Pleiotropic Locus for Emotion Recognition and Amygdala Volume Identified Using Univariate and Bivariate Linkage
Abstract
Objective:
The role of the amygdala in emotion recognition is well established, and amygdala volume and emotion recognition performance have each been shown separately to be highly heritable traits, but the potential role of common genetic influences on both traits has not been explored. The authors investigated the pleiotropic influences of amygdala volume and emotion recognition performance.
Method:
In a sample of randomly selected extended pedigrees (N=858), the authors used a combination of univariate and bivariate linkage to investigate pleiotropy between amygdala volume and emotion recognition performance and followed up with association analysis.
Results:
The authors found a pleiotropic region for amygdala volume and emotion recognition performance on chromosome 4q26 (LOD score=4.40). Association analysis conducted in the region underlying the bivariate linkage peak revealed a variant meeting the corrected significance level (Bonferroni-corrected p=5.01×10−5) within an intron of PDE5A (rs2622497, p=4.4×10−5) as being jointly influential on both traits. PDE5A has been implicated previously in recognition-memory deficits and is expressed in subcortical structures that are thought to underlie memory ability, including the amygdala.
Conclusions:
This study extends our understanding of the shared etiology between the amygdala and emotion recognition by showing that the overlap between amygdala volume and emotion recognition performance is due at least in part to common genetic influences. Moreover, this study identifies a pleiotropic locus for the two traits and an associated variant, which localizes the genetic signal even more precisely. These results, when taken in the context of previous research, highlight the potential utility of PDE5 inhibitors for ameliorating emotion recognition deficits in individuals suffering from mental or neurodegenerative illness.
The ability to successfully process and label the emotions of others is crucial to human social interaction (1). Impaired emotion recognition is a hallmark of a number of psychiatric disorders, including schizophrenia, bipolar disorder, and major depressive disorder (2, 3). Emotion recognition deficits also occur in neurodegenerative illnesses like Parkinson’s disease and Alzheimer’s disease (4, 5). Furthermore, there is considerable evidence for individual differences in emotion recognition in healthy populations (6), as well as evidence that a substantial portion of that variation appears to be under genetic influence (7). While multiple neural systems putatively subserve emotion recognition, the amygdala appears to have a preferential role in affect processing in both healthy and mentally ill individuals (1, 8–10). As the structure of the amygdala is influenced by genetic factors (11, 12), it is possible that the same genes that influence emotion recognition also influence amygdala volume and vice versa. However, it remains unclear whether the association between amygdala volume and emotion recognition is due to common genetic influences, and if so, which genes in particular are involved. Identifying genes with pleiotropic influence on both traits might reveal those molecular mechanisms that alter brain architecture or function, which in turn affect emotion recognition performance. In an effort to further our understanding of the molecular underpinnings of emotion recognition, our aim in this study was to isolate genes that jointly influence emotion recognition and amygdala volume in randomly selected extended pedigrees.
Numerous neural systems are implicated in emotion recognition, and those systems reside in particular in the frontal and temporal lobes, which together make up the neural pathways responsible for the interpretation of visual emotional stimuli (9, 10). After a visual stimulus is processed via the lateral geniculate nucleus and the primary visual cortex (V1), the amygdala becomes the focus of further processing in the brain, receiving input from both cortical and subcortical streams (13). The amygdala was first implicated in emotional capacity by Brown and Shafer (14), who noted that monkeys with bilateral temporal lobe lesions were rendered tame and docile. Later this type of lesion was linked to emotion processing and, more specifically, to fearful responses (15, 16). Subsequent work in primates and rodents narrowed the region of interest for emotion processing down to the amygdala (17, 18). The role of the amygdala in emotion recognition, and in particular the recognition of negative emotions, was confirmed in humans by lesion studies (19, 20) as well as functional imaging studies (10, 21, 22). While there is evidence that variation in the serotonin transporter gene influences the amygdala’s response to emotive faces (23), the complete genetic architecture of amygdala function and structure is largely unknown (24).
There have been a handful of candidate gene studies with a focus on emotion recognition ability (25, 26) as well as amygdala volume in healthy and depressed individuals (27–31). A genome-wide linkage study isolated a significant quantitative trait locus (QTL) for emotion recognition on chromosome 1p36 in a sample selected for schizophrenia (32). However, there have been no genome-wide searches related to amygdala volume, nor have there been attempts to disentangle the pleiotropic effects on the traits using multivariate analyses, which are statistically more powerful than univariate ones if traits are genetically correlated (33–35).
We report here on bivariate linkage and association analysis in a sample of Mexican American individuals from extended pedigrees. Using bivariate linkage analysis, we identified a region of chromosome 4 as being truly pleiotropic for bilateral amygdala volume and emotion recognition performance. Using association analysis of common variants within that linkage region, we identified a gene, PDE5A, as being influential on both traits.
Method
Participants
The sample comprised 858 Mexican American individuals from extended pedigrees (115 families; average family size, 7.53 people; range, 1–89 people), from the San Antonio Family Study. The sample was 63% female and had a mean age of 44.78 years (SD=15.19, range=18–97). Individuals in this cohort have actively participated in research for more than 18 years and were randomly selected from the community, with the constraints that they had to be of Mexican American ancestry, be part of a large family, and live within the San Antonio region (see reference 36 for recruitment details).
All participants provided written informed consent on forms approved by the institutional review board at the University of Texas Health Science Center at San Antonio.
Neuropsychological Assessment
As part of the “Genetics of Brain Structure and Function” protocol, each participant completed a 90-minute neuropsychological test battery consisting of standard and computerized measures (37, 38), including the Penn Emotion Recognition Task (39). This computer-based emotion recognition task consists of 40 color photographs of facial expression of emotions, including happy, sad, angry, fearful, and neutral (Figure 1). The stimuli are balanced for gender and ethnicity across emotions. During the task, participants are required to identify which of the five emotions best describes each face stimulus. The emotion recognition phenotype in the present study is a summed score across all stimuli.

FIGURE 1. Examples of Facial Emotion Stimuli From the Penn Emotion Recognition Taska
a The emotions depicted, from left to right (where the upper image is a mild expression of emotion and the lower is intense), are happy, sad, angry, fearful, and neutral.
MRI Acquisition and Processing
All images were acquired on a research-dedicated Siemens 3-T Tim Trio scanner and a high-resolution phase array head coil housed in the Research Imaging Institute, University of Texas Health Science Center at San Antonio. Seven high-resolution T1-weighted three-dimensional turbo-flash sequences with an adiabatic inversion contrast pulse were acquired in each subject using the following parameters: TE=3.04 ms, TR=2100 ms, TI=785 ms, flip angle=13°; 800 μm isotropic resolution (40).
The software package FreeSurfer (41, 42; http://surfer.nmr.mgh.harvard.edu/), as implemented in our group (12), was used to extract amygdala volume for subsequent genetic analyses. These methods have been described elsewhere; briefly, Fischl and colleagues (43, 44) developed a procedure for automatically and accurately labeling each voxel in the brain as part of one of 40 subcortical structures (thalamus, hippocampus, amygdala, and so on). This procedure is based on modeling the segmentation as a nonstationary anisotropic Markov random field, in which the probability of a neuroanatomic label is modulated by that of its neighbors. Probabilities were computed separately at each position in an atlas, resulting in a maximum a posteriori estimation of each voxel’s label in each image. Amygdala volume was averaged across hemispheres, yielding an average volume phenotype for each subject.
Data Analysis
Genotyping.
Subjects were genotyped for approximately 1 million single-nucleotide polymorphisms (SNPs) using Illumina HumanHap550v3, HumanExon510Sv1, Human1Mv1, and Human1M-Duov3 BeadChips, according to the Illumina Infinium protocol (Illumina, San Diego). SNP loci were checked for Mendelian consistency using SimWalk2 (45). SNPs or samples exhibiting high calling rate failures or requiring excessive blanking (i.e., if <95% of the genotypes are retained) were eliminated from analyses. Missing genotypes were imputed according to Mendelian laws based on available pedigree data using MERLIN (46). Maximum likelihood techniques accounting for pedigree structure were used to estimate allelic frequencies (47). For linkage analyses, multipoint identity-by-descent matrices were calculated based on 28,387 SNPs selected from the 1 million-SNP genome-wide association study panel as follows. Using genotypes for 345 founders, SNPs on each chromosome were selected to be at least 1 kb apart, with a minor allele frequency ≥5% and linkage disequilibrium (LD) within a 100-kb sliding window not exceeding an absolute value of 0.15 for rho. The resulting selection averaged seven to eight SNPs per centimorgan. For each centimorgan location in the genome, multipoint identity-by-descent probability matrices were calculated using a stochastic Markov chain Monte Carlo procedure implemented in the software package LOKI (48).
Confirmatory factor analysis.
Given that the aim of this study was to identify genes with pleiotropic effects on both amygdala volume and emotion recognition performance, it was important to be able to conduct multivariate analyses beyond bivariate linkage. To this end, a simple one-factor confirmatory factor model was built using two items, amygdala volume and emotion recognition performance, in which, to ensure that each trait contributed to the factor score equally, factor loadings were constrained to be equal, which is also a requirement of the model being identified. This model was built using Mplus (49), in which family structure was taken into account using the “cluster” command.
Quantitative genetic analyses.
All genetic analyses were performed in SOLAR (33). SOLAR implements a maximum likelihood variance decomposition to determine the contribution of genes and environmental influence to a trait by modeling the covariance among family members as a function of expected allele sharing given the pedigree. In the simplest such decomposition, the additive genetic contribution to a trait is represented by the heritability index (h2). First, univariate variance decomposition analysis was applied to both left and right amygdala volume and emotion recognition performance. Both traits were normalized using an inverse Gaussian transformation. Age, age squared, sex, and their interactions were included as covariates. Second, bivariate analysis was applied to the two variables, with the phenotypic covariance between the traits decomposed into its genetic and environmental constituents to determine the extent to which they are influenced by shared genetic effects (e.g., genetic correlation, rhog).
Linkage and association analyses.
Quantitative trait linkage analysis was performed to localize specific chromosomal locations influencing amygdala volume and emotion recognition ability (33). Initially this was done under a univariate model for each trait. Model parameters were estimated using maximum likelihood. The hypothesis of significant linkage was assessed by comparing the likelihood of a classical additive polygenic model with that of a model allowing for both a polygenic component and a variance component due to linkage at a specific chromosomal location (as evidenced by the location-specific identity-by-descent probability matrix). The LOD score, given by the log10 of the ratio of the likelihoods of the linkage and the polygenic null models, served as the test statistic for linkage. Genome-wide thresholds for linkage evidence were computed for this exact pedigree structure and density of markers, using a method derived from Feingold et al. (50): a LOD score of 1.69 is required for suggestive significance (likely to happen by chance less than once in a genome-wide scan), and a LOD score of 2.9 is required for genome-wide significance. Regions showing potential pleiotropy were subjected to bivariate linkage analysis; for comparison to the univariate results, the resulting LOD score was converted to a one degree of freedom equivalent based on the p value for the two degrees of freedom test (linkage to both traits versus linkage to neither) (51). To ensure that the bivariate LOD scores were truly driven by both and not one of the traits, we tested the null hypothesis of the absence of pleiotropy (that is, co-occurrence of linkage is by chance) versus the alternative of complete pleiotropy by comparing the likelihoods of the relevant nested models. To this end, we maximized two models, one in which the genetic correlation between linkage peaks was allowed to vary freely and a null in which this correlation was constrained to be zero; the likelihoods of these two models were then compared, with twice the difference between these two log-likelihoods being distributed as a chi-square with one degree of freedom. This method has been established as a powerful approach to detecting pleiotropic effects (52).
Genomic regions meeting bivariate genome-wide significance for linkage were investigated in greater detail using association analysis of the emotion and amygdala confirmatory factor score and the genetic variants encapsulated by the linkage peak. Statistical significance levels were established according to the effective number of tested variants given the LD structure in the region; to this end, the pairwise genotypic correlations are calculated in an effort to establish the effective number of independent tests carried out during association analysis. This method, proposed by Moskvina and Schmidt (53), is considered to be conservative and entails computing the eigenvalues of the genotypic correlation matrix. A corrected p value is obtained from a Bonferroni correction based on the nominal alpha (0.05) and the total number of independent tests.
Results
Heritability and Linkage Analysis
Both amygdala volume (h2=0.72, SE=0.07, p=4.95×10−5; mean=3066.72, SD=434.47) and emotion recognition (h2=0.32, SE=0.06, p=5.12×10−10; happy: mean=7.78, SD=1.47; sad: mean=6.15, SD=1.40; fear: mean=6.61, SD=1.36; anger: mean=5.56, SD=2.21; neutral: mean=5.58, SD=4.37) were highly heritable, and a bivariate model indicated significant genetic overlap between the two traits (rhog=0.25, SE=0.13, p=0.048). For amygdala volume and emotion recognition, age and sex were significant covariates (see Table S1 in the data supplement that accompanies the online edition of this article), and hence the effect of age and sex was covaried in all subsequent analyses. The factor score derived for emotion recognition and amygdala volume was also highly heritable (h2=0.45, SE=0.06, p=5.68×10−22), and the factor loadings for both traits were deemed to be significant at the p<0.001 level; goodness-of-fit statistics were not available because of saturation of the model.
For amygdala volume, one genome-wide significant locus was observed on chromosome 4 at 120 cM (LOD score=4.065). A LOD score of 2.175 was observed for the emotion recognition task on chromosome 2 at 71 cM, meeting criteria for suggestive significance, while the chromosome 4 locus showed some evidence for linkage to emotion recognition (LOD score=1.217) (Figure 2). Bivariate linkage revealed a genome-wide significant QTL for both amygdala and emotion recognition on chromosome 4 at 122 cM (1 df-equivalent LOD score=4.399), which suggests that this region of chromosome 4 mediates both amygdala volume and performance on the emotion recognition task. The test for pleiotropy versus coincident linkage confirmed the presence of pleiotropy for the two traits at this locus (χ2=20.12, df=1, p=3.6×10−6). The factor score, derived from the one-factor model of emotion recognition and amygdala volume, showed genome-wide significant linkage at precisely the same region on chromosome 4 at 122 cM (LOD score=3.336).
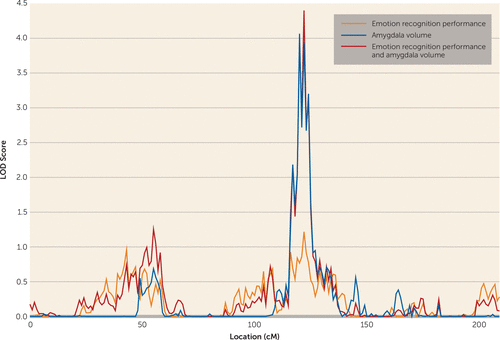
FIGURE 2. Chromosome 4 Multipoint Plot for Univariate and Bivariate Linkage Analysesa
a Univariate analysis reveals a genome-wide significant quantitative trait locus for amygdala volume and near suggestive significance for emotion recognition performance, and bivariate analysis reveals a genome-wide significant linkage signal for both traits.
The possible confounding role of intracranial volume at this locus was investigated using trivariate linkage. Univariate linkage revealed a QTL of suggestive significance for intracranial volume on chromosome 16 at 37 cM (LOD score=2.65) with little evidence for genetic influence on this trait on chromosome 4 at 122 cM (LOD score=0.47). Moreover, in a trivariate linkage model of the emotion recognition task, amygdala volume and intracranial volume on chromosome 4 at 122 cM showed genome-wide significance (1 df-equivalent LOD score=3.546), and even within this trivariate model, the pleiotropy test supported complete pleiotropy between amygdala and emotion recognition (χ2=7.74, p=2.7×10−3). Furthermore, the possibility that the right or left amygdala might be driving the result was addressed by running univariate and bivariate linkage in the same region. Left amygdala volume (h2=0.7019, SE=0.0788, p=2.03×10−21) had a univariate LOD score of 3.315 on chromosome 4 at 120 cM, and right amygdala volume (h2=0.6958, SE=0.0754, p=1.43×10−23) had a univariate LOD score of 3.103 in the same location. Moreover, bivariate linkage analysis with left amygdala volume and emotion recognition (rhog=0.24) revealed a bivariate LOD score of 3.282 on chromosome 4 at 122 cM, while bivariate linkage analysis with right amygdala volume and emotion recognition (rhog=0.27) revealed a bivariate LOD score of 3.6812 on chromosome 4 at 122 cM. These results support the idea that the shared genetic influence on amygdala volume and emotion recognition is not lateralized to either the left or right amygdala.
Association Analysis
Association analysis was conducted for all genetic variants under the 1 – LOD score confidence interval of the bivariate linkage peak (defined as 120–124 cM) and a factor score derived from amygdala volume and emotion recognition. In total there were 2,053 SNPs in this region, but after taking LD into account (53), there were 1,023 effective SNPs, necessitating a Bonferroni-corrected alpha of 5.01×10−5. One variant met the adjusted-significance level: rs2622497 (p=4.40×10−5), located within an intron of the gene PDE5A (phosphodiesterase 5A, cGMP specific). Several other variants met a suggestive level of significance that also fell within PDE5A in addition to a number of variants downstream of the gene, all of which were in varying degrees of LD with the top-ranked variant (see Table 1 and Figure 3). Univariate association analysis for each individual trait for rs2622497 did not reach significance either for amygdala volume (p=1.0×10−3) or for emotion recognition (p=4.1×10−3).
| SNP | χ2 | p | β | Variance Explained | Minor Allele Frequency | pa |
|---|---|---|---|---|---|---|
| rs2622497 | 16.67 | 0.000044 | –0.07 | 0.02 | 0.008 | 0.84 |
| rs2715021 | 16.42 | 0.000051 | –0.07 | 0.02 | 0.008 | 0.73 |
| rs9884801 | 15.76 | 0.000072 | –0.07 | 0.02 | 0.006 | 0.88 |
| rs2389894 | 14.10 | 0.000173 | –0.05 | 0.01 | 0.014 | 0.13 |
| rs2714982 | 12.22 | 0.000472 | –0.05 | 0.01 | 0.015 | 0.62 |
TABLE 1. Estimates for the Top Five Single-Nucleotide Polymorphisms (SNPs) From the Quantitative Trait Locus-Specific Association Analysis for the Bivariate Emotion Recognition and Amygdala Volume Factor Score
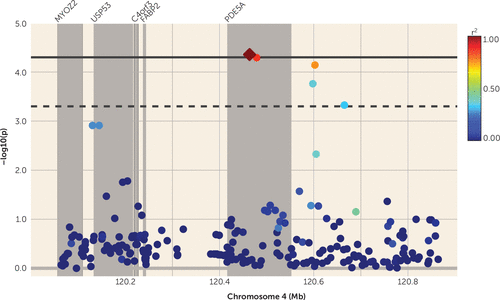
FIGURE 3. Quantitative Trait Locus (QTL)-Specific Association Analysis for the Genome-Wide Significant QTL Region Associated With Amygdala Volume and Emotion Recognition Performance on Chromosome 4a
a Intergenic regions are pale gray, and genes are represented by dark gray bars with the gene name shown at the top of the plot. The top-ranked variant in this region is represented by a diamond, and the degree of linkage disequilibrium (r2) with this variant is represented by the color scale.
When the SNP rs2622497 was included as a covariate in the linkage analysis of the factor score derived from emotion recognition and amygdala volume, the LOD score observed without the covariate (3.336) was reduced (2.506) and no longer significant. This linkage conditional on association test gives additional support for the association between rs2622497, emotion recognition performance, and amygdala volume.
Given the significant effect of age and sex on amygdala volume, the interactive effects of genotype, sex, and age were included as covariates in the association analysis. For our top SNPs, there was a significant interaction with sex (sex by rs2622497: β=0.9823664, p=0.0032331; sex by rs2715021: β=0.9887387, p=0.0032051; sex by rs9884801: β=0.9857738, p=0.0038682; see Figures S1–S3 in the online data supplement) but not with age. In each case, the sex-by-SNP interactions indicated that the effect was marginally more pronounced in men than in women, although the direction of the effect was the same in both groups. Consequently, an interaction term for sex by genotype was included in all association analyses.
Discussion
While numerous studies have highlighted an association between the amygdala and emotion recognition (10, 19–22), the present study extends this finding by providing evidence for a pleiotropic locus on chromosome 4 using bivariate linkage. Furthermore, by examining variants within the quantitative locus, we identified a variant (rs2622497) within an intron of PDE5A that appears to jointly influence amygdala volume and emotion recognition performance. To our knowledge, this is the first study to formally test the common genetic influences on amygdala volume and emotion recognition ability using a bivariate model. It is well established that the implementation of a bivariate as opposed to a univariate model is beneficial, as the joint analysis of multiple traits confers greater power and precision in the mapping of a QTL (33, 54, 55).
The cyclic nucleotide phosphodiesterases (PDEs) are a family of enzymes with two main subtypes, cAMP- and cGMP-specific nucleotides. The PDE5A gene codes for PDE5, a cGMP-specific PDE (56). cGMP, a second messenger, is crucial to signal transduction between cells as well as synapse communication and synaptic plasticity (57). Hence PDEs are important for effective cell-to-cell communication in the central nervous system (56). Indeed, there is widespread expression of PDE5A throughout the body, and it is expressed in various regions of the brain, including in the amygdala (58), the pyramidal cells of the hippocampus, Purkinje cells in the cerebellum, and some areas of the cortex (59, 60). Thus, PDE5 has emerged as a potential drug target for treating cognitive deficits (56, 61). Through the use of mouse models, it has emerged that administration of sildenafil, a PDE5 inhibitor, improves memory, including recognition memory as well as spatial and fear-conditioning memory, in aged rats (62, 63). Sildenafil also ameliorates cognitive deficits associated with Huntington’s chorea and Alzheimer’s disease by increasing cGMP levels in the hippocampus (64–66). Thus the results of the present study are in line with previous research. As in previous studies, the results presented here show an association between recognition memory—of which emotion recognition could be considered a sub-type (67)—and PDE5.
Recognition is predicated on knowledge retention, which is enhanced by contextual association. In the case of emotion recognition, this might include associations formed by previous experiences in which a particular facial configuration has come to be associated with a particular emotion. Such examples might include life events that precede or co-occur with the expression of a particular emotion, with what a person said while experiencing that emotion or what was said about them during that time, with how one felt on seeing the expression, and so on (67). If this is the case, then emotion recognition should require access to long-term memory, even when the emotional stimulus is portrayed by a stranger, so it seems plausible that emotion recognition is supported by a distributed neural network that includes those brain regions typically implicated in memory performance in addition to the amygdala (10, 68). In this context, PDE5A, a gene expressed particularly in the cerebellum and hippocampus, is especially interesting. Indeed, the cerebellum and the hippocampus, and for that matter the amygdala, have been implicated in long-term memory activation in humans (69). It is of note that in the present sample there was a significant genetic correlation between amygdala and both cerebellum (rhog=0.30, p=2.5×10−3) and hippocampus (rhog=0.66, p=2.15×10−14). A tentative hypothesis generated from the results of this study is that emotion recognition might be improved with the administration of a PDE5 inhibitor, which would be in line with some of the research cited above (62, 63) and would have substantial implications for those individuals for whom emotion recognition proves difficult, such as schizophrenia patients (2).
Rare variation is a likely source of family-based linkage signals associated with complex traits (70, 71). Therefore, it is unsurprising that those SNPs showing the strongest association with amygdala volume and emotion recognition in the present study are relatively rare (Table 1). The use of extended pedigrees, such as those used this study, improves the chances of detecting association with rare variants, as pedigree-based studies represent an implicit enrichment strategy for identifying rare variants. Mendelian transmissions from parents to offspring maximize the chances that multiple copies of rare variants exist in the pedigree. Thus, pedigree-based studies have optimal power to detect effects of rare variants, so it is unlikely that the associations we observed here are false positives, particularly given that the variants in question do not show a significant departure from the Hardy-Weinberg equilibrium (Table 1). Our top-ranked variant (rs2622497) appears to be similarly rare across populations (72; see also Table S2 in the online data supplement).
The results of this study could be called into question if the genetic effects detected were in fact univariate in nature (e.g., driven by only one of the phenotypes, perhaps in particular by amygdala volume). However, the linkage signal was subjected to a pleiotropy test and was shown to be truly pleiotropic. Furthermore, the factor score derived from the factor model can be said to be driven by both traits equally, as the factor loadings, which are used to determine each individual’s factor score, were constrained to be equal. It is true, however, that no formal test can be applied to the association analysis to further underscore the bivariate underpinnings of the signal.
Some evidence from previous research is suggestive of a modulatory effect of age on amygdala volume and emotion recognition (73–75) as well as interactions between sex and genotype on amygdala volume (76). In the present study, the effects of age and sex were controlled for in all analyses, including in the association analysis, where an additional interaction covariate (sex by SNP) was included. A significant sex-by-SNP interaction was evident for amygdala volume for our top three SNPs (see Figures S1–S3 in the online data supplement), such that the effect of genotype was slightly more pronounced in men than in women, but the direction of effect of was the same.
Patients with schizophrenia exhibit substantial and robust impairments in emotion recognition ability (39, 77). It is interesting, then, that the gene implicated in this study, PDE5, codes for a member of the phosphodiesterase enzyme family, as phosphodiesterase genes, and in particular cAMP-specific PDE4, have been implicated in schizophrenia risk (78, 79). Moreover, administration of rolipram (a PDE4 inhibitor) reduces phencyclidine-induced cognitive impairments in humans (phencyclidine is an established pharmacological model of schizophrenia symptomatology) (80). Phosphodiesterases have also been shown to have potential utility in the treatment of Alzheimer’s disease and in particular the associated cognitive impairment (81, 82). Furthermore, the administration of a PDE5 inhibitor was shown in a recent placebo-controlled study (83) to reduce symptoms of depression and cognitive impairment. The established role of phosphodiesterases in psychopathology and cognitive impairment in psychiatric illness, taken together with the results of the present study, highlight the potential utility of PDE5 inhibitors in the treatment of emotion recognition impairments in schizophrenia, depression, and Alzheimer’s disease.
In summary, the linkage and association findings presented here highlight a pleiotropic gene, PDE5A, for amygdala volume and emotion recognition ability. To our knowledge, this is the first study to identify a common genetic locus that influences these two traits. Although this study was conducted in healthy individuals, when taken in the context of previous research, which has shown the potential utility of PDE5 inhibitors as cognitive enhancers, it suggests that PDE5A may be an important target for ameliorating emotion recognition deficits in patients suffering from mental or neurodegenerative illness.
1 : Emotion circuits in the brain. Annu Rev Neurosci 2000; 23:155–184Crossref, Medline, Google Scholar
2 : Abnormal recognition of facial expression of emotions in depressed patients with major depression disorder and schizotypal personality disorder. Biol Psychiatry 1996; 40:697–705Crossref, Medline, Google Scholar
3 : Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biol Psychiatry 2000; 48:127–136Crossref, Medline, Google Scholar
4 : A meta-analysis of performance on emotion recognition tasks in Parkinson’s disease. Neuropsychology 2010; 24:176–191Crossref, Medline, Google Scholar
5 : Impaired recognition of facial expressions of emotion in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 2002; 14:64–71Crossref, Medline, Google Scholar
6 : Exploring issues of personality measurement and structure through the development of a short form of the Eysenck Personality Profiler. J Pers Assess 2003; 81:271–280Crossref, Medline, Google Scholar
7 : Heritability of individual differences in cortical processing of facial affect. Behav Genet 2010; 40:178–185Crossref, Medline, Google Scholar
8 : Neurobiology of emotion perception, II: implications for major psychiatric disorders. Biol Psychiatry 2003; 54:515–528Crossref, Medline, Google Scholar
9 : Cortical systems for the recognition of emotion in facial expressions. J Neurosci 1996; 16:7678–7687Crossref, Medline, Google Scholar
10 : Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002; 16:331–348Crossref, Medline, Google Scholar
11 : Genes contributing to subcortical volumes and intellectual ability implicate the thalamus. Hum Brain Mapp 2014; 35:2632–2642Crossref, Medline, Google Scholar
12 : Influence of age, sex, and genetic factors on the human brain. Brain Imaging Behav 2014; 8:143–152Crossref, Medline, Google Scholar
13 : Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci 2010; 11:773–783Crossref, Medline, Google Scholar
14 : An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philos Trans R Soc Lond B Biol Sci 1888; 179:303–327Crossref, Google Scholar
15 : “Psychic blindness” and other symptoms following bilateral temporal lobectomy in rhesus monkeys. Am J Physiol 1937; 119:352–353Google Scholar
16 : Preliminary analysis of functions of the temporal lobes in monkeys: 1939. J Neuropsychiatry Clin Neurosci 1997; 9:606–620Crossref, Medline, Google Scholar
17 : Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol 1956; 49:381–391Crossref, Medline, Google Scholar
18 : Emotion and the amygdala, in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Edited by Aggleton JP. New York, Wiley-Liss, 1992, pp 339–351Google Scholar
19 : Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 1994; 372:669–672Crossref, Medline, Google Scholar
20 : Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cogn Neuropsychol 1996; 13:699–745Crossref, Google Scholar
21 : A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 1996; 383:812–815Crossref, Medline, Google Scholar
22 : A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 1998; 121(Pt 1):47–57Crossref, Medline, Google Scholar
23 : Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 2005; 8:20–21Crossref, Medline, Google Scholar
24 : Amygdala-enriched genes identified by microarray technology are restricted to specific amygdaloid subnuclei. Proc Natl Acad Sci USA 2001; 98:5270–5275Crossref, Medline, Google Scholar
25 : MET and AKT genetic influence on facial emotion perception. PLoS ONE 2012; 7:e36143Crossref, Medline, Google Scholar
26 : The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol Psychiatry 2010; 67:543–549Crossref, Medline, Google Scholar
27 : Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology 2011; 36:891–897Crossref, Medline, Google Scholar
28 : Human amygdala volume is predicted by common DNA variation in the stathmin and serotonin transporter genes. Transl Psychiatr 2013; 3:e283Crossref, Medline, Google Scholar
29 : MAO A VNTR polymorphism and amygdala volume in healthy subjects. Psychiatry Res 2011; 191:87–91Crossref, Medline, Google Scholar
30 : The BDNF Val66Met polymorphism impacts parahippocampal and amygdala volume in healthy humans: incremental support for a genetic risk factor for depression. Psychol Med 2009; 39:1831–1839Crossref, Medline, Google Scholar
31 : 5-HTTLPR genotype influences amygdala volume. Eur Arch Psychiatry Clin Neurosci 2009; 259:212–217Crossref, Medline, Google Scholar
32 : Genome-wide linkage analyses of 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry 2013; 170:521–532Link, Google Scholar
33 : Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 1998; 62:1198–1211Crossref, Medline, Google Scholar
34 : Endophenotypes for psychiatric disorders: ready for primetime? Trends Genet 2006; 22:306–313Crossref, Medline, Google Scholar
35 : High dimensional endophenotype ranking in the search for major depression risk genes. Biol Psychiatry 2012; 71:6–14Crossref, Medline, Google Scholar
36 : Common genetic influences on depression, alcohol, and substance use disorders in Mexican-American families. Am J Med Genet B Neuropsychiatr Genet 2011; 156B:561–568Crossref, Medline, Google Scholar
37 : Adjudicating neurocognitive endophenotypes for schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2007; 144B:242–249Crossref, Medline, Google Scholar
38 : Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Arch Gen Psychiatry 2010; 67:168–177Crossref, Medline, Google Scholar
39 : Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry 2003; 160:1768–1774Link, Google Scholar
40 : Retrospective motion correction protocol for high-resolution anatomical MRI. Hum Brain Mapp 2006; 27:957–962Crossref, Medline, Google Scholar
41 : Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage 1999; 9:179–194Crossref, Medline, Google Scholar
42 : Cortical surface-based analysis, II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999; 9:195–207Crossref, Medline, Google Scholar
43 : Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355Crossref, Medline, Google Scholar
44 : Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004; 23(suppl 1):S69–S84Crossref, Medline, Google Scholar
45 : Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker-sharing statistics. Am J Hum Genet 1996; 58:1323–1337Medline, Google Scholar
46 : Merlin: rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 2002; 30:97–101Crossref, Medline, Google Scholar
47 : Allele frequency estimation from data on relatives. Am J Hum Genet 1991; 48:22–25Medline, Google Scholar
48 : Markov chain Monte Carlo segregation and linkage analysis for oligogenic models. Am J Hum Genet 1997; 61:748–760Crossref, Medline, Google Scholar
49 : Mplus User’s Guide, 6th ed. Los Angeles, Muthén & Muthén, 2011Google Scholar
50 : Gaussian models for genetic linkage analysis using complete high-resolution maps of identity by descent. Am J Hum Genet 1993; 53:234–251Medline, Google Scholar
51 : Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet Epidemiol 1997; 14:953–958Crossref, Medline, Google Scholar
52 : Joint multipoint linkage analysis of multivariate qualitative and quantitative traits, II: alcoholism and event-related potentials. Am J Hum Genet 1999; 65:1148–1160Crossref, Medline, Google Scholar
53 : On multiple-testing correction in genome-wide association studies. Genet Epidemiol 2008; 32:567–573Crossref, Medline, Google Scholar
54 : Multiple trait analysis of genetic mapping for quantitative trait loci. Genetics 1995; 140:1111–1127Medline, Google Scholar
55 : Multitrait least squares for quantitative trait loci detection. Genetics 2000; 156:899–911Medline, Google Scholar
56 : Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov 2006; 5:660–670Crossref, Medline, Google Scholar
57 : Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav 2011; 99:130–145Crossref, Medline, Google Scholar
58 Allen Institute for Brain Science: Allen Human Brain Atlas. 2012. http://human.brain-map.org/microarray/search/show?exact_match=false&search_term=PDE5&search_type=geneGoogle Scholar
59 : Cloning and localization of the cGMP-specific phosphodiesterase type 9 in the rat brain. J Neurocytol 2002; 31:729–741Crossref, Medline, Google Scholar
60 : Specific localized expression of cGMP PDEs in Purkinje neurons and macrophages. Neurochem Int 2004; 45:853–857Crossref, Medline, Google Scholar
61 : Improving memory: a role for phosphodiesterases. Curr Pharm Des 2006; 12:2511–2523Crossref, Medline, Google Scholar
62 : Effects of sildenafil on long-term retention of an inhibitory avoidance response in mice. Behav Pharmacol 1999; 10:731–737Crossref, Medline, Google Scholar
63 : Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochem Int 2004; 45:915–928Crossref, Medline, Google Scholar
64 : Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J Neurosci 2009; 29:8075–8086Crossref, Medline, Google Scholar
65 : Sildenafil restores cognitive function without affecting β-amyloid burden in a mouse model of Alzheimer’s disease. Br J Pharmacol 2011; 164:2029–2041Crossref, Medline, Google Scholar
66 : Regulation of hippocampal cGMP levels as a candidate to treat cognitive deficits in Huntington’s disease. PLoS ONE 2013; 8:e73664Crossref, Medline, Google Scholar
67 : Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behav Cogn Neurosci Rev 2002; 1:21–62Crossref, Medline, Google Scholar
68 : The distributed human neural system for face perception. Trends Cogn Sci 2000; 4:223–233Crossref, Medline, Google Scholar
69 : The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia 2006; 44:2189–2208Crossref, Medline, Google Scholar
70 : Molecular basis of a linkage peak: exome sequencing and family-based analysis identify a rare genetic variant in the ADIPOQ gene in the IRAS Family Study. Hum Mol Genet 2010; 19:4112–4120Crossref, Medline, Google Scholar
71 : Congenital heart disease, in Genomic and Personalized Medicine, 2nd ed, vols 1–2. Edited by Ginsburg GS, Willard HF. London, Academic Press, 2012, pp 624–634Google Scholar
72 : The International HapMap Project. Nature 2003; 426:789–796Crossref, Medline, Google Scholar
73 : Interpretation of emotionally ambiguous faces in older adults. J Gerontol B Psychol Sci Soc Sci 2008; 63:337–343Crossref, Google Scholar
74 : The human amygdala in normal aging and Alzheimer’s disease, in The Human Amygdala. Edited by Whalen PJ, Phelps EA. New York, Guilford Press, 2009, pp 382–402Google Scholar
75 : Cognitive aging explains age-related differences in face-based recognition of basic emotions except for anger and disgust. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2013; 20:253–270Crossref, Medline, Google Scholar
76 : 5-HTTLPR, anxiety, and gender interaction moderates right amygdala volume in healthy subjects. Soc Cogn Affect Neurosci (Epub ahead of print, Sept 29, 2013)Google Scholar
77 : Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull 2010; 36:1009–1019Crossref, Medline, Google Scholar
78 : DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 2005; 310:1187–1191Crossref, Medline, Google Scholar
79 : The PDE4B gene confers sex-specific protection against schizophrenia. Psychiatr Genet 2007; 17:129–133Crossref, Medline, Google Scholar
80 : The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1999; 20:201–225Crossref, Medline, Google Scholar
81 : The role of phosphodiesterases in schizophrenia: therapeutic implications. CNS Drugs 2008; 22:983–993Crossref, Medline, Google Scholar
82 : Phosphodiesterases as therapeutic targets for Alzheimer’s disease. ACS Chem Neurosci 2012; 3:832–844Crossref, Medline, Google Scholar
83 : Effects of daily low-dose treatment with phosphodiesterase type 5 inhibitor on cognition, depression, somatization, and erectile function in patients with erectile dysfunction: a double-blind, placebo-controlled study. Int J Impot Res 2014; 26:76–80Crossref, Medline, Google Scholar



