In Vivo Measurement of GABA Transmission in Healthy Subjects and Schizophrenia Patients
Abstract
Objective:
Postmortem studies in schizophrenia reveal alterations in gene products that regulate the release and extracellular persistence of GABA. However, results of in vivo studies of schizophrenia measuring total tissue GABA with magnetic resonance spectroscopy (MRS) have been inconsistent. Neither the postmortem nor the MRS studies directly address the physiological properties of GABA neurotransmission. The present study addresses this question through an innovative positron emission tomography (PET) paradigm.
Method:
The binding of [11C]flumazenil, a benzodiazepine-specific PET radiotracer, was measured before and after administration of tiagabine (0.2 mg/kg of body weight), a GABA membrane transporter (GAT1) blocker, in 17 off-medication patients with schizophrenia and 22 healthy comparison subjects. Increased extracellular GABA, through GAT1 blockade, enhances the affinity of GABAA receptors for benzodiazepine ligands, detected as an increase in [11C]flumazenil tissue distribution volume (VT).
Results:
[11C]Flumazenil VT was significantly increased across all cortical brain regions in the healthy comparison group but not in the schizophrenia group. This lack of effect was most prominent in the antipsychotic-naive schizophrenia group. In this subgroup, [11C]flumazenil ΔVT in the medial temporal lobe was correlated with positive symptoms, and baseline [11C]flumazenil VT in the medial temporal lobe was negatively correlated with visual learning. In the healthy comparison group but not the schizophrenia group, [11C]flumazenil ΔVT was positively associated with gamma-band oscillation power.
Conclusions:
This study demonstrates, for the first time, an in vivo impairment in GABA transmission in schizophrenia, most prominent in antipsychotic-naive individuals. The impairment in GABA transmission appears to be linked to clinical symptoms, disturbances in cortical oscillations, and cognition.
One of the most consistent and replicated postmortem findings in schizophrenia is the reduced expression of mRNA encoding the 67-kD isoform of glutamic acid decarboxylase (GAD67), the enzyme principally responsible for the synthesis of gamma-aminobutyric acid (GABA) (1). The deficit in GAD67 mRNA appears to be prominent in the parvalbumin-containing subset of GABA neurons and to be conserved with similar magnitude across multiple cortical regions (2). These findings may be interpreted as a reduced capacity for cortical GABA production. GABA neurotransmission plays a key role in sustaining synchronous oscillations in cortical networks (3), which in turn is thought to be a critical neural mechanism for supporting a number of cognitive and perceptual processes (4–7). Thus, lower GABA synthesis in schizophrenia has been hypothesized to contribute to the altered prefrontal cortical oscillations and functional activation associated with impaired performance on working memory or cognitive control tasks (8–15), as well as to contribute to abnormalities in a range of other cognitive, affective, sensory, and motor functions that depend on GABA neurotransmission in a number of other cortical regions (16–19). However, to date, there is no direct, in vivo evidence that cortical GABA transmission is altered in schizophrenia or linked to cognitive and neurophysiological disturbances in this illness.
We recently validated a method for in vivo measurement of GABA neurotransmission in human subjects, using positron emission tomography (PET) to measure binding of [11C]flumazenil, a radiotracer that binds to the benzodiazepine site of the GABAA receptor (20, 21). This relationship reflects findings from in vitro, preclinical, and previous imaging experiments suggesting that increased GABA levels enhance the affinity of GABAA receptors for benzodiazepine ligands via a conformational change (22, 23). While in certain circumstances—for example, in the case of benzodiazepine receptor inverse agonists—increased GABA levels can decrease the affinity (24), our previous studies demonstrate that increases in GABA levels increase the affinity at the benzodiazepine receptor, detected as an increase in the binding of the GABAA benzodiazepine receptor site-specific PET radioligand [11C]flumazenil, consistent with findings from preclinical work (22, 25).
In the present study, we utilized this approach to explore, in vivo, the evidence that GABA transmission disturbances span multiple cortical brain regions in schizophrenia and relate these abnormalities to clinical symptoms, disturbances in cortical oscillations, and cognition. We hypothesized that in individuals with schizophrenia, in response to elevated extracellular GABA levels induced by blockade of the GABA membrane transporter (GAT1) with tiagabine (20), the increase in [11C]flumazenil binding would be blunted relative to healthy comparison subjects and that this blunted transmission would be correlated with abnormalities across a range of cognitive functions. In addition, as previous studies (26, 27) have linked PET measurements of benzodiazepine receptors to positive symptoms, we sought to replicate these correlations.
Method
Participants
Nineteen patients with schizophrenia were recruited and enrolled in this study between May 31, 2008, and June 24, 2013. (The inclusion and exclusion criteria are listed in the data supplement that accompanies the online edition of this article.) Two patients were unable to complete the study (in both cases, the second PET scan was terminated at the patient’s request because of discomfort), leaving a final cohort of 17 schizophrenia patients. Eight of the schizophrenia patients were antipsychotic naive; the other nine had been treated in the past and had been off of psychotropic medications for at least 5 weeks (mean=154 weeks, SD=217, range=5–624). Twenty-two healthy comparison subjects, 10 of whom overlapped with our previously published data (20, 21), matched for age, gender, and ethnicity, were recruited contemporaneously with the schizophrenia patients (see the data supplement for inclusion and exclusion criteria). Menstrual phase was not taken into account during recruitment or scanning in either group, representing a limitation, given previous work showing an effect of menstrual cycle on GABA level (28). The University of Pittsburgh Institutional Review Board approved the study. All participants provided written informed consent after receiving a full explanation of the study procedures. For the schizophrenia patients, capacity to provide informed consent was evaluated by a psychiatrist not associated with the study.
Clinical Assessment
Diagnosis was assessed using the Structured Clinical Interview for DSM-IV (SCID) (29). Absence of psychiatric history and/or symptoms in healthy comparison subjects was determined after administration of the nonpatient version of the SCID by a trained rater. Severity of symptoms in schizophrenia patients was assessed with the Positive and Negative Syndrome Scale (PANSS) (30), and cognitive functioning was assessed with the MATRICS Consensus Cognitive Battery (MCCB) (31).
PET Protocol and Image Analysis
The PET scanning protocol was identical to that used in our previous studies (20, 21). All participants underwent scanning twice with [11C]flumazenil on the same day. First, a baseline PET scan was performed, followed by oral administration of tiagabine, and the second scan was begun 30 minutes after tiagabine administration. The tiagabine dose was rounded to the nearest even number (thus avoiding any splitting of the 2-mg tablets) to provide a dose of approximately 0.2 mg/kg body weight (mean=0.20 mg/kg, SD=0.04; N=39). The plasma level of tiagabine was calculated as the average of three measurements taken 30 minutes, 50 minutes, and 90 minutes after dosing. Participants remained in the Montefiore University Hospital Clinical and Translational Research Center overnight after the study for monitoring of tiagabine side effects.
Regions of interest were drawn on each individual’s MRI as described previously (20, 21) and applied to the co-registered dynamic PET data to generate time-activity curves. Three functionally based cortical regions of interest were obtained as weighted averages of component regions of interest: the association cortex, comprising the dorsolateral prefrontal, orbital frontal, medial prefrontal, and anterior cingulate cortices; the sensory cortex, comprising the parietal and occipital cortices; and the limbic medial temporal lobe, comprising the amygdala, hippocampus, entorhinal cortex, and parahippocampal gyrus.
Derivation of Distribution Volumes
Derivation of [11C]flumazenil regional tissue distribution volume (VT, mL/g) was performed with an unconstrained two-tissue compartment model using arterial input (20, 21). In previous studies, including our own (20), the pons has been used as the region of reference because activity in this region has been reported to represent predominantly nonspecific binding (32, 33). However, postmortem studies (34, 35) as well as previous receptor imaging studies (36), including unpublished human imaging data from our laboratory, have demonstrated that a significant percentage (up to 60%) of the signal from the pons is due to specific binding. We elected to utilize VT as our main outcome measure because it has been shown to be a more reliable and robust outcome measure for [11C]flumazenil, given that variability in the measurement of the nondisplaceable distribution volume, VND, will be propagated to measurements of binding potential (BPND or BPP) (37). The change in [11C]flumazenil binding induced by tiagabine was calculated as ΔVT = (posttiagabine VT – baseline VT)/baseline VT.
Electrophysiology Measurement
In all participants, the electrophysiology study was performed approximately 1 week before the PET scans. EEG data were acquired during the “preparing to overcome prepotency” task, a cued stimulus-response reversal paradigm that requires increases in cognitive control to overcome prepotent response tendencies. The methodology was identical to that used in our previous work (20, 21) and resulted in one summary measure of frontal gamma activity for each participant. This measure of gamma oscillations was then compared with the individual measurements of tiagabine-induced increase in [11C]flumazenil binding as well as baseline [11C]flumazenil binding by PET within each subject. An experimenter blind to the PET data performed the determinations of these EEG measures of frontal gamma. (For more details, see the online data supplement.)
Statistical Analysis
Comparisons between scan parameters and VND (pons VT) were assessed with a two-tailed paired t test with a significance level of 0.05. Baseline and posttiagabine VT for the three functional cortical regions were compared using a two-tailed paired t test with a Bonferroni-corrected probability value of 0.02 (0.05/3 regions). For the analysis of the tiagabine-induced change in VT (ΔVT) in the component regions of interest (N=10), a univariate repeated-measures analysis of variance (ANOVA) with brain regions as the within-scan factor and condition (baseline or posttiagabine) as the between-scan factor. Comparisons between the three groups initially utilized a repeated-measures ANOVA with brain regions (N=10) as the within-scan factor and diagnosis (healthy, antipsychotic-exposed schizophrenia, or antipsychotic-naive schizophrenia) as the between-subject factor, with subsequent group-by-group repeated-measures ANOVAs performed when the initial analysis indicated a significant difference between the three groups. Although there was no correlation between plasma tiagabine level and ΔVT in any group (see Figure S1 in the online data supplement), when comparing ΔVT across groups, plasma tiagabine level was included as a covariate because of the high variability of the plasma levels and the lower plasma level in the antipsychotic-naive compared with the antipsychotic-exposed schizophrenia group, which fell short of significance. Paired t tests were performed when appropriate to determine which regions accounted for significant effects observed in the repeated-measures ANOVAs. The relationship between the regional PET scan outcome parameters and PANSS and MCCB scores were analyzed with the Pearson product moment correlation coefficient after first confirming normal distribution of the data using the Kolmogorov-Smirnov test with a Bonferroni-corrected probability value of 0.02 for the three functional cortical regions, as noted above, and a value of 0.005 when examining the component regions (0.05/10 regions).
Results
The schizophrenia group included 10 African Americans and seven Caucasians; 11 of the group were male, and the mean age was 27.5 years (SD=6.8). The healthy comparison group included seven African Americans, 14 Caucasians, and one Asian; 13 of the group were male, and the mean age was 28.4 years (SD=8.7). There were no significant differences between groups on any demographic measure. The antipsychotic-naive group included five African Americans and three Caucasians; six of the group were male, and the mean age was 26.8 (SD=7.7). The antipsychotic-exposed group included five African Americans and four Caucasians; five of the group were male, and the mean age was 28.2 (SD=6.2). There were no significant differences between these subgroups on any demographic measure, nor between either of these groups and the healthy comparison group.
Participants’ scores on the PANSS and the MCCB are summarized in Table 1.
| Schizophrenia Group (N=17) | Antipsychotic-Naive Group (N=8) | Antipsychotic-Exposed Group (N=9) | Antipsychotic-Exposed Versus Antipsychotic-Naive Groupa | ||||
|---|---|---|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD | Mean | SD | p |
| Positive and Negative Syndrome Scale | |||||||
| Total score | 83 | 19 | 93 | 13 | 71 | 18 | <0.001 |
| Positive score | 21 | 7.5 | 25 | 6 | 15 | 6 | 0.004 |
| Negative score | 21 | 6.6 | 23 | 6 | 19 | 7 | 0.001 |
| General score | 41 | 9.3 | 46 | 7 | 36 | 9 | 0.25 |
| MATRICS Consensus Cognitive Battery composite score | 37.5 | 13 | 34.1 | 16 | 40.1 | 11 | 0.39 |
TABLE 1. Baseline Clinical Measures for Schizophrenia Patients
PET Scan Parameters
Neither the injected dose, specific activity, injected mass, free plasma fraction, nor VND differed between the baseline and posttiagabine scan for any of the groups (Tables 2 and 3). Tiagabine administration resulted in a slight increase in the plasma clearance of [11C]flumazenil in the healthy comparison group (Table 2). No significant differences were detected between the schizophrenia and healthy comparison groups for either the baseline or the posttiagabine scan, with the exception of slightly higher injected mass in both conditions for the schizophrenia group (Table 2). Comparison of the healthy comparison group with the antipsychotic-exposed schizophrenia group and with the antipsychotic-naive schizophrenia group revealed that the antipsychotic-exposed group had a higher injected mass than the healthy comparison group in both conditions (Table 3). However, although numerically higher, the injected mass for all scans for all subjects remained within tracer dose range of <10 μg (38) and would not be expected to affect the measurement of VT. The free fraction was lower in the antipsychotic-naive schizophrenia group than in the antipsychotic-exposed group in the baseline condition, and lower than the healthy comparison group in both conditions (Table 3).
| Schizophrenia Group (N=17) | Healthy Comparison Group (N=22) | Schizophrenia Group Versus Healthy Comparison Groupb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Posttiagabine | Baseline | Posttiagabine | Baseline | Posttiagabine | |||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p |
| Tiagabine | ||||||||||
| Dose (mg/kg) | 0.20 | 0.04 | 0.21 | 0.04 | 0.33 | |||||
| Plasma levelc (ng/mL) | 244 | 157 | 223 | 152 | 0.67 | |||||
| [11C]Flumazenil | ||||||||||
| Injected dose (mCi) | 20.7 | 1.2 | 20.8 | 1.6 | 19.6 | 2.9 | 19.9 | 2.4 | 0.19 | 0.21 |
| Specific activity (Ci/mmol) | 1,642 | 993 | 1,386 | 616 | 1,763 | 739 | 1,943 | 1,381 | 0.67 | 0.13 |
| Injected mass (μg) | 5.1 | 3.0 | 5.8 | 3.2 | 3.8 | 1.2 | 3.9 | 1.6 | 0.05 | 0.02 |
| Free plasma fraction (%) | 52.7 | 9.1 | 52.8 | 9.4 | 55.8 | 7.7 | 56.2 | 8.3 | 0.26 | 0.25 |
| Clearanced (L/h) | 60 | 16 | 64 | 19 | 50 | 18 | 62 | 23 | 0.08 | 0.74 |
| Pons VT (or VND)e (mL/g) | 1.0 | 0.2 | 1.0 | 0.2 | 1.0 | 0.2 | 1.0 | 0.2 | 0.90 | 0.68 |
TABLE 2. Positron Emission Tomography-Related Measures for Schizophrenia Patients and Healthy Comparison Subjects at Baseline and After Tiagabine Administrationa
| Comparisonsa | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antipsychotic-Naive Schizophrenia Group (N=8) | Antipsychotic-Exposed Schizophrenia Group (N=9) | Antipsychotic-Naive Versus Antipsychotic-Exposed Group | Antipsychotic-Naive Versus Healthy Comparison Group | Antipsychotic-Exposed Versus Healthy Comparison Group | ||||||||||
| Baseline | Posttiagabine | Baseline | Posttiagabine | Baseline | Posttiagabine | Baseline | Posttiagabine | Baseline | Posttiagabine | |||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p | p | p | p | p |
| Tiagabine | ||||||||||||||
| Dose (mg/kg) | 0.19 | 0.02 | 0.20 | 0.04 | 0.83 | 0.36 | 0.53 | |||||||
| Plasma level (ng/mL) | 173 | 80 | 308 | 184 | 0.07 | 0.39 | 0.20 | |||||||
| [11C]Flumazenil | ||||||||||||||
| Injected dose (mCi) | 20.6 | 1.4 | 21.1 | 0.7 | 20.8 | 1.1 | 20.5 | 2.1 | 0.75 | 0.48 | 0.41 | 0.19 | 0.28 | 0.53 |
| Specific activity (Ci/mmol) | 1,745 | 732 | 1,479 | 671 | 1,550 | 1,217 | 1,303 | 590 | 0.70 | 0.57 | 0.95 | 0.37 | 0.55 | 0.19 |
| Injected mass (μg) | 4.5 | 2.9 | 5.4 | 2.9 | 5.7 | 3.1 | 6.1 | 3.7 | 0.43 | 0.65 | 0.31 | 0.08 | 0.02 | 0.02 |
| Free plasma fraction (%) | 46.9 | 2.8 | 48.8 | 5.6 | 57.9 | 9.7 | 56.5 | 10.8 | 0.01 | 0.09 | <0.01 | 0.03 | 0.53 | 0.94 |
| Clearance (L/h) | 61 | 16 | 58 | 22 | 59 | 16 | 69 | 15 | 0.79 | 0.25 | 0.14 | 0.72 | 0.22 | 0.38 |
| Pons VT (or VND)b (mL/g) | 1.1 | 0.2 | 1.1 | 0.2 | 0.9 | 0.2 | 1.0 | 0.1 | 0.06 | 0.32 | 0.25 | 0.38 | 0.36 | 0.79 |
TABLE 3. Positron Emission Tomography-Related Measures for Antipsychotic-Naive and Antipsychotic-Exposed Schizophrenia Patients at Baseline and After Tiagabine Administration, and Comparison With Each Other and the Healthy Comparison Group
Regional Distribution Volumes and Benzodiazepine Receptor Availability
Healthy comparison subjects.
Tiagabine administration significantly increased VT in the large cortical regions, with a Bonferroni-corrected p value of 0.02 (Table 4). Examination of VT across the component regions of interest revealed a significant regional effect (F=151, df=12, 31, p<0.001), no region-by-condition interaction, and a significant difference across conditions (F=7.2, df=1, 42, p=0.01). On a region-by-region basis, significant increases in all regions were seen in the posttiagabine condition (Table 4).
| Healthy Comparison Group (N=22) | Schizophrenia Group (N=17) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline VT (mL/g) | Posttiagabine VT (mL/g) | ΔVT (%) | Analysisb | Baseline VT (mL/g) | Posttiagabine VT (mL/g) | ΔVT (%) | Analysisb | |||||||||
| Subdivision and Component Region of Interest | Mean | SD | Mean | SD | Mean | SD | d | p | Mean | SD | Mean | SD | Mean | SD | d | p |
| Association cortex | 6.5 | 0.6 | 7.1 | 0.8 | 9.2 | 11.6 | 0.80 | 0.001 | 7.0 | 0.8 | 7.3 | 0.8 | 5.9 | 11.4 | 0.46 | 0.08 |
| Dorsolateral prefrontal cortex | 6.5 | 0.6 | 7.1 | 0.8 | 9.7 | 12.1 | 0.85 | 0.001 | 6.9 | 0.9 | 7.3 | 0.8 | 6.3 | 11.9 | 0.48 | 0.07 |
| Orbital frontal cortex | 6.5 | 0.7 | 7.0 | 0.9 | 9.0 | 12.2 | 0.71 | 0.002 | 6.9 | 0.9 | 7.3 | 0.8 | 6.4 | 11.4 | 0.46 | 0.06 |
| Medial prefrontal cortex | 6.8 | 0.7 | 7.4 | 0.8 | 9.1 | 11.0 | 0.80 | 0.001 | 7.3 | 0.9 | 7.6 | 0.7 | 4.6 | 10.3 | 0.37 | 0.13 |
| Anterior cingulate cortex | 6.7 | 0.6 | 7.2 | 0.9 | 7.1 | 12.6 | 0.60 | 0.020 | 7.2 | 0.8 | 7.4 | 0.8 | 3.0 | 10.3 | 0.23 | 0.34 |
| Sensory cortex | 6.4 | 0.5 | 7.0 | 0.8 | 9.1 | 11.0 | 0.86 | 0.001 | 6.8 | 0.8 | 7.1 | 0.7 | 4.8 | 10.6 | 0.38 | 0.11 |
| Parietal cortex | 6.2 | 0.5 | 6.8 | 0.8 | 9.4 | 11.1 | 0.88 | 0.001 | 6.6 | 0.8 | 6.9 | 0.7 | 5.8 | 11.8 | 0.44 | 0.09 |
| Occipital cortex | 6.7 | 0.6 | 7.3 | 0.9 | 8.3 | 11.1 | 0.77 | 0.002 | 7.1 | 0.8 | 7.3 | 0.7 | 4.2 | 9.7 | 0.34 | 0.13 |
| Medial temporal lobe | 5.1 | 0.4 | 5.5 | 0.6 | 9.0 | 12.9 | 0.83 | 0.003 | 5.4 | 0.6 | 5.6 | 0.6 | 4.5 | 11.5 | 0.37 | 0.17 |
| Amygdala | 5.0 | 0.5 | 5.4 | 0.7 | 9.1 | 13.3 | 0.75 | 0.004 | 5.2 | 0.6 | 5.4 | 0.7 | 3.3 | 12.1 | 0.24 | 0.35 |
| Hippocampus | 5.1 | 0.5 | 5.5 | 0.7 | 9.3 | 15.3 | 0.77 | 0.009 | 5.3 | 0.6 | 5.5 | 0.7 | 3.7 | 13.8 | 0.25 | 0.40 |
| Entorhinal cortex | 5.1 | 0.6 | 5.5 | 0.7 | 8.3 | 14.2 | 0.61 | 0.012 | 5.3 | 0.6 | 5.6 | 0.7 | 6.6 | 11.8 | 0.50 | 0.05 |
| Parahippocampus | 5.2 | 0.4 | 5.6 | 0.7 | 8.8 | 12.4 | 0.82 | 0.003 | 5.5 | 0.6 | 5.8 | 0.6 | 4.9 | 11.2 | 0.42 | 0.12 |
TABLE 4. Tiagabine-Induced Change in [11C]Flumazenil VT in the Healthy Comparison Group and the Schizophrenia Groupa
Schizophrenia patients.
No significant change in VT was observed in any of the large cortical regions after administration of tiagabine (Table 4). Examination of VT across the component regions of interest revealed no significant difference across conditions with the omnibus test or with region-by-region contrasts (Table 4).
Comparison of healthy comparison and schizophrenia groups.
No difference was observed when comparing [11C]flumazenil ΔVT between the healthy comparison and schizophrenia groups. Performing the repeated-measures ANOVA with the three groups (healthy, antipsychotic-exposed schizophrenia, and antipsychotic-naive schizophrenia) revealed a significant difference between groups (F=3.6, df=2, 33, p=0.04). Further analysis revealed that the antipsychotic-naive group had significantly lower ΔVT compared with the healthy comparison group (F=5.93, df=1, 26, p=0.02). This difference reached significance in the dorsolateral prefrontal, orbital frontal, medial prefrontal, and parietal cortices (Table 5). No significant differences were observed in [11C]flumazenil ΔVT between the healthy comparison and antipsychotic-exposed schizophrenia groups or between the antipsychotic-naive and antipsychotic-exposed schizophrenia groups.
| ΔVT (%) | Comparisonsb | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy Comparison Group (N=22) | Antipsychotic-Naive Schizophrenia Group (N=8) | Antipsychotic-Exposed Schizophrenia Group (N=9) | Antipsychotic-Exposed Versus Antipsychotic-Naive Group | Healthy Comparison Versus Antipsychotic-Exposed Group | Healthy Comparison Versus Antipsychotic-Naive Groupc | ||||
| Subdivision and Component Region of Interest | Mean | SD | Mean | SD | Mean | SD | p | p | p |
| Association cortex | 9.2 | 11.6 | –0.26 | 7.51 | 11.31 | 11.76 | 0.03 | 0.66 | 0.04 |
| Dorsolateral prefrontal cortex | 9.7 | 12.1 | –0.19 | 7.39 | 12.07 | 12.41 | 0.03 | 0.62 | 0.04 |
| Orbital frontal cortex | 9.0 | 12.2 | –0.88 | 7.35 | 12.92 | 10.64 | 0.01 | 0.41 | 0.04 |
| Medial prefrontal cortex | 9.1 | 11.0 | 0.17 | 8.05 | 8.48 | 10.90 | 0.10 | 0.90 | 0.05 |
| Anterior cingulate cortex | 7.1 | 12.6 | –0.28 | 9.01 | 5.85 | 11.04 | 0.23 | 0.80 | 0.14 |
| Sensory cortex | 9.1 | 11.0 | –0.07 | 7.06 | 9.17 | 11.57 | 0.07 | 0.99 | 0.04 |
| Parietal cortex | 9.4 | 11.1 | –0.08 | 6.98 | 10.99 | 13.12 | 0.05 | 0.74 | 0.03 |
| Occipital cortex | 8.3 | 11.1 | 0.03 | 7.47 | 7.95 | 10.38 | 0.09 | 0.94 | 0.06 |
| Medial temporal lobe | 9.0 | 12.9 | –0.43 | 9.68 | 8.94 | 11.65 | 0.09 | 1.00 | 0.07 |
| Amygdala | 9.1 | 13.3 | –0.54 | 12.05 | 6.74 | 11.63 | 0.22 | 0.64 | 0.08 |
| Hippocampus | 9.3 | 15.3 | –0.90 | 11.64 | 7.73 | 14.94 | 0.21 | 0.80 | 0.10 |
| Entorhinal cortex | 8.3 | 14.2 | 0.41 | 10.58 | 12.01 | 10.47 | 0.04 | 0.49 | 0.16 |
| Parahippocampus | 8.8 | 12.4 | –0.08 | 8.71 | 9.32 | 11.68 | 0.08 | 0.92 | 0.07 |
TABLE 5. Effects of Previous Treatment on Tiagabine-Induced Change in [11C]flumazenil VT in the Healthy Comparison Group and the Antipsychotic-Naive and Antipsychotic-Exposed Schizophrenia Groups, and Group Comparisonsa
To examine the proximal source of the low ΔVT in the antipsychotic-naive schizophrenia group, the baseline and posttiagabine VT values were compared with those of the healthy comparison group and the antipsychotic-exposed schizophrenia group. The repeated-measures ANOVA across the three groups on baseline [11C]flumazenil VT revealed a significant difference between groups (F=5.81, df=2, 36, p=0.007), with the antipsychotic-naive schizophrenia group demonstrating elevated baseline [11C]flumazenil VT compared with the healthy comparison group (F=10.39, df=1, 28, p=0.003) and the antipsychotic-exposed group (F=6.68, df=1, 15, p=0.02). No differences in posttiagabine [11C]flumazenil VT were observed in the initial analysis or when the groups were compared separately (Table 6 and Figure 1). No differences were observed when comparing [11C]flumazenil binding in the healthy comparison group and the overall schizophrenia group at baseline or after tiagabine administration, or between the healthy comparison group and the antipsychotic-exposed group in baseline or posttiagabine [11C]flumazenil VT.
| VT (mL/g) | Comparisonsb | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy Comparison Group (N=22) | Antipsychotic-Naive Schizophrenia Group (N=8) | Antipsychotic-Exposed Schizophrenia Group (N=9) | Antipsychotic-Exposed Versus Antipsychotic-Naive Groupc | Healthy Comparison Versus Antipsychotic-Exposed Group | Healthy Comparison Versus Antipsychotic-Naive Groupc | ||||
| Subdivision and Component Region of Interest | Mean | SD | Mean | SD | Mean | SD | p | p | p |
| Association cortex | 6.5 | 0.6 | 7.4 | 0.9 | 6.6 | 0.6 | 0.053 | 0.84 | 0.009 |
| Dorsolateral prefrontal cortex | 6.5 | 0.6 | 7.3 | 0.9 | 6.6 | 0.7 | 0.088 | 0.76 | 0.013 |
| Orbital frontal cortex | 6.5 | 0.7 | 7.4 | 0.9 | 6.4 | 0.6 | 0.014 | 0.73 | 0.006 |
| Medial prefrontal cortex | 6.8 | 0.7 | 7.7 | 0.9 | 7.0 | 0.7 | 0.123 | 0.53 | 0.013 |
| Anterior cingulate cortex | 6.7 | 0.6 | 7.6 | 0.8 | 6.8 | 0.7 | 0.053 | 0.63 | 0.004 |
| Sensory cortex | 6.4 | 0.5 | 7.2 | 0.8 | 6.4 | 0.6 | 0.039 | 0.98 | 0.003 |
| Parietal cortex | 6.2 | 0.5 | 7.1 | 0.8 | 6.2 | 0.7 | 0.029 | 0.88 | 0.003 |
| Occipital cortex | 6.7 | 0.6 | 7.5 | 0.8 | 6.7 | 0.7 | 0.052 | 0.95 | 0.008 |
| Medial temporal lobe | 5.1 | 0.4 | 5.7 | 0.5 | 5.1 | 0.5 | 0.014 | 0.97 | 0.001 |
| Amygdala | 5.0 | 0.5 | 5.6 | 0.5 | 4.9 | 0.5 | 0.007 | 0.61 | 0.007 |
| Hippocampus | 5.1 | 0.5 | 5.7 | 0.4 | 5.0 | 0.6 | 0.022 | 0.75 | 0.006 |
| Entorhinal cortex | 5.1 | 0.6 | 5.7 | 0.4 | 4.9 | 0.5 | 0.001 | 0.44 | 0.008 |
| Parahippocampus | 5.2 | 0.4 | 5.8 | 0.5 | 5.3 | 0.5 | 0.041 | 0.57 | 0.002 |
TABLE 6. Baseline [11C]flumazenil VT in the Healthy Comparison Group and the Antipsychotic-Naive and Antipsychotic-Exposed Schizophrenia Groups, and Group Comparisonsa
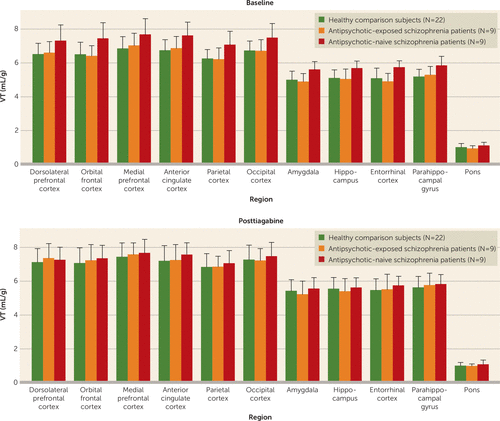
FIGURE 1. [11C]Flumazenil Regional Tissue Distribution Volumes (VT) at Baseline and After Tiagabine Administration in Healthy Comparison Subjects and Antipsychotic-Naive and Antipsychotic-Exposed Schizophrenia Patientsa
a Error bars indicate standard deviations.
Given the finding of elevated [11C]flumazenil VT at baseline in the antipsychotic-naive schizophrenia group, we examined the relationship between time off medications in the antipsychotic-exposed schizophrenia subjects and [11C]flumazenil VT at baseline to see whether treatment had an effect on this measurement. A correlation was seen between weeks off of medications and baseline [11C]flumazenil VT in the medial temporal lobe, although it did not meet the Bonferroni-corrected significance threshold of p<0.02 (r=0.73, p=0.026).
Clinical and Cognitive Measures
PANSS positive score was correlated with [11C]flumazenil ΔVT in the medial temporal lobe (r=0.76, p=0.02) and the medial temporal lobe subregion of the amygdala (r=0.88, p=0.002) in the antipsychotic-naive schizophrenia group, using a Bonferroni-corrected p<0.02 for the medial temporal lobe functional region and p<0.005 for the amygdala (see Figure S2 in the online data supplement). No correlations were noted for [11C]flumazenil ΔVT with PANSS score or subscores in the antipsychotic-exposed schizophrenia group or the schizophrenia group as a whole. Baseline [11C]flumazenil binding in the antipsychotic-exposed group was negatively correlated with PANSS positive score in the orbital frontal cortex (r=−0.80, p=0.009), but the correlation did not survive Bonferroni correction (p<0.005). No other correlations were observed between [11C]flumazenil binding (baseline, posttiagabine, or ΔVT) and PANSS measures.
Baseline [11C]flumazenil VT in the antipsychotic-naive schizophrenia group, but not in the antipsychotic-exposed group or the schizophrenia group as a whole, was negatively correlated with the visual learning cognitive domain of the MCCB (using a Bonferroni-corrected threshold of p<0.02 for the functional regions and p<0.005 for the subregions). This negative correlation was seen in the medial temporal lobe (r=−0.74, p=0.02) as well as in one of its subregions, the entorhinal cortex (r=−0.87, p=0.002). In the antipsychotic-naive schizophrenia group, other regions had similar negative correlations, albeit falling short of significance, between baseline [11C]flumazenil VT and the MCCB visual learning domain. In addition, negative correlations with baseline [11C]flumazenil VT, also falling short of significance, were observed across several of the other MCCB domains in various regions in the antipsychotic-naive schizophrenia group but not in the antipsychotic-exposed group or the schizophrenia group as a whole.
Cortical Oscillations
For the healthy comparison group, the association between gamma-band power and the ability to increase extracellular GABA levels was significant in the large cortical area of the association cortex (r=0.69, p=0.04; see Figure S3 in the online data supplement) and the dorsolateral prefrontal cortex (r=0.69, p=0.04), although these relationships did not survive Bonferroni correction. No association between [11C]flumazenil ΔVT and gamma-band power was noted in the schizophrenia group as a whole or in the antipsychotic-exposed and antipsychotic-naive groups.
In the antipsychotic-naive group, but not in the antipsychotic-exposed group, the schizophrenia group as a whole, or the healthy comparison group, gamma-band power was strongly correlated with baseline [11C]flumazenil VT in the medial temporal lobe (r=0.94, p<0.001), the association cortex (r=0.78, p=0.01), and the sensory cortex (r=0.79 p=0.01) (see Figure S4 in the data supplement).
Discussion
After acute GAT1 blockade in healthy subjects and in antipsychotic-naive and antipsychotic-exposed schizophrenia patients, we detected the predicted elevated extracellular GABA levels as increased binding of the benzodiazepine receptor ligand, measured as [11C]flumazenil ΔVT, in the healthy comparison group. However, schizophrenia patients did not exhibit this same increase in [11C]flumazenil VT after GAT1 blockade, indicating impaired GABA transmission in this population. Antipsychotic-naive schizophrenia patients showed an absence of change in [11C]flumazenil binding after acute increase in GABA and increased baseline [11C]flumazenil binding, indicating that this subgroup contributed disproportionally to the group effect. In contrast, schizophrenia patients who had past treatment with antipsychotics were indistinguishable from healthy comparison subjects on the PET scan measurements.
There are two potential interpretations of the finding of increased [11C]flumazenil binding at baseline with no change in binding after tiagabine administration in antipsychotic-naive schizophrenia patients. The first possibility is that elevated [11C]flumazenil binding at baseline reflects greater affinity due to higher extracellular GABA levels prior to tiagabine exposure. Consequently, tiagabine administration does not result in significant additional elevations of synaptic GABA in this population. Alternatively, elevated baseline [11C]flumazenil binding may indicate a compensatory increase in GABAA receptors in response to a deficit in GABA transmission early in the illness, with lower presynaptic GABA synthesis limiting the effect of tiagabine on extracellular GABA levels.
The postmortem findings of lower levels of GAD67 mRNA and protein, which are most prominent in parvalbumin-containing interneurons, as well as alterations in other markers of GABA function, suggest that impairment in inhibitory neurotransmission could contribute to the symptoms of schizophrenia (see reference 1 for a review). However, reductions in GAD67 mRNA and protein do not, in and of themselves, support lower GABA levels, as these findings could result from a down-regulation of GAD67 transcription in parvalbumin neurons in response to elevated GABA levels. Furthermore, lower GABA synthesis in parvalbumin neurons does not exclude the possibility of other interneurons releasing greater-than-normal levels of GABA (39) in response to elevated activity of excitatory pyramidal neurons, as proposed in the N-methyl-d-aspartate receptor hypofunction hypothesis of schizophrenia (40). Thus GABA may be elevated globally to maintain homeostasis in the face of perturbations in circuit activity. Since GAT1 is widespread across the neocortex and present in both GABA neurons and glial cells (41), the technique employed in our study is well suited to detect alterations in global extracellular GABA levels, but it does not have the resolution to detect localized, circuit-specific perturbations in GABA levels.
Similarly, the other brain imaging technique used to measure GABA levels in vivo, magnetic resonance spectroscopy (MRS), provides a measurement of global GABA levels generated from the average tissue concentration of GABA in all pools (intra- and extracellular), as opposed to PET, which allows the detection of alterations in GABA levels in the extracellular space. Of the published MRS studies exploring GABA in schizophrenia, three demonstrated increased GABA measures in schizophrenia (42–44), three demonstrated decreased GABA measures (45–47), and three showed no difference (42, 48, 49). Two of the MRS studies examined the effect of treatment with antipsychotic medications; Kegeles et al. (42) found increased GABA measures in the medial prefrontal cortex and normal GABA levels in the dorsolateral prefrontal cortex in unmedicated patients, with no effect of previous treatment. Tayoshi et al. (49) found that the lower the dosage of antipsychotic medication, the higher the GABA levels in the anterior cingulate cortex. Both of these findings are consistent with one interpretation of our findings, that basal GABA levels are elevated in antipsychotic-naive schizophrenia patients. They are also consistent with the observed association (falling short of significance) between time off antipsychotic medications and baseline [11C]flumazenil binding in antipsychotic-exposed schizophrenia patients.
Alternatively, our findings could be viewed as reflecting a compensatory increase in GABAA receptors in response to a deficit in GABA transmission early in the illness, such that a lower pool of presynaptic GABA limits the effect of tiagabine on extracellular GABA levels. We believe this second interpretation is more likely to be the case, for the following reasons. First, this interpretation is consistent with postmortem studies showing increases in GABAA receptor binding (50) in schizophrenia. Although findings from postmortem studies of benzodiazepine receptors have been mixed—reporting no change (51), decreases (52), or increases (53)—these findings of variable differences in benzodiazepine binding could be due to differences in previous treatment, and if so, they are in line with the differences we observed in relation to the effects of the presence or absence of previous treatment on [11C]flumazenil binding at baseline, as the postmortem studies do not report on antipsychotic-naive individuals. Previous imaging studies of benzodiazepine receptor densities in schizophrenia have found no differences (26, 27, 54–56). Only the study by Asai et al. (56) reported on medication-naive individuals separately and found no difference; however, the study used the pons as a reference region and used [11C]Ro15-4513, a ligand that measures separate, albeit overlapping, populations of benzodiazepine receptors (24), making direct comparison with our results difficult. Second, in our study, schizophrenia patients who had never received antipsychotic treatment had elevated benzodiazepine binding, whereas in those who had received treatment, benzodiazepine binding was no different from that in healthy subjects, with a notable association, approaching significance, between time off medication and benzodiazepine binding. These findings may be explained by low GABA transmission in the illness initially, with a compensatory increase in GABAA receptors. Subsequent treatment with antipsychotics may increase GABA transmission, resulting in normalization of GABAA receptor levels, perhaps through a reduction of excess dopamine D2 receptor stimulation at the convergence of cortical glutamatergic afferents and dopamine projections on GABA-ergic medium spiny neurons (57), although some preclinical work suggests that antipsychotic medications reduce GABA transmission (58). However, continued impairment in other, as yet unknown, processes prevents this normalization from being effective in overcoming the deficits in cognition.
Third, the lack of correlation between gamma power and [11C]flumazenil ΔVT in the schizophrenia group but not the healthy comparison group further supports impaired GABA neurotransmission in the illness. Consistent with the hypothesis that GABA transmission is critical for various types of perceptual and cognitive processes, in the antipsychotic-naive schizophrenia group, we observed negative correlations between the visual learning cognitive domain of the MCCB and baseline [11C]flumazenil binding (again, interpreted as increased in response to reduced GABA transmission). This association between impaired GABA transmission and impaired cognition in schizophrenia is supported by experimental models (59) that suggest that GABAA receptor-mediated transmission is required for the induction of network oscillations. In turn, synchronous gamma activity has been proposed to be critical for perceptual feature binding and to be associated with higher cognitive processes (4, 60). We measured oscillatory activity during a cognitive load in an attempt to directly link the measurement of GABA in vivo with this phenomenon in our subjects (see the online data supplement). We found no association between GABA transmission and the ability to increase oscillatory activity in the gamma-band range in the schizophrenia group as a whole or when broken down to the antipsychotic-exposed and antipsychotic-naive groups. Interestingly, we noted a strong relationship between baseline [11C]flumazenil binding in antipsychotic-naive schizophrenia patients and gamma-band power, perhaps indicating that a compensatory increase in GABAA receptors is effective in increasing the ability to entrain cortical networks, albeit not to a sufficient degree to improve cognitive performance. Alternatively, baseline GABA increases could result from increased baseline activity of parvalbumin-positive interneurons, which in turn could give rise to network gamma oscillations (61). In fact, increases in baseline gamma activity have been reported in schizophrenia and have been invoked to explain decreases in task-activated gamma activity, given the standard practice of subtracting prestimulus baseline activity (62). However, on this account, the increases in baseline [11C]flumazenil binding would negatively correlate with task-activated gamma. In other words, a higher baseline gamma activity, resulting from increased baseline GABA, would result in decreased task related-activated gamma when subtracting prestimulus activity from the task-activity measure, meaning that subjects with higher baseline GABA (i.e., higher prestimulus activity) would have the lower gamma, contrary to our findings of a positive relationship.
Our finding of a relationship between clinical symptoms and markers of GABA-ergic transmission is consistent with and extends previous findings. Although it did not survive correction for multiple comparisons, the negative correlation between PANSS positive symptom score and baseline [11C]flumazenil VT in the orbital frontal cortex is consistent with the findings reported by Busatto et al (26); however, Schröder et al. (27) found a positive correlation with the total score on the Brief Psychiatric Rating Scale. The finding of a positive relationship between [11C]flumazenil ΔVT and PANSS positive symptom score in the antipsychotic-naive but not the antipsychotic-exposed schizophrenia group is difficult to interpret in the context of a minimal change in VT in the antipsychotic-naive group and a lower degree of positive symptoms in the antipsychotic-exposed group; further studies are necessary to explore this finding. Interestingly, we did not observe any relationship between negative symptoms and [11C]flumazenil binding parameters, in contrast to the report of Asai et al. (56), who reported a negative correlation between benzodiazepine binding and PANSS negative symptom score.
Taken together, the results of this study are consistent with postmortem studies suggesting lower cortical GABA neurotransmission in schizophrenia (see reference 1 for a review). Our data indicate that impairment in GABA transmission and reduced GABA signaling are most pronounced prior to treatment; with treatment, the abnormalities in the receptor parameters and GABA transmission measured by this paradigm appear to normalize.
The strengths of this study include measurement of the arterial input function, allowing for the assessment of the effects of tiagabine on VND and free plasma fraction. While the absence of change in these variables after tiagabine administration validates the use of either BPP or BPND as an outcome measure, we chose to use VT as our primary outcome measure. We were concerned that differential effects of increasing GABA levels on [11C]flumazenil-specific binding in the pons would have an effect on the comparison across groups. Differences in specific binding within the pons would affect either BPP or BPND to a greater degree in one group relative to the other, potentially obscuring group differences in tiagabine-induced change in [11C]flumazenil binding, despite the fact that, on average, no changes were seen in the pons VT after tiagabine administration in either group. Moreover, VT has been shown to be a more reliable and robust outcome measure for [11C]flumazenil than either BPND or BPP (37).
This study also has several limitations, among which is the fact that only minimal information on total exposure to antipsychotic medications was available for the previously treated group, thereby limiting our ability to explore the relationship between our outcome measures and time and type of medication. In addition, our previously published studies noted a high variability in the percent change in [11C]flumazenil binding across subjects (20, 21). The present study was consistent with this, as we again saw a high degree of variability in [11C]flumazenil ΔVT across all of the regions of interest. Detecting differences between individuals with a psychiatric disorder and healthy comparison subjects remains challenging with this level of variability; however, comparing the groups in this study, we noted the increase in [11C]flumazenil VT to be greater in the healthy comparison group than in the antipsychotic-naive schizophrenia group, in which there was a near absence of tiagabine-induced increase in [11C]flumazenil VT. In other words, this relatively large between-group difference could be detected with the present method (the average increase in VT in the healthy comparison group was 8.3% [SD=2.2%], whereas it was −0.51% [SD=1.1%] for the antipsychotic-naive schizophrenia group), but more subtle differences in GABA availability between the antipsychotic-exposed group and the healthy comparison or antipsychotic-naive group may be difficult to detect without improvements in the methods to reduce the variability.
The results of this study suggest that GABA abnormalities in schizophrenia are widespread across cortical domains, consistent with recent postmortem studies (2), and are linked to clinical symptoms and cognitive impairments of the illness. In addition, treatment with antipsychotic medications appears to normalize the measured abnormalities in GABA signaling; however, the clinical impact of this normalization appears minimal at best with regard to cognitive functioning.
1 : Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6:312–324Crossref, Medline, Google Scholar
2 : Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol 2014; 26:22–26Crossref, Medline, Google Scholar
3 : Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007; 8:45–56Crossref, Medline, Google Scholar
4 : Gamma oscillations correlate with working memory load in humans. Cereb Cortex 2003; 13:1369–1374Crossref, Medline, Google Scholar
5 : Coherence of gamma-band EEG activity as a basis for associative learning. Nature 1999; 397:434–436Crossref, Medline, Google Scholar
6 : Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci 1995; 18:555–586Crossref, Medline, Google Scholar
7 : Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis Neurosci 1999; 16:449–459Crossref, Medline, Google Scholar
8 : Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943–958Crossref, Medline, Google Scholar
9 : Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry 1998; 155:1285–1287Link, Google Scholar
10 : Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia, III: a new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry 1988; 45:609–615Crossref, Medline, Google Scholar
11 : Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional cerebral blood flow evidence. Arch Gen Psychiatry 1986; 43:114–124Crossref, Medline, Google Scholar
12 : Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci 1996; 351:1495–1503Crossref, Medline, Google Scholar
13 : Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078–1092Crossref, Medline, Google Scholar
14 : Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 2000; 48:99–109Crossref, Medline, Google Scholar
15 : Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry 1999; 45:1128–1137Crossref, Medline, Google Scholar
16 : Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry 2001; 158:1126–1133Link, Google Scholar
17 : Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry 2001; 158:1423–1428Link, Google Scholar
18 : Functional magnetic resonance imaging in schizophrenia: cortical response to motor stimulation. Psychiatry Res 2004; 130:227–243Crossref, Medline, Google Scholar
19 : Cognitive and motor impairments are related to gray matter volume deficits in schizophrenia. Biol Psychiatry 1996; 39:234–240Crossref, Medline, Google Scholar
20 : Tiagabine increases [11C]flumazenil binding in cortical brain regions in healthy control subjects. Neuropsychopharmacology 2009; 34:624–633Crossref, Medline, Google Scholar
21 : [11C]Flumazenil binding is increased in a dose-dependent manner with tiagabine-induced elevations in GABA levels. PLoS ONE 2012; 7:e32443Crossref, Medline, Google Scholar
22 : “GABA shift” in vivo: enhancement of benzodiazepine binding in vivo by modulation of endogenous GABA. Eur J Pharmacol 1988; 148:123–130Crossref, Medline, Google Scholar
23 : GABAergic modulation of benzodiazepine binding site sensitivity. Nature 1978; 274:383–385Crossref, Medline, Google Scholar
24 : Acute increases in synaptic GABA detectable in the living human brain: a [¹¹C]Ro15-4513 PET study. Neuroimage 2014; 99:158–165Crossref, Medline, Google Scholar
25 : [3H]Ro 15-1788 binding to benzodiazepine receptors in mouse brain in vivo: marked enhancement by GABA agonists and other CNS drugs. Eur J Pharmacol 1987; 142:373–384Crossref, Medline, Google Scholar
26 : Correlation between reduced in vivo benzodiazepine receptor binding and severity of psychotic symptoms in schizophrenia. Am J Psychiatry 1997; 154:56–63Link, Google Scholar
27 : Benzodiazepine receptor distribution and diazepam binding in schizophrenia: an exploratory study. Psychiatry Res 1997; 68:125–131Crossref, Medline, Google Scholar
28 : Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 2002; 59:851–858Crossref, Medline, Google Scholar
29 : The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
30 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
31 : The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008; 165:203–213Link, Google Scholar
32 : Measurement of benzodiazepine receptor number and affinity in humans using tracer kinetic modeling, positron emission tomography, and [11C]flumazenil. J Cereb Blood Flow Metab 1993; 13:656–667Crossref, Medline, Google Scholar
33 : Compartmental analysis of [11C]flumazenil kinetics for the estimation of ligand transport rate and receptor distribution using positron emission tomography. J Cereb Blood Flow Metab 1991; 11:735–744Crossref, Medline, Google Scholar
34 : Benzodiazepine receptor: demonstration in the central nervous system. Science 1977; 198:849–851Crossref, Medline, Google Scholar
35 : High densities of benzodiazepine receptors in human cortical areas. Nature 1977; 269:702–704Crossref, Medline, Google Scholar
36 : SPECT measurement of benzodiazepine receptors in human brain with iodine-123-iomazenil: kinetic and equilibrium paradigms. J Nucl Med 1994; 35:228–238Medline, Google Scholar
37 : Measurement of GABAA receptor binding in vivo with [11C]flumazenil: a test-retest study in healthy subjects. Neuroimage 2008; 41:260–269Crossref, Medline, Google Scholar
38 : Parametric images of benzodiazepine receptor concentration using a partial-saturation injection. J Cereb Blood Flow Metab 1997; 17:343–355Crossref, Medline, Google Scholar
39 : Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 2012; 35:57–67Crossref, Medline, Google Scholar
40 : From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012; 37:4–15Crossref, Medline, Google Scholar
41 : Neuronal and glial localization of GAT-1, a high-affinity gamma-aminobutyric acid plasma membrane transporter, in human cerebral cortex: with a note on its distribution in monkey cortex. J Comp Neurol 1998; 396:51–63Crossref, Medline, Google Scholar
42 : Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 2012; 69:449–459Crossref, Medline, Google Scholar
43 : Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry 2010; 68:667–670Crossref, Medline, Google Scholar
44 : In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull 2013; 39:1096–1104Crossref, Medline, Google Scholar
45 : GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci 2010; 30:3777–3781Crossref, Medline, Google Scholar
46 : Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T proton MRS study. Schizophr Res 2009; 112:192–193Crossref, Medline, Google Scholar
47 : Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: the potential impact of GABA concentration in the visual cortex. Prog Neuropsychopharmacol Biol Psychiatry 2013; 47:13–19Crossref, Medline, Google Scholar
48 : GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin 2014; 4:531–539Crossref, Medline, Google Scholar
49 : GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res 2010; 117:83–91Crossref, Medline, Google Scholar
50 : Up-regulation of GABAA receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience 1996; 75:1021–1031Crossref, Medline, Google Scholar
51 : Uncoupling of GABA(A) and benzodiazepine receptor binding activity in the hippocampal formation of schizophrenic brain. Brain Res 1997; 755:121–129Crossref, Medline, Google Scholar
52 : Reduced [3H]flunitrazepam binding in cingulate cortex and hippocampus of postmortem schizophrenic brains: is selective loss of glutamatergic neurons associated with major psychoses? Neurochem Res 1993; 18:219–223Crossref, Medline, Google Scholar
53 : Benzodiazepine receptors increase in post-mortem brain of chronic schizophrenics. Eur Arch Psychiatry Neurol Sci 1989; 239:71–78Crossref, Medline, Google Scholar
54 : [123I]Iomazenil SPECT benzodiazepine receptor imaging in schizophrenia. Psychiatry Res 1999; 91:163–173Crossref, Medline, Google Scholar
55 : No evidence of altered in vivo benzodiazepine receptor binding in schizophrenia. Neuropsychopharmacology 1999; 20:650–661Crossref, Medline, Google Scholar
56 : GABAA/benzodiazepine receptor binding in patients with schizophrenia using [11C]Ro15-4513, a radioligand with relatively high affinity for alpha5 subunit. Schizophr Res 2008; 99:333–340Crossref, Medline, Google Scholar
57 : Mechanism of action of antipsychotic drugs: from dopamine D(2) receptor antagonism to glutamate NMDA facilitation. Clin Ther 2005; 27(suppl A):S16–S24Crossref, Medline, Google Scholar
58 : The effects of haloperidol and clozapine on extracellular GABA levels in the prefrontal cortex of the rat: an in vivo microdialysis study. Cereb Cortex 1994; 4:69–77Crossref, Medline, Google Scholar
59 : When inhibition not excitation synchronizes neural firing. J Comput Neurosci 1994; 1:313–321Crossref, Medline, Google Scholar
60 : Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 1999; 3:151–162Crossref, Medline, Google Scholar
61 : Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009; 459:698–702Crossref, Medline, Google Scholar
62 : Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 2012; 62:1504–1518Crossref, Medline, Google Scholar



