for the unaffected individual, and the y-axis shows the value for the individual with schizophrenia. Points below the line indicate a lower value in the individual with schizophrenia relative to their matched unaffected individual. In all graphs, data are shown for 29 subject pairs in each layer. PV=parvalbumin; SOX6=SRY-box transcription factor 6; SST=somatostatin; VGAT=vesicular GABA transporter.
In addition, consistent with previous findings that VGAT and SOX6 mRNA levels in total DLPFC gray matter are not markedly altered in schizophrenia (32–34), we did not find evidence of lower VGAT (F=2.0, df=1,51, p=0.17) or SOX6 (F=2.1, df=1,51, p=0.15) mRNA levels per VGAT+/SOX6+ neuron in schizophrenia in either layer (see Figure S5 in the online supplement).
Influence of Comorbid Factors on SST and PV Levels per Neuron in Schizophrenia
Levels of SST mRNA per neuron in layer 2 or 4, or levels of PV mRNA per neuron in layer 4, in individuals with schizophrenia did not appear to differ on the basis of death by suicide, a diagnosis of schizoaffective disorder, comorbid substance or alcohol use disorder at time of death, or nicotine, antipsychotic, antidepressant, or benzodiazepine/antiepileptic use at time of death (see Figures S6 and S7 in the online supplement). Here, the comparison for antipsychotics is limited given the small number of individuals with schizophrenia who were not on antipsychotics at time of death. However, previous studies in larger cohorts of individuals with schizophrenia on and off antipsychotics (15), in individuals with mood disorders taking antipsychotics at time of death (57), and in monkeys chronically treated with antipsychotics (16, 20, 21) did not show evidence of altered levels of either SST or PV mRNAs with antipsychotic exposure, strongly suggesting that the findings here do not reflect confounding from antipsychotic use.
Discussion
Deficits in DLPFC tissue levels of SST and PV mRNA have been reported in multiple cohorts of subjects with schizophrenia by several research groups using different quantitative methods (15–17, 20, 58–60). However, the nature of those deficits has been unclear, with some reviews suggesting that schizophrenia is associated with fewer GABA neurons (5, 61) and others suggesting that GABA neurons are functionally altered but not fewer in number in this illness (2, 62). However, all of the studies on which these interpretations are based lacked the technical capacity to distinguish between these alternative views. This issue similarly extends to recent studies using new techniques. An analysis of the DLPFC transcriptome at the single-nucleus level of resolution led to the conclusion that there was a lower abundance of GABA neurons in the upper cortical layers, most notably in SST neurons (63), yet similarly relied on markers of GABA neurons known to be altered in schizophrenia as the identifying features.
Here, using a multilabel fluorescent in situ technique that overcomes earlier technical limitations of brightfield in situ approaches, we demonstrate that schizophrenia is associated with lower SST and PV mRNA levels per neuron in the DLPFC, without any difference in the relative density of either neuron subtype. Even in individuals with schizophrenia who exhibit pronounced deficits in SST and PV mRNA levels in tissue homogenates, we found strong evidence that there are not fewer neurons in those individuals. Thus, the basis for lower tissue levels of SST and PV mRNAs in schizophrenia is lower gene expression per neuron and not fewer neurons. As discussed below, these findings have important implications both for understanding the pathogenesis of GABA neuron alterations in schizophrenia and for the design of therapeutic interventions targeting these alterations.
Novel Multiplex In Situ Hybridization Approaches Clarify the Nature of GABA Neuron Disturbances in Schizophrenia
Previous postmortem studies of SST+ and PV+ neurons have produced mixed findings as to whether the density of these neurons is lower in the DLPFC in schizophrenia. These mixed findings likely reflect multiple technical limitations of these studies (1). Specifically, in all published studies to date of SST or PV neuron density in schizophrenia, lower gene expression per neuron could have rendered some SST or PV neurons undetectable using the single-label methods employed.
The approach used in the present study includes several important advances that provide a clear picture of the nature of SST and PV mRNA deficits in schizophrenia. First, relative to previous radiolabeled in situ hybridization assays, fluorescent in situ hybridization assays have markedly higher sensitivity and specificity, in part as a result of lower background grain density (64, 65). For example, using single-label in situ hybridization for SST mRNA in schizophrenia, we previously reported a lower density of SST mRNA–positive neurons (20); however, the high background labeling of mRNA grains in that study rendered low-expressing neurons difficult to detect. The findings of the present study demonstrate that neurons with low SST expression can be detected with the appropriate experimental design and more sensitive techniques.
We also found that the density of PV mRNA–expressing neurons was not altered in the illness. Thus, previous studies reporting lower densities of PV-immunoreactive neurons in schizophrenia (5) may have been confounded by a limited ability to detect those neurons given the techniques employed. For example, in the same tissue sections from schizophrenia and unaffected comparison individuals, a deficit in PV neuron density that appeared to be present at lower magnification was clearly not present at higher magnification (29). Similarly, previous studies that reported a deficit in PV-immunoreactive neuron density using paraffin-embedded sections (66) were likely confounded by the reduced immunoreactivity associated with that approach (31), an interpretation supported by findings of no schizophrenia-associated deficits in PV-immunoreactive neuron density in studies using immunohistochemical methods with more robust antigen detection (27–29).
Second, even with methodological advances that improve sensitivity for detecting labeled neurons, approaches that rely on visualizing SST or PV alone still conflate the expression level of the indexing transcript with the presence of the neuron. Here, simultaneously labeling for both SST and PV in concert with their colocalization with independent proxy markers of these neurons (VGAT and SOX6) has demonstrated that the densities of SST and PV neurons are not altered in schizophrenia. This interpretation is particularly robust given that we used a cohort of individuals with pronounced deficits in SST and PV mRNA levels in DLPFC total gray matter (15), which would have maximized the likelihood of detecting a deficit in SST or PV neuron density in schizophrenia if either existed.
Finally, proper interpretation of null results requires more evidence than is available from p values generated by frequentist statistical tests (53). Bayes factors (67) provide a quantitative metric for evaluating the strength of the null hypothesis over the alternative. Here, for all findings of neuron density, the statistical null hypothesis was at least three times more likely than the alternative hypothesis, providing strong evidence for no difference in neuron density between subject groups. In contrast, the evidence for the findings of lower SST or PV mRNA per neuron can be considered decisive.
Together, the methods employed here provide multiple advances over previous methods to strongly support the conclusion that SST and PV mRNA expression is lower, but that SST and PV neurons are not missing, in the DLPFC of individuals with schizophrenia. We note that the present study only indexes neuron density, not absolute neuron number, which can only be assessed using stereological methods. However, consistent with the findings here, the only such study to date found no difference in total neuron number in the frontal lobe of people with schizophrenia (38). Furthermore, we note that our study was not designed to estimate the true effect size of the SST or PV mRNA per neuron deficit, given that we deliberately selected subjects with schizophrenia known to have deficits of SST and PV mRNA levels in tissue homogenates.
Implications for Understanding the Etiology of GABA Neuron Dysfunction in Schizophrenia
Our finding of a normal complement of SST and PV neurons in DLPFC layers 2 and 4 strongly argues that neither excessive neuronal death nor aberrant migration of these GABA neurons is operative in the disease process of schizophrenia. In contrast, the presence of lower levels of both SST and PV mRNAs in schizophrenia is more likely to reflect upstream alterations in the cortical circuit shared by SST and PV neurons. The expression of both SST (68–72) and PV (73, 74) transcripts appears to be influenced by neuronal activity, suggesting that deficient excitatory drive to these neurons in schizophrenia could account for lower SST and PV mRNA levels. In monkey DLPFC, ∼50% of local axon collaterals from layer 3 pyramidal neurons innervate GABA neurons (75), with most of these synapses targeting PV neurons in layer 4 and most likely SST neurons in layer 2 (76). In schizophrenia, convergent lines of evidence suggest that layer 3 pyramidal neurons are hypoactive in the DLPFC (77–79), suggesting that weaker excitatory drive from layer 3 pyramidal neurons might contribute to the activity-dependent downregulation of SST and PV mRNAs.
Alternatively, these alterations in SST and PV neurons might emerge in early development in individuals who are later diagnosed with schizophrenia. Early environmental insults, such as maternal immune activation, have been reported to alter the properties of SST (80) and PV (81) neurons in rodent models, including lower expression of both transcripts. Finally, altered gene expression in SST and PV neurons could reflect independent, cell type–specific alterations in schizophrenia. For example, altered alternative splicing of ErbB4 in PV neurons could lead to fewer excitatory synapses onto these cells and lower PV expression (28, 82); consistent with this idea, ErbB4 splicing shifts are correlated with PV levels in schizophrenia (82). Alterations to N-methyl-d-aspartate receptors (NMDARs) have been posited to principally affect SST neurons (reviewed in reference 83) and not PV neurons (84), suggesting that hypofunction of NMDARs in SST neurons may contribute to lower activation of these cells and subsequent downregulation of activity-dependent SST expression.
Finally, it appears unlikely from these and other data that SST and PV mRNA deficits in schizophrenia are secondary to features frequently comorbid with schizophrenia, and thus it appears that they likely reflect the underlying disease process. First, the present study failed to find any significant associations between comorbid factors measured here and SST or PV levels per neuron among individuals diagnosed with schizophrenia. Second, previous studies failed to find evidence of altered SST or PV mRNA levels in monkeys chronically exposed to antipsychotic medications (16, 21). Third, the levels of VGAT and SOX6 mRNAs were not significantly altered within these same neurons, arguing that the deficit in SST and PV mRNAs is due to the disease process of schizophrenia rather than to ante- or postmortem factors in schizophrenia that contribute to RNA degradation.
Implications for Therapeutic Strategies Targeting Cognitive Dysfunction in Schizophrenia
If deficient inhibitory signaling from DLPFC SST and PV neurons represents part of the neural substrate for cognitive dysfunction in schizophrenia (85–87), augmenting the excitability of these neurons could enhance inhibitory capacity and improve cognitive function in the disorder. Our findings of a normal complement of SST and PV neurons suggests that these neurons are viable targets for novel procognitive therapeutic interventions (88–91). Some preclinical models provide support for this strategy. For example, a recent study found that positive allosteric modulators acting at mGluR1 receptors preferentially target SST neurons and enhance performance on certain working memory tasks in rodents (92). Alternatively, if SST and PV neuron alterations are a consequence of diminished excitatory drive from layer 3 pyramidal neurons (77–79), then augmentation of pyramidal neuron function, and in turn more robust engagement of inhibitory neurotransmission from SST and PV neurons, could restore normal circuitry activity in the DLPFC of individuals with schizophrenia.
Conclusions
Our study elucidates the nature of SST and PV neuron mRNA deficits in schizophrenia by finding robust evidence for lower gene expression per neuron of both transcripts without deficits in the density of either type of neuron in the DLPFC. These findings have important implications for understanding the schizophrenia-associated pathogenesis of GABA neuron dysfunction, designing and validating animal models useful for the study of the disorder (10–12), and informing novel strategies for procognitive therapeutic interventions. The finding that both SST and PV neurons are present in normal complement in schizophrenia encourages the use of methodological strategies employed in the present study to identify novel therapeutic targets that are shared between these cell types or specific to either cell type. The methodological strategies used in this study offer a road map for the conduct of such cell type–specific investigations in the context of schizophrenia.
1. : Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiol Dis 2019; 131:104208Crossref, Medline, Google Scholar
2. : GABAergic mechanisms in schizophrenia: linking postmortem and in vivo studies. Front Psychiatry 2017; 8:118Crossref, Medline, Google Scholar
3. : White matter neuron biology and neuropathology in schizophrenia. NPJ Schizophr 2019; 5:10Crossref, Medline, Google Scholar
4. : Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry 1996; 53:425–436Crossref, Medline, Google Scholar
5. : Pre-frontal parvalbumin interneurons in schizophrenia: a meta-analysis of post-mortem studies. J Neural Transm (Vienna) 2019; 126:1637–1651Crossref, Medline, Google Scholar
6. : Losing your inhibition: linking cortical GABAergic interneurons to schizophrenia. Neurobiol Dis 2013; 53:36–48Crossref, Medline, Google Scholar
7. : Interneurons from embryonic development to cell-based therapy. Science 2014; 344:1240622Crossref, Medline, Google Scholar
8. : GABA targets for the treatment of cognitive dysfunction in schizophrenia. Curr Neuropharmacol 2005; 3:45–62Crossref, Medline, Google Scholar
9. : GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharmacol Sin 2018; 39:733–753Crossref, Medline, Google Scholar
10. : Neural circuit dysfunction in schizophrenia: insights from animal models. Neuroscience 2016; 321:42–65Crossref, Medline, Google Scholar
11. : Animal models of neuropsychiatric disorders. Nat Neurosci 2010; 13:1161–1169Crossref, Medline, Google Scholar
12. : Animal models of psychiatric disorders. Neuroscience 2016; 321:1–2Crossref, Medline, Google Scholar
13. : Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 1995; 52:258–266Crossref, Medline, Google Scholar
14. : Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 1991; 48:996–1001Crossref, Medline, Google Scholar
15. : Cortical GABA markers identify a molecular subtype of psychotic and bipolar disorders. Psychol Med 2016; 46:2501–2512Crossref, Medline, Google Scholar
16. : Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 2008; 13:147–161Crossref, Medline, Google Scholar
17. : Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry 2010; 167:1479–1488Link, Google Scholar
18. : Expression of transcripts selective for GABA neuron subpopulations across the cortical visuospatial working memory network in the healthy state and schizophrenia. Cereb Cortex 2019; 29:3540–3550Crossref, Medline, Google Scholar
19. : Relationship between somatostatin and death receptor expression in the orbital frontal cortex in schizophrenia: a postmortem brain mRNA study. NPJ Schizophr 2015; 1:14004Crossref, Medline, Google Scholar
20. : Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex 2008; 18:1575–1587Crossref, Medline, Google Scholar
21. : Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 2003; 23:6315–6326Crossref, Medline, Google Scholar
22. : Transcriptional dysregulation of γ-aminobutyric acid transporter in parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. Psychiatry Res 2014; 220:1155–1159Crossref, Medline, Google Scholar
23. : GABAergic neuronal subtypes in the human frontal cortex: development and deficits in schizophrenia. J Chem Neuroanat 2001; 22:95–100Crossref, Medline, Google Scholar
24. : Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology 2008; 28:143–150Crossref, Medline, Google Scholar
25. : Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res 1997; 24:349–355Crossref, Medline, Google Scholar
26. : Neurons expressing calcium-binding proteins in the prefrontal cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28:273–278Crossref, Medline, Google Scholar
27. : Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry 1997; 154:1013–1015Link, Google Scholar
28. : Pathological basis for deficient excitatory drive to cortical parvalbumin interneurons in schizophrenia. Am J Psychiatry 2016; 173:1131–1139Link, Google Scholar
29. : Reduced labeling of parvalbumin neurons and perineuronal nets in the dorsolateral prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology 2016; 41:2206–2214Crossref, Medline, Google Scholar
30. : Schizophrenia and bipolar disorder show both common and distinct changes in cortical interneuron markers. Schizophr Res 2014; 155:26–30Crossref, Medline, Google Scholar
31. : Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Curr Pharm Biotechnol 2012; 13:1557–1562Crossref, Medline, Google Scholar
32. : Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry 2012; 169:1082–1091Link, Google Scholar
33. : Altered cortical expression of GABA-related genes in schizophrenia: illness progression vs developmental disturbance. Schizophr Bull 2015; 41:180–191Crossref, Medline, Google Scholar
34. : Lack of change in markers of presynaptic terminal abundance alongside subtle reductions in markers of presynaptic terminal plasticity in prefrontal cortex of schizophrenia patients. Biol Psychiatry 2011; 69:71–79Crossref, Medline, Google Scholar
35. : Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry 2000; 57:237–245Crossref, Medline, Google Scholar
36. : Distribution of GABA-containing neurons in human frontal cortex: a quantitative immunocytochemical study. Anat Embryol (Berl) 1994; 189:139–145Crossref, Medline, Google Scholar
37. : Distinct laminar and cellular patterns of GABA neuron transcript expression in monkey prefrontal and visual cortices. Cereb Cortex 2021; 31:2345–2363Crossref, Medline, Google Scholar
38. : No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J Psychiatr Res 2001; 35:15–21Crossref, Medline, Google Scholar
39. : Proxy measures of premortem cognitive aptitude in postmortem subjects with schizophrenia. Psychol Med 2020; 50:507–514Crossref, Medline, Google Scholar
40. : SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci 2009; 12:1238–1247Crossref, Medline, Google Scholar
41. : The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron 2009; 63:466–481Crossref, Medline, Google Scholar
42. : Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci 2013; 16:1588–1597Crossref, Medline, Google Scholar
43. : Laminar distribution of subsets of GABAergic axon terminals in human prefrontal cortex. Front Neuroanat 2018; 12:9Crossref, Medline, Google Scholar
44. : Markedly lower glutamic acid decarboxylase 67 protein levels in a subset of boutons in schizophrenia. Biol Psychiatry 2016; 79:1006–1015Crossref, Medline, Google Scholar
45. : CellProfiler 4: improvements in speed, utility, and usability. BMC Bioinformatics 2021; 22:433Crossref, Medline, Google Scholar
46. : Fitting linear mixed-effects models using lme4. J Stat Soft 2015; 67:1–48 Crossref, Google Scholar
47. : lmerTest package: tests in linear mixed effects models. J Stat Soft 2017; 82:1–26 Crossref, Google Scholar
48. : emmeans: Estimated marginal means, aka least-squares means. 2020. https://CRAN.R-project.org/package=emmeans Google Scholar
49. : R: A Language and Environment for Statistical Computing, version 3.5.2. 2018. https://www.r-project.org/ Google Scholar
50. : BayesFactor: computation of Bayes factors for common designs. 2021. https://CRAN.R-project.org/package=BayesFactor Google Scholar
51. : rstatix: Pipe-friendly framework for basic statistical tests. 2021. https://CRAN.R-project.org/package=rstatix Google Scholar
52. : Statistical Power Analysis for the Behavioral Sciences. Oxfordshire, UK, Routledge, 1988 Google Scholar
53. : Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat Neurosci 2020; 23:788–799Crossref, Medline, Google Scholar
54. : Theory of Probability, 3rd ed. Oxford, UK, Oxford University Press, 1961 Google Scholar
55. : Vasoactive intestinal polypeptide containing neurones in monkey medial prefrontal cortex (mPFC): colocalisation with calretinin. Brain Res 1997; 744:179–184Crossref, Medline, Google Scholar
56. : Neocortical neuronal diversity: chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cereb Cortex 1993; 3:273–289Crossref, Medline, Google Scholar
57. : Altered ErbB4 splicing and cortical parvalbumin interneuron dysfunction in schizophrenia and mood disorders. Neuropsychopharmacology 2018; 43:2478–2486Crossref, Medline, Google Scholar
58. : Putative psychosis genes in the prefrontal cortex: combined analysis of gene expression microarrays. BMC Psychiatry 2008; 8:87Crossref, Medline, Google Scholar
59. : Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci 2016; 19:1442–1453Crossref, Medline, Google Scholar
60. : A combined analysis of microarray gene expression studies of the human prefrontal cortex identifies genes implicated in schizophrenia. J Psychiatr Res 2012; 46:1464–1474Crossref, Medline, Google Scholar
61. : The neurochemical pathology of schizophrenia: post-mortem studies from dopamine to parvalbumin. J Neural Transm (Vienna) 2022; 129:643–647Crossref, Medline, Google Scholar
62. : Transforming discoveries about cortical microcircuits and gamma oscillations into new treatments for cognitive deficits in schizophrenia. Am J Psychiatry 2022; 179:267–276Link, Google Scholar
63. : Upper cortical layer-driven network impairment in schizophrenia. Sci Adv 2022; 8:eabn8367Crossref, Medline, Google Scholar
64. : Detection and quantification of multiple RNA sequences using emerging ultrasensitive fluorescent in situ hybridization techniques. Curr Protoc Neurosci 2019; 87:e63Crossref, Medline, Google Scholar
65. : RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2012; 14:22–29Crossref, Medline, Google Scholar
66. : Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry 2002; 52:708–715Crossref, Medline, Google Scholar
67. : Bayes factors. J Am Stat Assoc 1995; 90:773–795 Crossref, Google Scholar
68. : Activity-regulated somatostatin expression reduces dendritic spine density and lowers excitatory synaptic transmission via postsynaptic somatostatin receptor 4. J Biol Chem 2013; 288:2501–2509Crossref, Medline, Google Scholar
69. : Postnatal development of somatostatin-containing neurons in the visual cortex of normal and dark-reared rats. Exp Brain Res 1993; 92:473–478Crossref, Medline, Google Scholar
70. : Differences in the regulation of neuropeptide Y, somatostatin, and parvalbumin levels in hippocampal interneurons by neuronal activity and BDNF. Prog Brain Res 2000; 128:193–202Crossref, Medline, Google Scholar
71. : The expression pattern of somatostatin and calretinin by postnatal hippocampal interneurons is regulated by activity-dependent and -independent determinants. Neuroscience 1997; 80:79–88Crossref, Medline, Google Scholar
72. : Gamma-aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res, 1995;689:172–182Crossref, Medline, Google Scholar
73. : Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 2013; 504:272–276Crossref, Medline, Google Scholar
74. : Regulation of calcium-binding protein immunoreactivity in GABA neurons of macaque primary visual cortex. Cereb Cortex 1996; 6:271–287Crossref, Medline, Google Scholar
75. : Synaptic targets of the intrinsic axon collaterals of supragranular pyramidal neurons in monkey prefrontal cortex. J Comp Neurol 2001; 430:209–221Crossref, Medline, Google Scholar
76. : Pyramidal neuron local axon terminals in monkey prefrontal cortex: differential targeting of subclasses of GABA neurons. Cereb Cortex 2003; 13:452–460Crossref, Medline, Google Scholar
77. : Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry 2015; 20:1397–1405Crossref, Medline, Google Scholar
78. : Altered markers of cortical γ-aminobutyric acid neuronal activity in schizophrenia: role of the NARP gene. JAMA Psychiatry 2015; 72:747–756Crossref, Medline, Google Scholar
79. : Diagnosis- and cell type-specific mitochondrial functional pathway signatures in schizophrenia and bipolar disorder. Am J Psychiatry 2020; 177:1140–1150Link, Google Scholar
80. : Reduced cortical somatostatin gene expression in a rat model of maternal immune activation. Psychiatry Res 2019; 282:112621Crossref, Medline, Google Scholar
81. : Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol Psychiatry 2016; 21:956–968Crossref, Medline, Google Scholar
82. : Dysregulated ErbB4 splicing in schizophrenia: selective effects on parvalbumin expression. Am J Psychiatry 2016; 173:60–68Link, Google Scholar
83. : Impaired tuning of neural ensembles and the pathophysiology of schizophrenia: a translational and computational neuroscience perspective. Biol Psychiatry 2017; 81:874–885Crossref, Medline, Google Scholar
84. : NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull 2012; 38:950–957Crossref, Medline, Google Scholar
85. : Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia. Neuropsychopharmacology 2022; 47:292–308Crossref, Medline, Google Scholar
86. : Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron 2018; 100:926–939.e3Crossref, Medline, Google Scholar
87. : Divisions of identified parvalbumin-expressing basket cells during working memory-guided decision making. Neuron 2016; 91:1390–1401Crossref, Medline, Google Scholar
88. : Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011; 10:685–697Crossref, Medline, Google Scholar
89. : RO4938581, a novel cognitive enhancer acting at GABAA alpha5 subunit-containing receptors. Psychopharmacology (Berl) 2009; 202:207–223Crossref, Medline, Google Scholar
90. : The role of α5 GABAA receptor agonists in the treatment of cognitive deficits in schizophrenia. Curr Pharm Des 2014; 20:5069–5076Crossref, Medline, Google Scholar
91. : Behavioral deficits induced by somatostatin-positive GABA neuron silencing are rescued by alpha 5 GABA-A receptor potentiation. Int J Neuropsychopharmacol 2021; 24:505–518Crossref, Medline, Google Scholar
92. : mGlu1 potentiation enhances prelimbic somatostatin interneuron activity to rescue schizophrenia-like physiological and cognitive deficits. Cell Rep 2021; 37:109950Crossref, Medline, Google Scholar



