Diagnosis- and Cell Type-Specific Mitochondrial Functional Pathway Signatures in Schizophrenia and Bipolar Disorder
Abstract
Objective:
The shared risk factors and clinical features in schizophrenia and bipolar disorder may be linked via mitochondrial dysfunction. However, the severity of mitochondrial dysfunction, and/or the specific mitochondrial functional pathways affected, may differ between diagnoses, especially at the level of individual cell types.
Methods:
Transcriptomic profiling data for a gene set indexing mitochondrial functional pathways were obtained for dorsolateral prefrontal cortex (DLPFC) gray matter and layer 3 and layer 5 pyramidal neurons of subjects with schizophrenia or bipolar disorder. Analyses were conducted using a dual strategy: identification of differentially expressed genes (DEGs) and their functional pathway enrichment, and application of weighted gene coexpression network analysis. These analyses were repeated in monkeys chronically exposed to antipsychotic drugs to determine their effect on mitochondrial-related gene expression.
Results:
In DLPFC gray matter, 41% of mitochondrial-related genes were differentially expressed in the schizophrenia group, whereas 8% were differentially expressed in the bipolar group. In the schizophrenia group, 83% of DEGs showed lower expression, and these were significantly enriched for three functional pathways, each indexing energy production. DEGs in the bipolar disorder group were not enriched for functional pathways. This disease-related pattern of findings was also identified in pyramidal neurons. None of the gene expression alterations disrupted coexpression modules, and DEGs were not attributable to antipsychotic medications.
Conclusions:
Schizophrenia and bipolar disorder do not appear to share similar mitochondrial alterations in the DLPFC. The selective and coordinated down-regulation of energy production genes in schizophrenia is consistent with the effects of chronic reductions in pyramidal neuron firing, and enhancement of this activity may serve as a therapeutic target.
Schizophrenia and bipolar disorder share some genetic (1) and environmental (2) risk factors, and for some individuals, certain clinical features such as psychosis (3). These shared attributes across diagnoses may be linked via similar functional (4), morphological (5), or molecular (6) brain alterations, many of which have been associated with evidence of mitochondrial dysfunction (7).
Mitochondria are responsible for multiple processes essential for brain function (8, 9). The primary role of mitochondria is energy production via synthesis of adenosine triphosphate (ATP) by oxidative phosphorylation (OXPHOS). In neurons, most of the ATP generated supports cell firing and synaptic neurotransmission (10). Mitochondria also participate in other key functional pathways, including reactive oxygen species generation, Ca2+ buffering, and apoptosis (11). Accumulating evidence implicates alterations in one or more of these mitochondrial functional pathways in schizophrenia (12) and bipolar disorder (13). Determining the presence, severity, and functional pathway specificity of mitochondrial alterations in schizophrenia and bipolar disorder at multiple anatomical levels of resolution can inform us on the contributors to, and possible therapeutic targets for, neuronal dysfunction in schizophrenia and bipolar disorder.
In vivo indices of cortical neuronal activity and energy metabolism indicate less neuronal OXPHOS in schizophrenia (14, 15), findings supported by lower transcriptomic, proteomic, and enzymatic measures of energy production in postmortem brain from schizophrenia subjects (6, 16). Transcriptomic profiling of two key circuit components in the dorsolateral prefrontal cortex (DLPFC), layer 3 pyramidal neurons (L3PNs) and layer 5 pyramidal neurons (L5PNs), revealed that the top affected functional pathways in schizophrenia were related to mitochondrial energy production (17–19). In bipolar disorder subjects, in vivo and postmortem findings regarding mitochondrial-related alterations from gray matter (6, 14, 20–23) and cell type-specific (18) studies are mixed. However, a meta-analysis identified oxidative damage to lipids in bipolar disorder subjects (24), suggesting altered reactive oxygen species regulation.
Together, these data suggest that cortical mitochondrial perturbations are present in both schizophrenia and bipolar disorder but that the severity of alterations and/or the affected mitochondrial functional pathways may differ across diagnoses, with such differences influenced by the anatomical resolution of analysis. To address this issue, we analyzed in schizophrenia and bipolar disorder subjects transcriptomic data that index mitochondrial functional pathways in samples of DLPFC total gray matter, L3PNs, and L5PNs using a dual strategy. First, we identified differentially expressed genes (DEGs) and assessed their enrichment for functional pathways, and second, we applied weighted gene coexpression network analysis for an unbiased examination of how disease-related differences in gene expression affect higher-order gene coexpression relationships.
Both DLPFC circuitry and mitochondrial functioning can be affected by chronic treatment with antipsychotic medications (25, 26), but whether these effects are related is unclear. For example, it is not known whether chronic treatment with first- or second-generation antipsychotic medications affects expression of genes in mitochondrial functional pathways within the DLPFC. Thus, we performed quantitative transcriptomic analysis of mitochondrial-related transcripts in DLPFC total gray matter, L3PNs, and L5PNs in separate cohorts of monkeys chronically exposed to antipsychotic drugs.
Methods
RNA Sequencing and Microarray Differential Gene Expression Analysis and Functional Pathway Enrichment in Human Subjects
Data were analyzed from the publicly available RNA sequencing analyses (27), completed as part of the CommonMind Consortium. Specifically, we analyzed gene expression in DLPFC gray matter samples from schizophrenia (N=57), bipolar disorder (N=35), and unaffected comparison (N=82) subjects. In order to eliminate confounding effects of donation site (27), analyses were restricted to samples provided by the University of Pittsburgh Brain Tissue Donation Program, because most samples from bipolar disorder subjects in the CommonMind Consortium were provided by that source. All schizophrenia and bipolar disorder subjects were matched to an unaffected subject on sex, age, and race. Matched pairs were processed together to mitigate the influence of library batch. Subject and tissue characteristics are presented in detail in the Methods section of the online supplement. Determination of differential gene expression relative to unaffected subjects is also described in detail in the Methods section of the online supplement. Briefly, the limma package for R was used for differential gene expression analysis of whole-gene count data (56,632 genes), with a Benjamini-Hochberg-corrected p value <0.05 considered significant. A basic linear regression model was used, along with the precision weights obtained during voom normalization. Covariates included in the final model were RNA integrity number, postmortem interval, age, and sex. This model is similar to that used in the initial analysis of the DLPFC in the entire CommonMind Consortium cohort (27). To capture the diversity of mitochondrial functional domains, we analyzed a specific gene set (1,033 genes) defined by the Gene Ontology (GO) project as “mitochondria” (GOMito), and 871 of these genes were identified via RNA sequencing.
We also reanalyzed data from two previously published microarray studies of DLPFC L3PNs and L5PNs (17, 18), from pools of 100 neurons per subject and layer. The first study (17) included 36 pairs of unaffected comparison and schizophrenia subjects matched for sex and matched as closely as possible for age and postmortem interval. After filtering of all data sets, 662 GOMito genes were included for analysis. Differential gene expression was determined as previously described (19). The second study (18) included a largely unique cohort of 19 triads of unaffected comparison, schizophrenia, and bipolar disorder subjects matched for sex and matched as closely as possible for age and postmortem interval. After filtering of all data sets, 634 GOMito genes were included for analysis. Differential gene expression was determined as previously described, including identification of DEGs at a 20% false discovery rate because of the smaller sample size (18). Subject and tissue characteristics for both cohorts are presented in detail in the Methods section of the online supplement.
To further analyze differences in gene expression across diagnostic groups and studies, correlations between the test statistics for each gene analyzed were determined. This approach avoids the issue of differences in statistical power due to different sample sizes and assesses the overall similarity or difference in gene expression alterations in a threshold-free manner. Correlation strength is described according to Evans (28).
Functionally related pathway enrichment analysis was performed using Ingenuity Pathway Analysis (IPA) software (Qiagen, Germantown, Md.). All DEGs, irrespective of direction of difference, were analyzed for pathway enrichment. Only IPA pathways comprising at least 15 genes were considered for enrichment analysis.
Weighted Gene Coexpression Network Analysis
The weighted network for unaffected subjects was constructed using a previously described weighted gene coexpression network analysis method (29), as detailed in the Methods section of the online supplement. The resulting modules were assigned a unique color identifier for further analysis. To compare network structures across diagnoses, the Zsummary module preservation algorithm was implemented (30).
RNA Sequencing and Microarray Differential Gene Expression Analysis in Monkeys Exposed to Antipsychotic Drugs
Analysis of DLPFC total gray matter was performed in a cohort (N=34) of monkeys previously reported by the CommonMind Consortium (27). The monkeys (Macaca mulatta) received oral doses of haloperidol (N=17), clozapine (N=9), or sham treatment (N=8) for 6 months. All procedures were conducted in accordance with National Institutes of Health (NIH) guidelines and with the approval of the Institutional Animal Care and Use Committee (IACUC) of Emory University and Wake Forest School of Medicine. These procedures and the RNA sequencing protocol are described by Fromer et al. (27). Of the GOMito genes, 691 were identified via RNA sequencing. Differential expression statistics were derived using the same approach described above for the human RNA sequencing data set.
Analysis of DLPFC L3PNs and L5PNs was performed in a cohort (N=18) of young adult male monkeys (Macaca fasicularis) exposed to twice-daily oral doses of haloperidol, olanzapine, or sham treatment (monkeys per group, N=6) for 17–27 months. All procedures regarding drug administration and euthanasia are described in detail by Dorph-Petersen et al. (31) and were conducted in accordance with NIH guidelines and with the approval of the University of Pittsburgh’s IACUC. Collection of pyramidal neurons using laser microdissection, microarray profiling, and analytic approach are described in detail in the Methods section of the online supplement. Of the GOMito genes present on the microarray platform, 507 survived data filtering and were included for analysis. Differential expression statistics were determined using the same approach applied to the human microarray data sets, except no covariates were included in the statistical model.
Results
Gray Matter Mitochondrial-Related Transcriptome Analyses in Schizophrenia and Bipolar Disorder Subjects
Differential GOMito gene expression and pathway enrichment in schizophrenia and bipolar disorder subjects.
In DLPFC gray matter, of the 871 GOMito genes detected by RNA sequencing, 356 (41%) were differentially expressed in schizophrenia subjects, whereas only 67 (8%) were differentially expressed in bipolar disorder subjects (Figure 1A; see also Table S1 in the online supplement for a list of DEGs in each diagnostic group). Compared with unaffected subjects, in schizophrenia subjects, 83% (N=296/356) of DEGs were down-regulated and 17% (N=60/356) were up-regulated, whereas in bipolar disorder subjects, 99% (N=66/67) of DEGs were down-regulated. Of the DEGs in bipolar disorder subjects, 53% (N=35/66) were not identified as differentially expressed in schizophrenia subjects (Figure 1A).
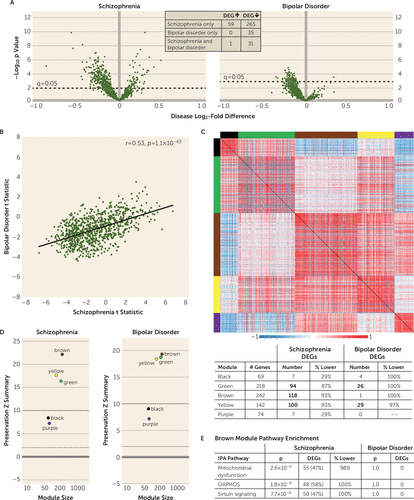
FIGURE 1. Differential expression, weighted gene coexpression network analysis, and module preservation and enrichment of GOMito genes in dorsolateral prefrontal cortex (DLPFC) gray matter in schizophrenia and bipolar disorder subjectsa
a Panel A shows volcano plots of gene expression for each Gene Ontology (GO) mitochondria (GOMito) gene detected by RNA sequencing, illustrating the mean log2-fold difference in schizophrenia and bipolar disorder subjects compared with unaffected subjects and the −log10 p value. Dashed horizontal lines denote statistical significance at a q value of 0.05, with data points above the line indicating differentially expressed genes (DEGs). The inset shows the number of shared and unique GOMito genes differentially expressed in schizophrenia and bipolar disorder subjects. Panel B shows scatterplots of the positive correlation between schizophrenia and bipolar disorder subjects of GOMito differential expression test statistics. Panel C shows that the weighted gene coexpression network analysis identified five GOMito gene coexpression modules in unaffected subjects. Colored bars on the top and left sides of the heat maps represent each identified module. The heat maps show pairwise correlation strength in unaffected subjects for each GOMito gene. For each module, the table (bottom) provides the number of genes present in each module, the number of genes that were differentially expressed, and the percentage of genes that were underexpressed in schizophrenia and bipolar disorder subjects compared with unaffected subjects. Bolded numbers represent modules enriched for DEGs. Panel D shows the module preservation Zsummary scores. Brown, yellow, and green modules were strongly preserved (Zsummary >10), and purple and black modules were moderately preserved (2 <Zsummary <10) in schizophrenia and bipolar disorder subjects. Panel E shows the Ingenuity Pathway Analysis (IPA) that identified significant mitochondrial dysfunction, oxidative phosphorylation (OXPHOS), and sirtuin-signaling pathway enrichment within the brown module. For each pathway, DEGs were significantly enriched in schizophrenia subjects, but none of these pathway genes were differentially expressed in bipolar disorder subjects.
To further interrogate the nature of mitochondrial-related alterations, we compared the differential expression test statistic for each GOMito gene across diagnoses. Although few GOMito genes were differentially expressed in bipolar disorder subjects, test statistics were moderately correlated (r=0.53, p=1.1×10−63) across diagnoses (Figure 1B), suggesting a shared disease effect; however, the much smaller range of test statistic values in bipolar disorder supports a weaker disease effect than in schizophrenia.
Analysis of the 356 DEGs in schizophrenia subjects using IPA identified three significantly affected functional pathways: mitochondrial dysfunction, OXPHOS, and sirtuin signaling (Table 1); in contrast, no functional pathways were identified as significantly affected in bipolar disorder subjects. Mitochondrial dysfunction, OXPHOS, and sirtuin signaling pathways show substantial overlap in constituent genes, with 100% of OXPHOS genes and 56% of sirtuin-signaling genes present in the mitochondrial dysfunction pathway. The OXPHOS pathway exclusively comprises components of the electron transport chain, complexes through which redox reactions synthesize ATP. Sirtuin signaling is implicated in regulating multiple aspects of cellular metabolism, including acting as “metabolic sensors” (32, 33); the sirtuin-signaling pathway largely comprises genes reflecting its role in regulating ATP synthesis and metabolism.
| Mitochondrial Dysfunction | Oxidative Phosphorylation | Sirtuin Signaling | |
|---|---|---|---|
| Gray matter | |||
| Number of genes | 118 | 83 | 107 |
| Number of DEGs | 82 | 66 | 67 |
| Percent down-/up-regulated | 96/4 | 100/0 | 91/9 |
| Top 10 DEGs | ATP5C1, COX7B, ATP5A1, ATP5F1A, COX7A2, NDUFA12, COX6A1, NDUFB3, UQCRQ, NDUFS4 | MAOB, ATP5F1C, VDAC2, COX7B, ATP5F1A, ATP5PB, VDAC1, COX7A2, NDUFA12, COX6A1 | STAT3, ATP5F1C, VDAC2, ATP5F1A, ATP5PB, VDAC1, NDUFA12, NDUFB3, NDUFS4, SDHB |
| L3PN | |||
| Number of genes | 103 | 74 | 85 |
| Number of DEGs | 64 | 55 | 46 |
| Percent down-/up-regulated | 100/0 | 100/0 | 100/0 |
| Top 10 DEGs | COX17, NDUFA2, NDUFAB1, NDUFB2, NDUFB8, ATP5F1C, UQCRB, COX7A2, ATP5MC3, COX5A | UQCRFS1, UQCR10, ATP5PB, COX11, COX7A1, SDHD, NDUFB7, NDUFA2, ATP5F1B, NDUFS2 | NDUFA2, NDUFAB1, NDUFB2, NDUFB8, ATP5F1C, TIMM10, NDUFA6, NDUFV1, NDUFB7, NDUFB3 |
| L5PN | |||
| Number of genes | 103 | 74 | 85 |
| Number of DEGs | 58 | 48 | 42 |
| Percent down-/up-regulated | 98/2 | 100/0 | 100/0 |
| Top 10 DEGs | COX7A1, NDUFB2, ATP5MC3, NDUFV1, COX5A, COX7A2, NDUFB3, NDUFAB1, ATP5F1A, ATP5F1B | COX7A1, NDUFB2, ATP5MC3, NDUFV1, COX5A, NDUFB3, NDUFAB1, COX7A2, PRDX5, ATP5F1A | NDUFB2, NDUFV1, NDUFB3, NDUFAB1, TIMM17A, ATP5F1A, ATP5F1B, SOD2, TIMM8B, NDUFS3 |
TABLE 1. Functional pathway enrichment of differentially expressed genes in schizophrenia subjectsa
Together, these data demonstrate the presence of a largely unique set of differentially expressed mitochondrial-related genes across diagnoses; a stronger disease effect on gene expression in schizophrenia than bipolar disorder; and selectively in schizophrenia subjects, the clustering of many DEGs in three overlapping mitochondrial functional pathways, each of which indexes energy production.
Preservation and pathway enrichment of network modules in schizophrenia and bipolar disorder subjects.
Weighted gene coexpression network analysis of GOMito gene expression in DLPFC gray matter of unaffected subjects identified five coexpression modules (Figure 1C). As expected, strong positive correlations were present across genes within a module (designated by a randomly assigned color). In addition, genes without any coexpression relationships (N=126) were not correlated with module genes. Notable positive and negative cross-module correlations were also found, which may reflect the interactive relationships between different mitochondrial functional domains. Module preservation analysis showed that the module structure present in unaffected subjects was highly (Zsummary >10) to moderately (2 <Zsummary <10) preserved (30) in both schizophrenia and bipolar disorder subjects (Figure 1D); thus, the fundamental architecture of mitochondrial-related gene expression was not affected in either diagnosis. In both schizophrenia and bipolar disorder subjects, DEGs were enriched in green and yellow modules, but neither module was enriched for specific functional pathways (Figure 1C table). In contrast, only schizophrenia subjects showed DEG enrichment in the brown module (Figure 1E), which contained genes in three functional pathways (mitochondrial dysfunction, OXPHOS, and sirtuin signaling). Therefore, we next sought to determine whether these schizophrenia-specific findings suggesting lower energy production were present in DLPFC L3PNs and/or L5PNs from schizophrenia subjects.
L3PN and L5PN Mitochondrial-Related Transcriptome Analyses in Schizophrenia Subjects
Differential GOMito gene expression and pathway enrichment in schizophrenia subjects.
Of the 662 GOMito genes detected by microarray, 185 (28%) in L3PNs and 167 (25%) in L5PNs were differentially expressed in schizophrenia subjects compared with unaffected subjects (Figure 2A; see Table S2 in the online supplement for a list of DEGs in each cell population). Unlike gray matter findings of up-regulated and down-regulated DEGs in schizophrenia subjects, in both L3PNs and L5PNs, 97% of DEGs were down-regulated. Of these DEGs, 47% (N=109/232) were lower in both cell populations (Figure 2A). In both L3PNs and L5PNs, IPA of the DEGs identified three significantly affected functional pathways: mitochondrial dysfunction, OXPHOS, and sirtuin signaling (Table 1). Differential expression test statistics showed a similar range of values and were strongly positively correlated (r=0.71, p=4.1×10−100) in L3PNs and L5PNs in schizophrenia subjects (Figure 2B), suggesting that a similar disease effect of comparable strength was present in both cell types.
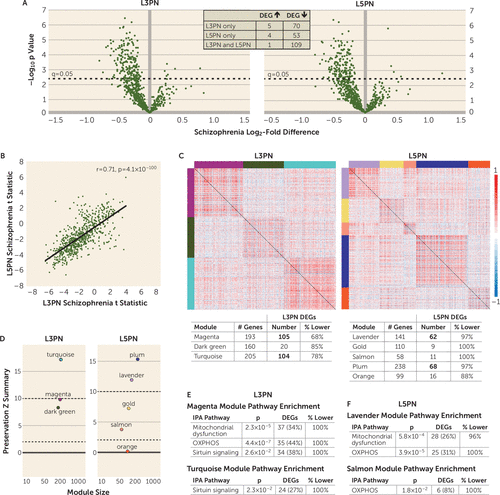
FIGURE 2. Differential expression, weighted gene coexpression network analysis, and module preservation and enrichment of GOMito genes in dorsolateral prefrontal cortex (DLPFC) layers 3 and 5 pyramidal neurons (L3PNs and L5PNs) of schizophrenia subjectsa
a Panel A shows volcano plots of gene expression for each Gene Ontology (GO) mitochondria (GOMito) gene detected by microarray illustrating the mean log2-fold difference in schizophrenia subjects compared with unaffected subjects and the −log10 p value. Dashed horizontal lines denote statistical significance at a q value of 0.05, with data points above the line indicating differentially expressed genes (DEGs). The inset shows the number of shared and unique DEGs in L3PNs and L5PNs. Panel B shows a scatterplot of the correlation between L3PNs and L5PNs of GOMito differential expression test statistics in schizophrenia subjects. Panel C shows the weighted gene coexpression network analysis of GOMito gene expression that identified three coexpression modules in unaffected subjects in L3PNs and five coexpression modules in L5PNs. Colored bars on the top and left sides of the heat maps indicate each identified module. The heat maps show pairwise correlation strength in unaffected subjects for each GOMito gene. For each module, the tables (bottom) provide the number of genes present in each module, the number of genes differentially expressed, and the percentage of genes that were underexpressed in L3PNs and L5PNs in schizophrenia subjects compared with unaffected subjects. Bolded numbers represent modules enriched for DEGs. Panel D shows the module preservation Zsummary scores. In L3PNs, the turquoise module was strongly preserved (Zsummary >10), and the magenta and dark green modules were moderately preserved (2 <Zsummary <10). In L5PNs, the plum and lavender modules were strongly preserved (Zsummary >10), gold and salmon modules were moderately preserved (2 <Zsummary <10), and the orange module was not preserved (Zsummary <2). Panel E shows the Ingenuity Pathway Analysis (IPA) in L3PNs illustrating significant mitochondrial dysfunction, oxidative phosphorylation (OXPHOS), and sirtuin-signaling pathway enrichment within the magenta module, and sirtuin-signaling pathway enrichment in the turquoise model. Panel F shows the IPA in L5PNs, illustrating significant mitochondrial dysfunction and OXPHOS pathway enrichment within the lavender module and OXPHOS pathway enrichment in the salmon module.
Preservation and pathway enrichment of network modules in schizophrenia subjects.
Weighted gene coexpression network analysis of GOMito gene expression identified three coexpression modules in unaffected subjects in L3PNs and five coexpression modules in L5PNs (Figure 2C). Positive correlations were present across genes within a module. In addition, genes that did not show any coexpression relationships (L3PN, N=104; L5PN, N=16) were not correlated with module genes. Module preservation analysis (Figure 2D) showed that the module structure was highly (Zsummary >10) to moderately (2 <Zsummary <10) preserved for all three modules in L3PNs and for four of five modules in L5PNs. DEGs were enriched in magenta and turquoise modules for L3PNs and in lavender and plum modules for L5PNs (Figure 2C table). Similar to gray matter, L3PN and L5PN modules showed selective enrichment of genes for functional pathways reflecting energy production, including within modules enriched with DEGs (Figure 2E–F). Thus, we next sought to determine whether schizophrenia and bipolar disorder subjects showed similar alterations in mitochondrial-related gene expression in L3PNs and L5PNs and whether gene expression differences in schizophrenia subjects were reproducible in a second, largely nonoverlapping but smaller cohort of schizophrenia subjects.
L3PN and L5PN Mitochondrial-Related Transcriptome Analyses in Schizophrenia and Bipolar Disorder Subjects
Comparison of GOMito gene expression in L3PNs and L5PNs in schizophrenia and bipolar disorder subjects.
Of the 634 GOMito genes detected by microarray, 80 (13%) in L3PNs and L5PNs were differentially expressed in schizophrenia subjects compared with unaffected subjects, but none were differentially expressed in either L3PNs or L5PNs (all q values ≥0.9) in bipolar disorder subjects. Direct comparison of differential expression test statistics in bipolar disorder and schizophrenia subjects (Figure 3A) showed very weak correlations in L3PNs (r=0.16, p=2.1×10−5) and weak correlations in L5PNs (r=0.28, p=5.7×10−13), confirming the greater effect of schizophrenia on gene expression. In contrast, comparison of the differential expression test statistic for each GOMito gene across the two pyramidal neuron studies including schizophrenia subjects (Figure 3B) showed moderate positive correlations in both L3PNs (r=0.51, p=9.3×10−43) and L5PNs (r=0.45, p=4.6×10−34), demonstrating similar schizophrenia-related findings across the two studies.
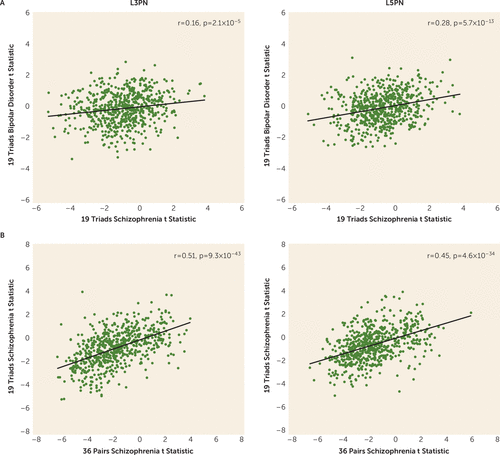
FIGURE 3. Comparison of differential expression test statistics in layers 3 and 5 pyramidal neurons (L3PNs and L5PNs) in schizophrenia and bipolar disorder subjectsa
a Panel A shows scatterplots illustrating the correlation within L3PNs (left) and L5PNs (right) of Gene Ontology (GO) mitochondria (GOMito) differential expression test statistics between schizophrenia and bipolar disorder subjects. Panel B shows scatterplots illustrating the correlation between L3PNs (left) and L5PNs (right) of GOMito differential expression test statistics in the two studies including schizophrenia subjects.
Gray Matter and L3PN and L5PN Mitochondrial-Related Transcriptome Analyses in Monkeys Chronically Exposed to Antipsychotic Drugs
None of the GOMito genes detected by RNA sequencing in DLPFC gray matter were differentially expressed in monkeys exposed to haloperidol or clozapine compared with sham-treated monkeys (all q values ≥0.8). Only seven of the DEGs in schizophrenia subjects showed even nominal significance (p<0.05) (see Table S3 in the online supplement) in monkeys exposed to either drug. Of these seven genes, five showed the same direction of change in schizophrenia subjects and in monkeys. Comparison of differential expression test statistics from gray matter in schizophrenia subjects and in monkeys exposed to antipsychotic drugs (Figure 4A) showed a very weak positive correlation with monkeys exposed to haloperidol (r=0.13, p=0.001) and no correlation with monkeys exposed to clozapine (r=0.04, p=0.4).
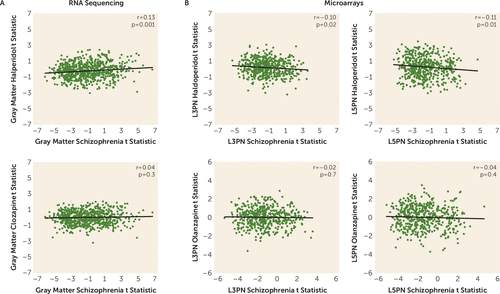
FIGURE 4. Comparison of differential expression test statistics in gray matter and layers 3 and 5 pyramidal neurons (L3PNs and L5PNs) in schizophrenia subjects and in monkeys chronically exposed to antipsychotic drugsa
a Panel A shows scatterplots illustrating the test statistic correlation in gray matter in schizophrenia subjects and in monkeys exposed to haloperidol (top) or clozapine (bottom). Panel B shows scatterplots illustrating the test statistic correlation in L3PNs and L5PNs in schizophrenia subjects and in monkeys exposed to haloperidol (top) or olanzapine (bottom).
None of the GOMito genes detected by microarray analysis in DLPFC L3PNs and L5PNs were differentially expressed in monkeys exposed to haloperidol or olanzapine compared with sham-treated monkeys (all q values ≥0.9). Only five DEGs in L3PNs and seven DEGs in L5PNs in schizophrenia subjects showed even nominal significance (p<0.05) in monkeys exposed to either drug (see Table S3 in the online supplement). Of these 12 genes, only two showed the same direction of change in the disease state and in monkeys. Comparison of L3PN and L5PN differential expression test statistics in schizophrenia subjects and in monkeys exposed to antipsychotic drugs (Figure 4B) showed very weak negative correlations with monkeys exposed to haloperidol (all r values <−0.11; all p values ≥0.01) and no correlation with monkeys exposed to clozapine (all r values <−0.02; all p values ≥0.4). Thus, analysis of independent cohorts of monkeys showed no effect of either first- or second-generation antipsychotic drug administration on expression of mitochondrial-related genes in either DLPFC gray matter or pyramidal neurons.
Discussion
Previous studies of postmortem human cortex suggested that there are mitochondrial perturbations in individuals with schizophrenia and bipolar disorder, but whether the severity of these alterations and/or the affected mitochondrial functional pathways differed across diagnoses was unclear. In this targeted analysis of mitochondrial-related gene expression, we found alterations in total DLPFC gray matter in both schizophrenia and bipolar disorder subjects, but the disease effect was substantially stronger in schizophrenia. Additionally, alterations in mitochondrial functional pathways, all of which were related to lower energy production, were found only in schizophrenia subjects. In pyramidal neurons, mitochondrial-related alterations were exclusively present in schizophrenia subjects and were also enriched for functional pathways indexing lower energy production. Together, these findings support the idea that mitochondrial perturbations are present in the DLPFC in both schizophrenia and bipolar disorder, but that the severity and nature of these alterations, and their apparent cell type-specificity, differ across diagnoses.
In the analyses of mitochondrial-related genes in total gray matter, the correlation of test statistics (an analysis that does not impose dichotomous statistical significance to each gene) showed a significant, moderately positive relationship across diagnoses, suggesting a shared disturbance in mitochondrial function. However, substantially more DEGs were down-regulated in schizophrenia than in bipolar disorder. These differences may reflect lower statistical power in the bipolar disorder group, but it is important to note that the weighted gene coexpression network analysis showed diagnostic differences similar to those in the DEG analyses. In addition, other differences by diagnosis included up-regulated DEGs solely in schizophrenia subjects and the presence of some DEGs in bipolar disorder subjects that were not altered in the schizophrenia group. Together, these data demonstrate that both the severity and the nature of mitochondrial-related gene expression alterations in the DLPFC differ between schizophrenia and bipolar disorder.
The presence of diagnosis-specific differences in mitochondrial-related gene expression was further supported by the absence of DEGs in L3PNs or L5PNs in bipolar disorder subjects. Because equal numbers of schizophrenia and bipolar disorder subjects were used in these analyses, the diagnostic differences were not confounded by differences in statistical power. Thus, the few DEGs identified in the gray matter of bipolar disorder subjects may reflect alterations in other cell types (34). For example, the RNA sequencing data reflect gene expression in all cell types, including highly metabolically active cells, such as parvalbumin interneurons. Parvalbumin interneurons have been reported to show down-regulated OXPHOS transcripts in schizophrenia (19), but studies of this cell type have not been conducted in bipolar disorder subjects. Moreover, the weakly correlated test statistics in each cell population between diagnoses suggests that few of the effects present in schizophrenia are evident in bipolar disorder. In schizophrenia subjects, test statistics were correlated between L3PNs and L5PNs, suggesting that the overall effects of schizophrenia on mitochondrial-related gene expression may be shared across neuronal types in the DLPFC. However, given that multiple up-regulated DEGs were present in gray matter but not in L3PNs or L5PNs in schizophrenia subjects, other neuronal or nonneuronal cell types in the DLPFC may be uniquely affected. Similarly, both neuronal populations also exhibited a unique set of DEGs, which may reflect cell type-specific alterations in mitochondrial-related gene expression resulting from intrinsic differences in the transcriptomes of L3PNs and L5PNs.
Peripheral metabolic effects of antipsychotic drug treatment, including impaired glucose metabolism, are well documented (reviewed in reference 35), and some cortical neural circuits and mitochondrial characteristics can be affected by chronic treatment with antipsychotic drugs (25, 26). However, whether mitochondrial-related gene expression, broadly or within specific metabolic pathways, is affected by chronic administration of first- or second-generation antipsychotics was unknown. Our findings that none of the mitochondrial-related genes were detected as differentially expressed at either level of anatomical resolution in monkeys chronically exposed to either first- or second-generation antipsychotics suggest that any effects antipsychotic drugs may have on the mitochondrial-related transcriptome do not occur in DLPFC gray matter or pyramidal neurons. Additionally, mitochondrial-related gene expression and lifetime chlorpromazine-equivalent dose (available for a subset of schizophrenia subjects [19]) were not significantly correlated in total gray matter, L3PNs, or L5PNs, with the exception of a single gene in L3PNs (RARS2, r=0.72, q=0.01). Thus, any role that antipsychotics may have in altering DLPFC functioning is unlikely to be mediated via changes in mitochondria.
In DLPFC gray matter and L3PNs and L5PNs in schizophrenia subjects, DEGs were significantly enriched for OXPHOS, mitochondrial dysfunction, and sirtuin-signaling pathways. Weighted gene coexpression network analysis showed that the coexpression network modules enriched for these functional pathways were unperturbed in schizophrenia, despite the number of DEGs. This selective and coordinated down-regulated expression of genes related to energy production suggests that schizophrenia is associated with less ATP synthesis via OXPHOS. One mechanism that can result in less OXPHOS is reduced cellular demand for ATP. Indeed, ATP is synthesized in mitochondria only on demand (36), and neurons normally make coordinated adjustments in the expression of OXPHOS-related genes to meet changes in energetic demand (37–40). In neurons, the most energetically demanding processes involve action potential generation and synaptic signaling (10, 41), and chronically lower rates of neuronal firing cause a coordinated reduction in OXPHOS-related transcripts (40). These coordinated alterations in OXPHOS-related transcript expression are unlike those identified in response to insults that impair or disrupt the energetic capacity of mitochondria to meet cellular demand (37, 38). For example, disrupting the expression of electron transport chain complex core subunits, accessory subunits, or assembly factors does not result in a coordinated reduction in the expression of OXPHOS-related transcripts (42–47). Moreover, this idea that less neuronal demand for energy production, and not defective mitochondrial function, is operative in the DLPFC in schizophrenia is consistent with existing anatomical and genetic data. For example, L3PNs have a smaller dendritic arbor and lower density of dendritic spines, the primary site of excitatory inputs (48). Fewer spines suggests that L3PNs receive fewer excitatory synapses in schizophrenia, and their resulting hypoactivity leads secondarily to less excitation of other pyramidal neurons. Indeed, given that L3PNs innervate L5PNs in the DLPFC (49), less firing of L3PNs is predicted to result in less excitation of this downstream cell population. Additionally, data from genome-wide association studies strongly implicate impaired synaptic processes in the etiology of schizophrenia and not primary insults to mitochondria (50–52).
In summary, our analysis across multiple data sets and cohorts of subjects of a large and specific gene set reflecting the multitude of constituent mitochondrial functions in the DLPFC showed a pronounced effect on energy production pathways selectively in schizophrenia subjects. The paucity of findings in bipolar disorder compared with schizophrenia may reflect fundamental differences in the underlying disease processes. Indeed, DLPFC dysfunction and cognitive impairments are thought to be much more prominent in schizophrenia than in bipolar disorder (53); however, examination of mitochondrial pathways in other brain regions or cell types may identify bipolar disorder-specific alterations. The lower measures of mitochondrial ATP production in schizophrenia may be a consequence of upstream pathological processes that result in less firing of L3PNs and L5PNs, which likely reflects an interaction of genetic vulnerabilities and environmental factors that affect synaptic integrity (48, 54). As such, therapeutics targeting enhancement of synaptic excitation within this circuit may prove to be beneficial in ameliorating cognitive dysfunction.
1 Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium: Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell 2018; 173:1705–1715.e1716.Crossref, Medline, Google Scholar
2 : Common or distinct pathways to psychosis? a systematic review of evidence from prospective studies for developmental risk factors and antecedents of the schizophrenia spectrum disorders and affective psychoses. BMC Psychiatry 2015; 15:205Crossref, Medline, Google Scholar
3 : Etiologic, phenomenologic, and endophenotypic overlap of schizophrenia and bipolar disorder. Annu Rev Clin Psychol 2015; 11:251–281Crossref, Medline, Google Scholar
4 : Shared and distinct functional architectures of brain networks across psychiatric disorders. Schizophr Bull 2019; 45:450–463Crossref, Medline, Google Scholar
5 : Are bipolar disorder and schizophrenia neuroanatomically distinct? an anatomical likelihood meta-analysis. Front Hum Neurosci 2010; 4:189Crossref, Medline, Google Scholar
6 : Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 2018; 359:693–697Crossref, Medline, Google Scholar
7 : Mitochondria, metabolism and redox mechanisms in psychiatric disorders. Antioxid Redox Signal 2018; 31:275–317Crossref, Google Scholar
8 : Mitochondria and neuronal survival. Physiol Rev 2000; 80:315–360Crossref, Medline, Google Scholar
9 : Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab 2012; 26:711–723Crossref, Medline, Google Scholar
10 : Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci 2012; 32:8940–8951Crossref, Medline, Google Scholar
11 : CNS energy metabolism as related to function. Brain Res Brain Res Rev 2000; 34:42–68Crossref, Medline, Google Scholar
12 : Mitochondrial multifaceted dysfunction in schizophrenia: complex I as a possible pathological target. Schizophr Res 2017; 187:3–10Crossref, Medline, Google Scholar
13 : Mitochondrial dysfunction in bipolar disorder: evidence, pathophysiology and translational implications. Neurosci Biobehav Rev 2016; 68:694–713Crossref, Medline, Google Scholar
14 : Neurometabolites in schizophrenia and bipolar disorder: a systematic review and meta-analysis. Psychiatry Res 2012; 203:111–125Crossref, Medline, Google Scholar
15 : Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 2009; 66:811–822Crossref, Medline, Google Scholar
16 : Defects in bioenergetic coupling in schizophrenia. Biol Psychiatry 2018; 83:739–750Crossref, Medline, Google Scholar
17 : Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry 2015; 20:1397–1405Crossref, Medline, Google Scholar
18 : Transcriptome alterations in prefrontal pyramidal cells distinguish schizophrenia from bipolar and major depressive disorders. Biol Psychiatry 2017; 82:594–600Crossref, Medline, Google Scholar
19 : Transcriptome alterations of prefrontal cortical parvalbumin neurons in schizophrenia. Mol Psychiatry 2018; 23:1606–1613Crossref, Medline, Google Scholar
20 : Brain lactate and pH in schizophrenia and bipolar disorder: a systematic review of findings from magnetic resonance studies. Neuropsychopharmacology 2018; 43:1681–1690Crossref, Medline, Google Scholar
21 : Abnormalities in high-energy phosphate metabolism in first-episode bipolar disorder measured using 31p-magnetic resonance spectroscopy. Biol Psychiatry 2018; 84:797–802Crossref, Medline, Google Scholar
22 : Proteomic analysis of the postsynaptic density implicates synaptic function and energy pathways in bipolar disorder. Transl Psychiatry 2016; 6:
23 : Regulators of mitochondrial complex I activity: a review of literature and evaluation in postmortem prefrontal cortex from patients with bipolar disorder. Psychiatry Res 2016; 236:148–157Crossref, Medline, Google Scholar
24 : An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res 2014; 218:61–68Crossref, Medline, Google Scholar
25 : The neuropathological effects of antipsychotic drugs. Schizophr Res 1999; 40:87–99Crossref, Medline, Google Scholar
26 : Postmortem studies on mitochondria in schizophrenia. Schizophr Res 2017; 187:17–25Crossref, Medline, Google Scholar
27 : Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci 2016; 19:1442–1453Crossref, Medline, Google Scholar
28 : Straightforward Statistics for the Behavioral Sciences. Pacific Grove, Calif, Thomson Brooks Cole Publishing, 1996Google Scholar
29 : A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol 2005; 4:Article17Crossref, Medline, Google Scholar
30 : Is my network module preserved and reproducible? PLOS Comput Biol 2011; 7:
31 : The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology 2005; 30:1649–1661Crossref, Medline, Google Scholar
32 : Sirtuins: from metabolic regulation to brain aging. Front Aging Neurosci 2013; 5:36Crossref, Medline, Google Scholar
33 : Mitochondrial sirtuins. Biochim Biophys Acta 2010; 1804:1645–1651Crossref, Medline, Google Scholar
34 : Transcriptomic evidence for alterations in astrocytes and parvalbumin interneurons in subjects with bipolar disorder and schizophrenia. Biol Psychiatry 2018; 84:787–796Crossref, Medline, Google Scholar
35 : Intrinsic and antipsychotic drug-induced metabolic dysfunction in schizophrenia. Front Neurosci 2017; 11:432Crossref, Medline, Google Scholar
36 : Oxidative phosphorylation, in Biochemistry. New York, WH Freeman and Company, 2002, pp 491–526Google Scholar
37 : Transcriptional co-expression and co-regulation of genes coding for components of the oxidative phosphorylation system. BMC Genomics 2008; 9:18Crossref, Medline, Google Scholar
38 : Mechanisms of mitochondrial response to variations in energy demand in eukaryotic cells. Am J Physiol Cell Physiol 2007; 292:C52–C58Crossref, Medline, Google Scholar
39 : Human primitive brain displays negative mitochondrial-nuclear expression correlation of respiratory genes. Genome Res 2018; 28:952–967Crossref, Medline, Google Scholar
40 : Bigenomic regulation of cytochrome c oxidase in neurons and the tight coupling between neuronal activity and energy metabolism. Adv Exp Med Biol 2012; 748:283–304Crossref, Medline, Google Scholar
41 : Synaptic energy use and supply. Neuron 2012; 75:762–777Crossref, Medline, Google Scholar
42 : Extensive respiratory chain defects in inhibitory interneurones in patients with mitochondrial disease. Neuropathol Appl Neurobiol 2016; 42:180–193Crossref, Medline, Google Scholar
43 : In vivo correction of COX deficiency by activation of the AMPK/PGC-1α axis. Cell Metab 2011; 14:80–90Crossref, Medline, Google Scholar
44 : Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 2016; 538:123–126Crossref, Medline, Google Scholar
45 : OXPHOS gene expression and control in mitochondrial disorders. Biochimica et Biophysica Acta (BBA). Molecular Basis of Disease 2009; 1792:1113–1121Crossref, Medline, Google Scholar
46 : Mitochondrial DNA depletion in respiratory chain-deficient Parkinson disease neurons. Ann Neurol 2016; 79:366–378Crossref, Medline, Google Scholar
47 : Mitochondrial protein synthesis adapts to influx of nuclear-encoded protein. Cell 2016; 167:471–483.e10Crossref, Medline, Google Scholar
48 : Dendritic spine pathology in schizophrenia. Neuroscience 2013; 251:90–107Crossref, Medline, Google Scholar
49 : Interlaminar connections in the neocortex. Cereb Cortex 2003; 13:5–14Crossref, Medline, Google Scholar
50 : A comprehensive analysis of nuclear-encoded mitochondrial genes in schizophrenia. Biol Psychiatry 2018; 83:780–789Crossref, Medline, Google Scholar
51 Schizophrenia Working Group of the Psychiatric Genomics Consortium: Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511:421–427Crossref, Medline, Google Scholar
52 : Developmental and genetic regulation of the human cortex transcriptome illuminate schizophrenia pathogenesis. Nat Neurosci 2018; 21:1117–1125Crossref, Medline, Google Scholar
53 : On the specificity of continuous cognitive decline in schizophrenia. Am J Psychiatry 2019; 176:774–776Link, Google Scholar
54 : Schizophrenia as a disorder of molecular pathways. Biol Psychiatry 2015; 77:22–28Crossref, Medline, Google Scholar



