Clinical and Cognitive Significance of Auditory Sensory Processing Deficits in Schizophrenia
Abstract
Objective:
Although patients with schizophrenia exhibit impaired suppression of the P50 event-related brain potential in response to the second of two identical auditory stimuli during a paired-stimulus paradigm, uncertainty remains over whether this deficit in inhibitory gating of auditory sensory processes has relevance for patients’ clinical symptoms or cognitive performance. The authors examined associations between P50 suppression deficits and several core features of schizophrenia to address this gap.
Method:
P50 was recorded from 52 patients with schizophrenia and 41 healthy comparison subjects during a standard auditory paired-stimulus task. Clinical symptoms were assessed with the Scale for the Assessment of Positive Symptoms and the Scale for the Assessment of Negative Symptoms. The MATRICS Consensus Cognitive Battery was utilized to measure cognitive performance in a subsample of 39 patients. Correlation and regression analyses were conducted to examine P50 suppression in relation to clinical symptom and cognitive performance measures.
Results:
Schizophrenia patients demonstrated a deficit in P50 suppression when compared with healthy subjects, replicating prior research. Within the patient sample, impaired P50 suppression covaried reliably with greater difficulties in attention, poorer working memory, and reduced processing speed.
Conclusions:
Impaired suppression of auditory stimuli was associated with core pathological features of schizophrenia, increasing confidence that P50 inhibitory processing can inform the development of interventions that target cognitive impairments in this chronic and debilitating mental illness.
Understanding the biological processes that accompany debilitating clinical symptoms holds great potential for identifying and ameliorating chronic mental illness, and exploring the biological aspects of basic cognitive systems is a priority within the National Institute of Mental Health Research Domain Criteria (RDoC) initiative (1). Consistent with these views, individuals diagnosed with schizophrenia reliably demonstrate dysfunction in inhibitory gating of auditory sensory processes. Specifically, using an S2/S1 [second or test stimulus/first or conditioning stimulus] ratio score to assess the degree of suppression of the P50 event-related potential in response to the second of paired auditory stimuli, patients with schizophrenia consistently exhibit higher P50 suppression scores compared with healthy individuals (2).
Disruption of the inhibitory mechanism activated by S1 is postulated to reflect a fundamental neural deficit in schizophrenia that contributes to attentional difficulties associated with the illness (3). Although P50 suppression deficits in schizophrenia appear to be robust, evidence relating this impairment to overt clinical symptoms and cognitive disturbances is mixed. Some studies demonstrate clear associations between P50 abnormalities and clinical symptom ratings (4–8), whereas other studies do not (9–12). In the domain of cognitive performance, P50 is associated with working memory and attention-related processes in schizophrenia (5, 13, 14), although again, contradictory findings cast doubt on these effects (15–17 [for a review, see reference 16]). Although evidence linking P50 to symptoms and cognition may be spurious, the null findings could reflect underpowered studies or dichotomized scores on key variables, thereby reducing statistical power and underestimating effect estimates. Reliance on heterogeneous clinical composite measures that include domains presumed to be minimally related to attention (e.g., affective flattening, anhedonia) may also obscure important relationships between P50 suppression and specific symptoms (9, 11).
In view of the apparent promise of P50 inhibitory processes as a candidate mechanism for further biological elaboration on the one hand and the ambiguous findings relating P50 to specific behavioral and cognitive components of the disorder on the other, the present study enrolled relatively large samples of clinically stable schizophrenia outpatients and demographically matched healthy comparison subjects to evaluate disturbances associated with compromised P50 processing. More critically, we tested whether higher P50 ratio scores covary with clinical observations of attentional difficulties in patients with schizophrenia and whether greater P50 suppression deficits accompany more pronounced impairments on performance-based measures of working memory, speed of processing, and attention. This second prediction was addressed with the MATRICS Consensus Cognitive Battery (18), a comprehensive and well-validated assessment battery specifically devised to support the development of interventions for prevalent cognitive difficulties in schizophrenia.
Method
Participants
Participants were 54 outpatients with schizophrenia and 45 healthy individuals who were screened with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (19). Eligible patients met DSM-IV diagnostic criteria for schizophrenia, schizophreniform disorder, or schizoaffective disorder, depressed type; were clinically stable; and were receiving antipsychotic medication at the time of enrollment in the study. Healthy comparison subjects had no history of a major psychiatric disorder, according to the SCID assessment, and no family history of psychotic disorder. Individuals with a premorbid IQ <70, evidence of a known neurological disorder or significant head injury, or substance abuse (in the past month) or dependence (in the past 6 months) were excluded. To avoid anticholinergic effects on dependent variables, antiparkinsonian medications were discontinued in nine patients 24–48 hours prior to EEG recording. Participants refrained from cigarette smoking during the hour preceding data acquisition given prior evidence of a brief, transient effect of nicotine on P50 suppression in schizophrenia (20). The study was approved by the institutional review board of the University of California, Los Angeles (UCLA), and participants provided written, informed consent.
Data for two patients and four healthy comparison subjects were excluded because the data did not meet P50 inclusionary criteria (see below). Of the remaining 52 patients, 45 were prescribed risperidone, whereas seven were stabilized with olanzapine, ziprasidone, fluphenazine, haloperidol, clozapine, or aripiprazole. There were no significant differences on any dependent variables as a function of medication type. Unless noted otherwise, analyses are reported for these 52 patients.
Clinical Assessment
Patients’ symptoms were assessed at the UCLA Aftercare Research Program with the clinician-rated Scale for the Assessment of Positive Symptoms (SAPS) (21) and the Scale for the Assessment of Negative Symptoms (SANS) (22). The summary score for positive symptoms was the sum of the global subscale scores for hallucinations, delusions, unusual behavior, and positive formal thought disorder. The summary score for negative symptoms was the sum of the global subscale scores for affective flattening, alogia, avolition/apathy, anhedonia/asociality, and inattention.
Cognitive Assessment
The MATRICS Consensus Cognitive Battery was administered to assess cognitive function in a subsample of 39 patients across the following seven domains: attention, working memory, speed of processing, verbal learning, visual learning, reasoning and problem solving, and social cognition. Performance on each domain was converted to age- and gender-corrected T scores with the battery’s scoring program, which also provided a composite T score (18).
Psychophysiological Recording Methods
EEG recordings were obtained with a SynAmps amplifier system (Neuroscan, Charlotte, N.C.) with a cap containing 124 Ag/AgCl-sintered electrodes, with an equidistant layout. Electrooculogram (EOG) was conducted with electrodes placed above and below the right eye and near the outer canthi of the eyes. Electrode sites were referenced to the left earlobe during data collection and re-referenced offline to averaged earlobes. All impedances were below 10 kΩ. The EEG was amplified 2,500 times and EOG signals 500 times, respectively. Signals were acquired at a sampling rate of 2,000 Hz, with filters from 0.5 Hz to 200 Hz.
Epochs were extracted from 200 ms before stimulus onset to 1,000 ms following stimulus presentation, with the first 200 ms used for baseline correction, and EEG trials were digitally filtered with a bandpass of 10 Hz–50 Hz for measuring the P50 event-related potential component. Blind source separation by independent component analysis was conducted with MATLAB (https://www.mathworks.com/products/matlab.html) and the open-source toolbox EEGLAB (https://sccn.ucsd.edu/eeglab/index.php), and eye movements, blink artifacts, and electrocardiographic activity were removed. Epochs containing artifacts (voltages exceeding ±100 µV) were rejected. The number of trials retained for patients (mean=78.73 [SD=4.62]) and healthy comparison subjects (mean=79.88 [SD=0.33]) did not differ (p >0.05). EEG trials were averaged across all trials for each participant, and P50 was identified as the maximum positivity between 40 ms and 80 ms after stimulus onset at the Cz site and was measured relative to the preceding N40. The P30 amplitude and latency were identified based on the most positive peak between 20 ms and 40 ms after stimulus onset, and the maximum negativity between P30 and P50 was identified as N40. Two raters (H. H. and E. O.), blind to participant group, independently verified scoring of each event-related potential. As noted above, six individuals were excluded from the analyses, since their P50 amplitudes in response to S1 did not exceed 0.5 μV. One P50 ratio value was truncated to 2.00 to prevent extreme scores from having a disproportionate effect on the results (e.g., see reference 23).
Procedure
All participants completed an audiometric screening, consisting of sound intensities presented in 5-dB increments at frequencies ranging from 500 Hz to 8,000 Hz, to ensure that they detected sounds at each frequency above a 30-dB sound pressure level with each ear. P50 recordings were collected during presentation of 80 trials of paired auditory stimuli that were each 3 ms in duration and 80 dB in sound pressure level, with a 500-ms interstimulus interval and a variable intertrial interval of 9–11 seconds between pairs of stimuli. Participants were instructed to sit comfortably in a sound-attenuated room while auditory stimuli were presented through foam-insert earphones. Clinical ratings were completed during a separate visit by trained clinicians who evaluated symptoms over the past 3 months, including the day of EEG data collection. For the patient subsample completing the MATRICS Consensus Cognitive Battery, the assessment was conducted within 1 month of EEG data collection as part of a separate study (24).
Statistical Analysis
Analyses of variance (ANOVAs) and chi-square tests were used to compare the schizophrenia group with the healthy comparison group on demographic characteristics. A repeated-measures ANOVA involving group (patient group compared with healthy comparison group) and P50 amplitude (S1 compared with S2) was used to provide a difference measure of P50 suppression to complement the more typical ratio score analysis. ANOVA effect size is reported with partial-eta2 (ηp2). Zero-order correlations and hierarchical linear regression analyses were performed to examine P50 relationships with clinical ratings and cognitive performance. The Benjamini-Hochberg approach was used to control the false discovery rate associated with multiple comparisons (25).
To examine potential confounds related to type and dosage of medication, all analyses were repeated with chlorpromazine-equivalent dosage included as a covariate for each schizophrenia patient and also in a sample restricted to the 45 patients receiving risperidone. To assess any confounding effects of nicotine, all analyses were repeated with the total number of cigarettes smoked in the past week as a covariate. To evaluate relationships with cognitive variables, all analyses were repeated to account statistically for SANS and SAPS symptom severity. Any deviations in results from the full sample are noted. An alpha level was set at 0.05 (two-tailed) for all statistical tests.
Results
Demographic and clinical characteristics of the study participants are summarized in Table 1. Because of group differences in age between schizophrenia patients and healthy comparison subjects, relevant analyses were repeated with age as a covariate. The covariate did not alter any significant results, and group differences without age as a covariate are reported below. The two groups were matched for highest parental level of education. As might be expected, schizophrenia patients and healthy comparison subjects differed in their years of education, given the likely influence of illness on the level of education achieved. Group comparisons were also repeated with level of education included as a covariate, which again did not alter the results. Symptom levels were generally mild-to-moderate for schizophrenia patients.
| Characteristic | Schizophrenia Patients (N=52) | Healthy Comparison Subjects (N=41) | Comparison | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | χ2 | df | p | |
| Demographic | |||||||
| Gender (female/male) | 15/37 | 28.8/71.2 | 6/35 | 14.6/85.4 | 2.65 | 1 | 0.136 |
| Cigarette smoker (yes/no) | 16/36 | 30.8/69.2 | 5/36 | 12.2/87.8 | 4.52 | 1 | 0.033 |
| Mean | M | Mean | SD | F | df | p | |
| Age (years) | 26.10 | 8/17 | 30.22 | 7.07 | 6.56 | 1, 91 | 0.012 |
| Education (years) | 13.10 | 2.19 | 14.44 | 1.98 | 9.40 | 1, 91 | 0.003 |
| Parental education (years) | 14.02 | 3.54 | 15.07 | 2.65 | 2.52 | 1, 91 | 0.116 |
| Chlorpromazine-equivalent dosage (mg/day) | 204.17 | 116.26 | |||||
| Age at psychosis onset (years) | 22.59 | 3.89 | |||||
| Number of cigarettes consumed in past week | 4.60 | 15.36 | 1.71 | 5.08 | 1.33 | 1, 91 | 0.251 |
| Symptoms (summary scores) | |||||||
| Scale for the Assessment of Positive Symptoms | 4.17 | 3.55 | |||||
| Scale for the Assessment of Negative Symptoms | 8.98 | 4.34 | |||||
| Cognition (T score)b | |||||||
| Working memory | 43.26 | 15.45 | |||||
| Speed of processing | 32.26 | 12.00 | |||||
| Attention/vigilancec | 37.39 | 12.29 | |||||
| Verbal learning | 42.41 | 10.16 | |||||
| Visual learning | 38.59 | 11.27 | |||||
| Reasoning/problem solving | 38.67 | 9.03 | |||||
| Social cognitionc | 41.34 | 12.49 | |||||
| MATRICS Consensus Cognitive Battery overall composited | 32.81 | 14.42 | |||||
| P50 measure | |||||||
| S1 amplitude (μV) | 2.88 | 1.65 | 3.50 | 1.84 | 2.93 | 1, 91 | 0.090 |
| S2 amplitude (μV) | 1.56 | 1.11 | 1.44 | 1.21 | 0.26 | 1, 91 | 0.612 |
| S2/S1 ratio score | 0.60 | 0.41 | 0.39 | 0.28 | 7.87 | 1, 91 | 0.006 |
TABLE 1. Demographic, Clinical, and Performance Characteristics of the Study Samplea
P50 Suppression
Grand-average event-related potential waveforms for each group are shown in Figure 1. P50 mean amplitudes and suppression ratios are presented in Table 1. Consistent with previous reports, P50 ratio scores indicated poorer suppression in schizophrenia patients compared with healthy comparison subjects (p=0.006) (Table 1). A main effect of stimulus (F=125.65, df=1, 91, p<0.001; ηp2=0.580) confirmed suppression and was qualified by a group-by-stimulus interaction (F=6.10, df=1, 91, p=0.015; ηp2=0.063). Post hoc tests determined that P50 amplitude in response to S1 tended to be attenuated in schizophrenia patients compared with healthy comparison subjects (F=2.93, df=1, 91, p=0.090; ηp2=0.031). Although the magnitude of the S2 amplitude response in the patient group was larger than that for the comparison group, the group difference was not statistically significant (p=0.612).
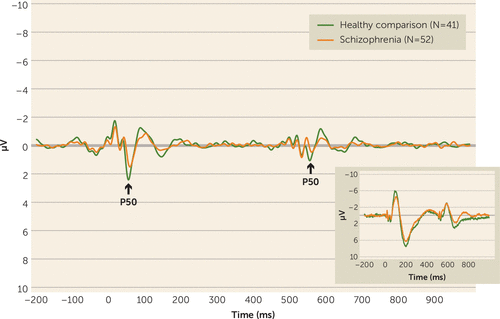
FIGURE 1. Grand Average Event-Related Potential Waveforms at the Cz Recording Site (Filtered 10 Hz–50 Hz)a
a The inset is filtered 0.5 Hz–200 Hz. The arrow indicates the P50 component.
P50 and Clinical Symptoms
As shown in Table 2, a significant positive association was observed among schizophrenia patients between P50 ratio scores and the SANS summary score but not the SAPS summary score. Correlations with each SANS subscale measure indicated that the association with P50 suppression was evident for only the global inattention subscale. Consistent with an inhibitory gating deficit, this effect was restricted to P50 amplitude in response to S2, such that the significant association between poorer suppression and clinical ratings of greater attentional impairment (r=0.338, p=0.014) did not extend to P50 in response to S1 (r=0.064, p=0.654).
| Symptom Scale | r | pa |
|---|---|---|
| Scale for the Assessment of Negative Symptoms Summary | 0.315 | 0.023 |
| Affective flattening | 0.201 | 0.153 |
| Alogia | 0.112 | 0.428 |
| Avolition-apathy | 0.248 | 0.077 |
| Anhedonia-asociality | 0.143 | 0.312 |
| Inattentionb | 0.408 | 0.003 |
| Scale for the Assessment of Positive Symptoms Summary | 0.043 | 0.760 |
| Hallucinations | 0.079 | 0.577 |
| Delusions | 0.152 | 0.282 |
| Unusual behavior | –0.124 | 0.385 |
| Positive formal thought disorder | –0.060 | 0.672 |
TABLE 2. Correlations Between P50 Ratio Scores and Clinician-Rated Negative and Positive Symptoms in Schizophrenia Patients (N=52)
To address the specificity of the association between P50 suppression and clinical ratings of attentional difficulties in schizophrenia patients, a hierarchical linear regression assessed the contribution of the inattention score after accounting for all other SANS subscale scores. This analysis revealed that inattention (β=0.394, p=0.009) uniquely explained 12.8% of the variance in P50 ratio scores, above and beyond the 7.2% of the variation accounted for by the other SANS subscale scores.
P50 and Cognitive Performance
The MATRICS Consensus Cognitive Battery performance results are summarized in Table 1. Zero-order correlations between P50 measures and cognitive variables of the consensus battery are presented in Table 3 for the 39 schizophrenia patients who underwent cognitive testing. P50 ratios correlated with overall composite scores on the battery, indicating that impaired suppression was associated with poorer cognitive performance. As predicted, this effect was evident in significant associations with speed of processing and working memory performance (Figure 2). Specifically, poorer P50 suppression was related to impaired performance during both verbal (r=–0.330, p=0.040) and visual (r=–0.381, p=0.017) working memory tasks. There were no statistically significant associations between the P50 ratio score and the other consensus battery domains (Table 3).
| Measure | r | pa |
|---|---|---|
| Working memoryb | –0.398 | 0.012 |
| Speed of processingb | –0.469 | 0.003 |
| Attention/vigilance | –0.104 | 0.533 |
| Verbal learning | –0.262 | 0.107 |
| Visual learning | –0.309 | 0.056 |
| Reasoning/problem solving | –0.270 | 0.096 |
| Social cognition | –0.319 | 0.051 |
| Overall composite scoreb | –0.414 | 0.011 |
TABLE 3. Relationships Between P50 Ratio Scores and the MATRICS Consensus Cognitive Battery Composite Score and Domains in Schizophrenia Patients (N=39)
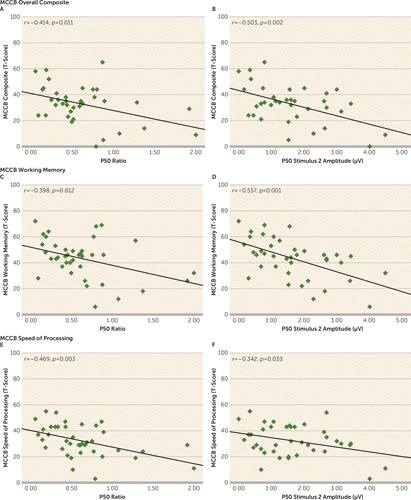
FIGURE 2. Correlations Between Cognitive Performance and P50 Ratio Scores and S2 Amplitudesa
a Panels A and B show a significant association between the P50 ratio and MATRICS Consensus Cognitive Battery (MCCB) composite score, which was largely explained by a significant relationship with S2 amplitude. Panels C and D show a significant association between P50 ratios and working memory performance on the MCCB, accounted for by a relationship with S2 amplitude. Panels E and F show a significant association between P50 ratios and speed of processing performance on the MCCB, again attributable to a relationship with S2 amplitude.
An examination of the relative contributions of S1 and S2 amplitudes to the relationship of P50 ratios and cognitive performance revealed that a significant correlation with P50 in response to S2 drove this relationship for working memory (r=–0.557, p<0.001) and speed of processing (r=–0.342, p=0.033) performance, as well as for the consensus battery overall composite score (r=–0.503, p=0.002). There were no significant associations involving P50 amplitude in response to S1 (working memory: r=–0.160, p=0.331; speed of processing: r=0.249, p=0.126; overall composite: r=–0.056, p=0.743).
Effects of Medication, Smoking, and Symptom Severity
When analyses were repeated and covaried for chlorpromazine-equivalent dosages of antipsychotic medications and the number of cigarettes smoked during the past week, all reported significant results remained statistically significant. Similarly, all symptom relationships remained statistically significant when the sample was restricted to the 45 patients receiving risperidone. Furthermore, symptom severity did not account for any of the observed relationships between P50 and cognitive performance, since all associations with clinical and cognitive variables remained significant when adjusting statistically for SAPS and SANS global scores (all p values <0.05).
Discussion
This study investigated linkages between clinical symptoms, cognitive dysfunction, and pathophysiological mechanisms associated with auditory sensory processing deficits in schizophrenia. Findings confirmed the hypothesis that P50 suppression abnormalities are associated with such core clinical characteristics of schizophrenia as symptoms of inattentiveness, thereby clarifying the clinical significance of P50 suppression deficits. Although analogous results involving clinician-rated attentional impairment measures have been described previously (5, 8), the present results are based on a larger patient sample, and a more powerful statistical approach was achieved by retaining the continuous range of information offered by SANS and P50 ratio scores. The results also underscore the specificity of the association between impaired P50 inhibitory processing and clinician-rated inattention in schizophrenia, since other negative symptom domains were not similarly implicated.
Beyond relating P50 suppression to a clinically observed core feature of schizophrenia, results from the present study link auditory sensory dysfunction to impairments involving working memory and speed of information processing. These findings corroborate the findings of previous studies (5, 13, 14, 16) and extend such work by use of standardized measures from the MATRICS Consensus Cognitive Battery, a cognitive test battery on which schizophrenia patients reliably show compromised performance (18). However, these findings contrast with those of one study in which P50 ratio scores were unrelated to cognitive performance in similar domains among medicated patients with chronic schizophrenia (17). Illness duration and prolonged exposure to antipsychotic medications may account for this discrepancy.
The pattern of findings observed in this study is substantiated by research on the neural mechanisms of P50 sensory impairments. Source localization studies implicate a neural network involving the prefrontal cortex—particularly the dorsolateral prefrontal cortex—the superior temporal gyrus, the hippocampus, and the thalamus in the generation and suppression of P50 (26, 27). Notably, the dorsolateral prefrontal cortex and hippocampus have been associated with working memory dysfunction in schizophrenia (28, 29), while prefrontal cortex, hippocampus, and superior temporal gyrus structural and functional abnormalities have been implicated in efficient information processing (30, 31). Furthermore, our results parallel a recent study, conducted by Tregellas et al. (29), which found that hippocampal dysfunction was associated with poorer working memory performance on the MATRICS Consensus Cognitive Battery, consistent with the suggestion that disrupted hippocampal activity contributes to impaired sensory information processing. Taken together, P50 suppression deficits appear to involve impaired interactions between frontal and temporal brain regions, and these early sensory processing difficulties may, in turn, contribute to cognitive symptoms in schizophrenia.
Another important consideration is the complex relationship between working memory and attention, which have been linked behaviorally and neurobiologically (32). Consistent with our findings of an association between working memory and enhanced P50 suppression, individuals with high working memory capacity may be more successful in resisting attentional capture by salient but irrelevant stimuli than those with lower capacity (33). Optimal performance on working memory tasks utilized in the present investigation may also rely on attentional processes that influence maintenance of the material to be remembered (34). Likewise, tasks measuring attention often include working memory demands to varying degrees (35), and clinically rated inattention is likely to involve temporary disruptions in working memory. Furthermore, impairments on purported tests of processing speed (e.g., coding tasks) appear to be attributable in part to attention and working memory deficits, since efficient performance requires the ability to rapidly bind representations in working memory to improve accurate speeded performance (36). Regardless, present results provide evidence for an important link between deficient P50 suppression and cognitive performance deficits in schizophrenia.
The absence of an association between P50 suppression and performance on the attention/vigilance task in this study highlights the possibility that P50 is related more closely to efficient information processing and working memory than to short-term, focused, sustained attention and vigilance as assessed, for example, by the Continuous Performance Test-Identical Pairs version. Specifically, P50 suppression may be more critical to tasks that involve rapid encoding, brief maintenance, and manipulation of stimuli than to tasks that require ongoing monitoring and sustained attention. Similarly, the lack of an association between P50 and performance on the MATRICS Consensus Cognitive Battery attention/vigilance task is not necessarily inconsistent with our finding of a relationship with inattentiveness on the SANS. Given that this global clinical rating is based on inattentiveness during social behavior and mental status testing, it may be that the construct better captures aspects of attention associated with speeded information processing and working memory rather than sustained attention and vigilance. This possibility is consistent with findings suggesting an absence of a relationship between P50 suppression and performance on the degraded stimulus Continuous Performance Test (17). In fact, the apparent divergence from studies reporting positive associations (5, 13) might be attributable to their reliance on tasks (i.e., digit vigilance and the Gordon Diagnostic System Continuous Performance Test) eliciting dimensions of attention other than those examined in this study (e.g., selective attention, vigilance, or orienting/shifting) or involving other task parameters (e.g., provision of feedback, time restrictions).
It is noteworthy that in addition to the P50 ratio score, our primary findings involved relationships between cognitive dysfunction and P50 S2 amplitude but not with the P50 S1 response. This pattern of findings is consistent with a dominant model of P50 sensory gating, whereby S1 activates inhibitory neuronal mechanisms that suppress or inhibit the response to the identical S2, resulting in attenuation of P50 in response to S2 (3). Findings regarding the relative importance of a P50 S1-amplitude deficit have been debated in the literature. A meta-analysis demonstrated substantial heterogeneity in S1-amplitude group differences across studies and suggested that S1 amplitude alone does not differentiate schizophrenia patients from healthy subjects as reliably as the P50 ratio (2). The present results support the possibility that patients with schizophrenia, and particularly those with greater working memory deficits, have insufficient activation of P50 inhibitory mechanisms.
The present research did not include medication-free patients, since at the time of participation, all patients were prescribed antipsychotic medications. Antipsychotic medications may affect some cognitive processes (37), although findings have been mixed (38). With respect to P50, neither first-generation nor second-generation antipsychotic medications—with the possible exception of clozapine (39) and, to a lesser extent, risperidone (8)—have been shown to improve sensory processing deficits in schizophrenia. Statistically accounting for chlorpromazine-equivalent dosages and limiting the study sample to recent-onset patients who were stabilized on risperidone did not modify the results. Therefore, the systematic impact of antipsychotic medications on our study findings was likely minimal. Additionally, although participants were assessed for substance abuse and/or dependence with the SCID, the study is limited by the lack of urine toxicology screens to verify abstinence prior to EEG assessment.
Taken together, results of this study implicate P50 inhibitory processing deficits with core features of schizophrenia. Furthermore, our findings substantiate P50 as a promising indicator of early sensory processing abnormalities by confirming the presence of inhibitory P50 deficits in schizophrenia and by demonstrating their associations with clinically rated inattention and working memory and processing speed performance. These results are consistent with the RDoC initiative and provide evidence that P50 suppression is a viable and promising manifestation of pathological mechanisms that can be used as targets for interventions that aim to improve cognitive impairments in schizophrenia (40). Indeed, there is encouraging evidence to suggest that inhibitory gating deficits in individuals with schizophrenia are modifiable by cognitive training (e.g., see reference 41). Further research will help to clarify whether P50 suppression deficits are amenable to other forms of interventions while also determining whether such benefits extend to other aspects of cognition.
1 : The NIMH Research Domain Criteria initiative: background, issues, and pragmatics. Psychophysiology 2016; 53:286–297Crossref, Medline, Google Scholar
2 : P50 sensory gating ratios in schizophrenics and controls: a review and data analysis. Psychiatry Res 2008; 158:226–247Crossref, Medline, Google Scholar
3 : Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull 1987; 13:669–678Crossref, Medline, Google Scholar
4 : Exploration of somatosensory P50 gating in schizophrenia spectrum patients: reduced P50 amplitude correlates to social anhedonia. Psychiatry Res 2004; 125:147–160Crossref, Medline, Google Scholar
5 : P50 abnormalities in schizophrenia: relationship to clinical and neuropsychological indices of attention. Schizophr Res 1998; 33:157–167Crossref, Medline, Google Scholar
6 : P50 inhibitory gating deficit is correlated with the negative symptomatology of schizophrenia. Psychiatry Res 2005; 136:27–34Crossref, Medline, Google Scholar
7 : Sensory gating deficit in a subtype of chronic schizophrenic patients. Psychiatry Res 2004; 125:237–245Crossref, Medline, Google Scholar
8 : P50 suppression in recent-onset schizophrenia: clinical correlates and risperidone effects. J Abnorm Psychol 1998; 107:691–698Crossref, Medline, Google Scholar
9 : Lack of relationship of auditory gating defects to negative symptoms in schizophrenia. Schizophr Res 1990; 3:131–138Crossref, Medline, Google Scholar
10 : Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am J Psychiatry 2000; 157:767–771Link, Google Scholar
11 : P50 gating in deficit and nondeficit schizophrenia. Schizophr Res 2010; 119:183–190Crossref, Medline, Google Scholar
12 : M50 sensory gating predicts negative symptoms in schizophrenia. Schizophr Res 2005; 73:311–318Crossref, Medline, Google Scholar
13 : Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res 1993; 10:131–141Crossref, Medline, Google Scholar
14 : Cognitive abilities and 50- and 100-msec paired-click processes in schizophrenia. Am J Psychiatry 2010; 167:1264–1275Link, Google Scholar
15 : Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. Am J Psychiatry 2003; 160:1595–1605Link, Google Scholar
16 : Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull 2006; 32:692–700Crossref, Medline, Google Scholar
17 : Neuropsychological correlates of P50 sensory gating in patients with schizophrenia. Schizophr Res 2013; 143:102–106Crossref, Medline, Google Scholar
18 : The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008; 165:203–213Link, Google Scholar
19 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders. New York, Biometrics Research, New York State Psychiatric Institute, 2002Google Scholar
20 : Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 1993; 150:1856–1861Link, Google Scholar
21 : Scale for the Assessment of Positive Symptoms. Iowa City, Iowa, University of Iowa, 1984Google Scholar
22 : Scale for the Assessment of Negative Symptoms. Iowa City, Iowa, University of Iowa, 1984Google Scholar
23 : Gating of auditory response in schizophrenics and normal controls: effects of recording site and stimulation interval on the P50 wave. Schizophr Res 1991; 4:31–40Crossref, Medline, Google Scholar
24 : Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia: a randomized clinical trial. JAMA Psychiatry 2015; 72:822–829Crossref, Medline, Google Scholar
25 : Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57:289–300Google Scholar
26 : Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res 2007; 92:262–272Crossref, Medline, Google Scholar
27 : Distinct neural generators of sensory gating in schizophrenia. Psychophysiology 2011; 48:470–478Crossref, Medline, Google Scholar
28 : Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 2001; 158:1809–1817Link, Google Scholar
29 : Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry 2014; 171:549–556Link, Google Scholar
30 : Prefrontal activation during verbal fluency tests in schizophrenia: a near-infrared spectroscopy (NIRS) study. Schizophr Res 2005; 77:65–73Crossref, Medline, Google Scholar
31 : Cognitive performance in schizophrenia: relationship to regional brain volumes and psychiatric symptoms. Psychiatry Res 2002; 116:1–23Crossref, Medline, Google Scholar
32 : Interactions between attention and working memory. Neuroscience 2006; 139:201–208Crossref, Medline, Google Scholar
33 : Human variation in overriding attentional capture. J Neurosci 2009; 29:8726–8733Crossref, Medline, Google Scholar
34 : Interference in immediate spatial memory. Mem Cognit 1994; 22:1–13Crossref, Medline, Google Scholar
35 : Comparison of the Continuous Performance Test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res 2001; 48:307–316Crossref, Medline, Google Scholar
36 : Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci 2012; 16:27–34Crossref, Medline, Google Scholar
37 : The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull 1999; 25:201–222Crossref, Medline, Google Scholar
38 : Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry 2007; 64:1115–1122Crossref, Medline, Google Scholar
39 : Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiology 1999; 39:10–17Crossref, Medline, Google Scholar
40 : Replacing DSM categorical analyses with dimensional analyses in psychiatry research: the Research Domain Criteria initiative. JAMA Psychiatry 2015; 72:1159–1160Crossref, Medline, Google Scholar
41 : Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biol Psychiatry 2011; 69:465–471Crossref, Medline, Google Scholar



