Effects of Psychotic State and Task Demand on Prefrontal Function in Schizophrenia: An fMRI Study of Overt Verbal Fluency
Abstract
OBJECTIVE: Impaired prefrontal cortical function is regarded as a central feature of schizophrenia. Although many neuroimaging studies have found evidence of abnormal prefrontal activation when patients with schizophrenia perform cognitive tasks, the extent to which this abnormality depends on the presence of active psychotic symptoms and on the demands of the task is unclear. The authors tested the hypothesis that prefrontal functional abnormalities in schizophrenia would be more evident in patients with active psychosis than in patients who were in remission and would become more apparent in the face of increasing task demands. METHOD: The authors used functional magnetic resonance imaging (fMRI) to examine prefrontal cortical activity during a paced letter verbal fluency task in three groups of subjects: acutely psychotic patients with schizophrenia, schizophrenia patients in remission, and healthy volunteers. Online subject performance was measured by utilizing a clustered fMRI acquisition sequence that allowed overt verbal responses to be made in the relative absence of scanner noise. RESULTS: Patients with schizophrenia showed less activation than the healthy comparison subjects in the anterior cingulate and the inferior frontal and right middle frontal cortices, independent of psychotic state and task demand. Acutely psychotic patients showed less activation than the healthy comparison subjects, but these differences were less marked than the differences between the patients in remission and the healthy comparison subjects. Acutely psychotic patients had less activation than the comparison subjects in the anterior cingulate but no significant difference in lateral prefrontal activation. Increasing task demand led to greater anterior cingulate and middle frontal activation in patients with active psychosis than in patients in remission. CONCLUSIONS: Schizophrenia is associated with impaired prefrontal function, but its manifestation depends on the severity of psychotic symptoms and the level of task difficulty.
Ingvar and Franzen (1) coined the term “hypofrontality” to describe a lower ratio of anterior to posterior cerebral activity in patients with schizophrenia, relative to healthy comparison subjects. Hypofrontality has since become the functional neuroimaging finding most associated with schizophrenia (2), notwithstanding dissenting views and contentious evidence (reviewed in references 3 and 4). Early studies examined subjects in the “resting state” (1, 5–10), but resting state hypofrontality has not been consistently replicated (11–17), nor is it specific to schizophrenia (reviewed in reference 4).
Attenuated prefrontal activation may also be evident when patients with schizophrenia perform cognitive tasks that engage the prefrontal cortex (8, 18). However, as patients with schizophrenia often perform such tasks more poorly than comparison subjects, this difference could simply reflect impaired performance (18–20). Researchers have thus sought to match the performance of schizophrenia patients and comparison subjects, usually by reducing the demands of the task so that patients can perform it as well as can the comparison subjects. The results of these studies have also been inconsistent, with reports of “hypofrontality” (21–23), no difference in frontal activation (24, 25), and “hyperfrontality” (26, 27) in the patients with schizophrenia. The potentially confounding effects of impaired performance can also be examined by experimentally manipulating the demands of the task. Recent studies suggest that prefrontal activity in schizophrenia depends not only on the nature of the task, but also on the level of task difficulty (23, 27–32).
Finally, prefrontal activation in schizophrenia may vary with the severity of symptoms at the time of scanning. Lower activity in the prefrontal cortex in patients with active psychosis during a joystick movement task resolves when these patients are subsequently in remission (33). The presence of positive psychotic symptoms such as auditory hallucinations, delusions of passivity, and thought disorder have each been associated with abnormal (usually attenuated) activation in prefrontal and other regions during a variety of cognitive paradigms (32–36).
The aim of the present study was to examine the effects of both task demand and current mental state on prefrontal function in schizophrenia. We utilized a letter verbal fluency task, which is associated with activation in the prefrontal cortex in healthy volunteers (37–39). Verbal fluency in schizophrenia has been reported to be associated with impaired activation in the middle and inferior frontal gyri and in the anterior cingulate gyrus (21, 25, 40, 41). However, some paced studies in which performance in patients was comparable to that in healthy subjects have failed to find differences in prefrontal activation (33, 36, 42–44). The effects of symptoms and task demand on activation during verbal fluency in schizophrenia have not been explicitly examined previously, although Sommer et al. (36) found that the current severity of hallucinations was correlated with greater engagement of the right prefrontal cortex.
We examined the influence of psychotic state on activation by comparing patients with schizophrenia who had active psychosis and patients who were in remission. To investigate the effects of task demand, we used both “easy” and “difficult” letters as cues; the letters differed in the number of words that subjects could generate from them (45). However, to minimize potentially confounding effects of between-group differences in task performance, we used a paced version of the task that allowed the patients to perform at a behavioral level similar to that of the comparison subjects, and we acquired overt verbal responses with a “clustered” functional magnetic resonance image (fMRI) acquisition sequence so that subjects could respond in the absence of scanner acoustic noise and task performance could be measured online.
We tested the hypothesis that, independent of psychopathology or task demand, patients with schizophrenia would show attenuated prefrontal activation, relative to healthy comparison subjects. As psychotic phenomena appear to be at least partly mediated by prefrontal areas, we predicted that this difference would be more marked in acutely psychotic patients than in those in remission. We also predicted that higher levels of task demand would exacerbate abnormalities of prefrontal activation in patients with active psychosis but would have little effect on activation in remitted patients.
Method
Subjects
Twenty subjects with a DSM-IV diagnosis of schizophrenia and no comorbid diagnoses and 11 healthy comparison subjects participated. Diagnoses were made by means of semistructured clinical interview with a research psychiatrist (C.H.Y.F. or P.K.M.), review of patients’ hospital records, and discussions with their clinical psychiatrist. All subjects were male, native English speaking, right-handed (46), and free of neurological illnesses and a history of head injury. All gave written informed consent, and the study was approved by the Institute of Psychiatry and South London and Maudsley National Health Services Trust Ethical Committee.
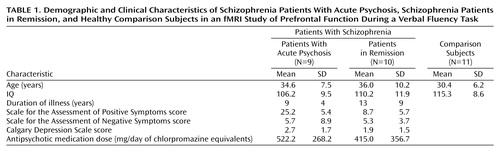
All subjects had normal premorbid intelligence, on the basis of school and work history, and normal premorbid IQ, estimated with the National Adult Reading Test (47). Severity of symptoms at the time of scanning was rated by a psychiatrist (C.H.Y.F.) with the Scale for the Assessment of Positive Symptoms (SAPS) (48) and the Scale for the Assessment of Negative Symptoms (SANS) (49), and presence of depressive symptoms was rated with the Calgary Depression Scale (50). The study was designed to recruit patients who either had marked positive symptoms or were in clinical remission from an acute psychotic episode; patients with formal thought disorder were excluded. The two subgroups were defined according to their SAPS scores: patients with acute psychosis, who were experiencing hallucinations or delusions weekly or more frequently and who scored a minimum of 3 (of 5) on these SAPS criteria, and patients in remission, who scored no more than 2 on any criterion of the SAPS. Analysis of behavioral responses during scanning revealed that one subject in the acute psychosis group did not perform the task correctly, as he repeated the same word for each letter cue, and his imaging data were excluded. As intended, the acute psychosis group and the group in remission had significantly different SAPS scores (t=5.71, df=17, p<0.001, independent t test) (Table 1) but did not differ in their scores on the SANS (t=1.54, df=17, p=0.14, independent t test) or the Calgary Depression Scale (t=1.03, df=17, p=0.32, independent t test). Patients were not specifically selected to have low levels of negative symptoms, but these symptoms were at a low level in both groups. SAPS and SANS ratings were corroborated with clinical information from patient case notes and the clinical teams.
All the patients had a duration of illness of more than 3 years (defined as time since first admission for psychosis), with no significant difference between the acute psychosis group and the group in remission (t=1.32, df=17, p=0.21, independent t test) (Table 1). All were taking regular doses of antipsychotic medication. In the acute psychosis group, three patients were receiving conventional depot neuroleptics, and six were taking atypical antipsychotics. In the acute psychosis group, three patients were receiving conventional depot neuroleptics, and six were receiving atypical antipsychotics. In the group in remission, one patient was receiving a conventional depot neuroleptic, three were taking oral conventional antipsychotics, and six were taking atypical antipsychotics. The average dose of medication in chlorpromazine equivalents was 522.2 mg/day (SD=268.2) for the acute psychosis group and 415.0 mg/day (SD=356.7) for the group in remission (t=0.73, df=17, p=0.47, independent t test). As for adjunctive medication for treatment of persistent psychotic symptoms, three patients in remission and one patient with active psychosis were also taking fluoxetine, two patients in remission were taking valproate, and one patient in remission was taking carbamazepine.
The comparison group included 11 healthy volunteers recruited from the local community. Exclusion of psychiatric and medical illnesses was performed by clinical interview (by C.H.Y.F. or P.K.M.). The three groups were matched for age and IQ, with no significant differences in mean age (F=1.42, df=2, 27, p=0.26, analysis of variance [ANOVA]) or premorbid IQ (F=2.04, df=2, 27, p=0.15, ANOVA) (Table 1).
Verbal Fluency Task
In the experimental condition, the subjects were instructed to overtly generate a word in response to a visually presented letter presented at a rate of one letter every 4 seconds. They were also told to not use proper names or a grammatical variation of the previous word and not to repeat previous responses (37, 38). If subjects were unable to think of a response they were asked to say “pass.” In the easy condition, one of two sets of letters consisting of T, L, B, R, S and T, C, B, P, S was presented; in the difficult condition, one of the following two sets was presented: O, A, N, E, G and I, F, N, E, G. This number of stimuli has provided sufficient power to detect regional activation in healthy individuals (45). Subjects were presented with one set of easy and difficult letters in separate blocked runs with the order of presentation randomized between subjects. Each condition was presented in blocks lasting 28 seconds, with seven presentations of a given letter per block and five blocks of each condition. Each subject participated in two runs, for a total of 10 block presentations of each condition (70 trials of each condition). The experimental condition alternated with the control condition, which consisted of repetition of the word “rest” presented at the same rate. Verbal responses were recorded on Cool Edit 2000 (Syntrillium Software Corp., [Adobe Systems Inc., San Jose, Calif.]) by means of a microphone that was compatible with the magnetic resonance imaging (MRI) apparatus. Incorrect responses were defined as pass responses and words that were proper names, repetitions, or grammatical variations of the previous word (37, 38).
fMRI Image Acquisition
Seventy-four T2*-weighted gradient-echo single-shot echo-planar images were acquired on a 1.5-T, neuro-optimized IGE LX System (General Electric, Milwaukee) at the Maudsley Hospital, South London National Health Services Trust, U.K. Twelve noncontiguous axial planes (7-mm thickness, slice skip: 1 mm) parallel to the anterior commissure-posterior commissure line were collected over 1100 msec in a “clustered” acquisition (TE=40 msec, flip angle=70°). Immediately after each acquisition a letter was presented (remaining visible for 750 msec, height: 7 cm, subtending a 0.4° field of view), and a single overt verbal response was made during the remaining silent portion (entire duration=2900 msec) of each repetition (TR=4000 msec).
Head movement was minimized by a forehead strap. To ensure that subjects heard their responses clearly, their speech was amplified by a computer sound card and then relayed to the subject through an acoustic MRI sound system (Ward Ray, Hampton Court, U.K.) and noise-insulated, stereo headphones at a mean volume of 91 dB (SD=2).
fMRI Data Analysis
The initial volume images of each time series, acquired while the MR signal was reaching steady state (four images) and responsible for triggering the software program (fifth image), were discarded. Subject movement was corrected by rigid-body realignment of the volumes to the series mean and residual magnetic history effects estimated by an autoregressive model followed by spatial smoothing with a Gaussian kernel (SD=3 mm). The amplitude of response to each event was then estimated by least-squares regression of a linear model, a convolution of the experimental design with two Poisson kernels (lambda=2 seconds and 4 seconds, respectively). A map of responses derived under the null hypothesis of no experimental effect was generated by regression of the model after repeated permutation of the data following orthogonal transformation into the wavelet domain (51).
Median observed and permuted responses, F*, were calculated after affine mapping of individual maps into standard stereotactic space (52). Group brain activation maps were created by inference against the permutation distribution. Probabilistic thresholds were set such that the estimated number of type I error voxels across the entire volume was 10, equivalent to p<0.0001 (2, 53).
Differences between task or group were inferred by regression of the general linear model: F=α0+α1H+α2X+e, where F is the response at a particular standard space voxel location, H is a dummy code for the condition or group, X is an optional covariate, and e is the residual error. Maps of the standardized coefficient of task difficulty (α1*), which is a measure of task demand, were tested for significance against a two-tailed distribution, generated by repeated randomization of H, sampling the null hypothesis of no difference between groups. To improve sensitivity, spatial information was introduced by thresholding the maps of α1* such that only voxels exceeding the critical value (CV0.05, corresponding to a voxel-wise test at p<0.05) were retained, and contiguous suprathreshold voxels were aggregated into three-dimensional clusters. For each cluster, the sum of the values of (α1*–CV0.05), the spatial extent statistic, was then tested for significance against the identically derived randomization distribution at a probabilistic threshold such that the estimated number of false positive clusters across the entire volume was <1 (51).
Group brain activation maps of the overall response (verbal fluency task versus control task, irrespective of difficulty level) were generated for each group. As the data were acquired in a blocked design for each condition, the easy and difficult conditions were contrasted within each group by using a categorical comparison and coding the covariate (X) as the number of erroneous responses produced.
To test our hypotheses, the following contrasts were performed:
| 1. | Prefrontal activation during verbal fluency (easy and difficult conditions combined) in the patients with schizophrenia (the acute psychosis group and the group in remission combined) was compared with prefrontal activation during verbal fluency in the healthy volunteers. | ||||
| 2. | The effects of psychotic state on prefrontal activation were examined by comparing the results for the acute psychosis group with those for the group in remission and by comparing the results for both patient groups with those for the healthy volunteers. | ||||
| 3. | The interaction between the effects of psychotic state (acute psychosis group versus remission group) and task demand (easy versus difficult conditions) on prefrontal activation was examined. To clarify the basis of this interaction, we also examined the effects of task demand in each group (remission, acute psychosis, and comparison groups). | ||||
Results
Behavioral Data
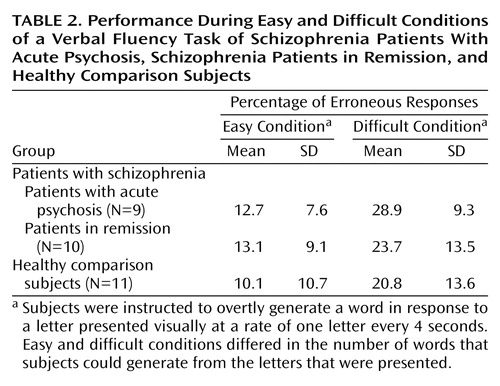
Patients were routinely debriefed about their experiences during scanning, and none reported hallucinations. Across all groups there was a main effect of task demand, with more erroneous responses produced for the difficult condition than for the easy condition (repeated measures F=37.5, df=1, 27, p<0.001) (Table 2). No difference in the number of errors generated was evident between the groups (repeated measures F=0.78, df=2, 27, p=0.50), and there was no significant interaction of group and task demand (F=0.79, df=2, 27, p=0.50).
fMRI Data
Activation during verbal fluency
In the healthy volunteers there was bilateral activation in the inferior and middle frontal gyri, the anterior and posterior cingulate gyri, and the insula. Further activation was evident in the middle and superior temporal gyri, inferior parietal lobule, precuneus, thalamus, caudate, and cerebellum (45). A qualitatively similar distribution of regional activation was observed in the acute psychosis group. In the group in remission, activation was less extensive and mainly restricted to the left hemisphere, with engagement of the left inferior frontal gyrus and insula, the left anterior and posterior cingulate gyri, and the left superior temporal gyrus. Further activation was evident in the left occipital cortex and precuneus and in the inferior parietal lobule bilaterally (coordinates are available upon request). The significance of these differences in activation between the subgroups was formally tested in the between-group comparisons that are reported later, in the section on the effect of psychotic state on activation.
Differences between patients with schizophrenia and healthy volunteers
When all patients with schizophrenia (remission and acute psychosis groups combined) were compared with the healthy comparison group across all verbal fluency trials (difficult and easy conditions combined), the patients showed less activation in the inferior frontal and anterior cingulate cortices bilaterally and in the right (but not the left) middle frontal gyrus. Patients also showed relatively attenuated activation bilaterally in the superior and middle temporal gyri, the medial temporal, inferior occipital, and posterior parietal cortices, and the caudate, thalamus, and cerebellum (Figure 1). There were no areas that were more activated in the patients than in the comparison subjects.
Effect of psychotic state on activation
Comparison of the acute psychosis subgroup and the healthy comparison subjects indicated that there were no significant differences in activation in the lateral prefrontal cortex. However, the acute psychosis group showed less activation bilaterally in the anterior and posterior cingulate cortices, parahippocampal region, basal ganglia, thalamus, lingual gyri, brainstem, and cerebellum. The group in remission showed less activation than the comparison subjects in the right middle frontal gyrus, left anterior and posterior cingulate gyri, inferior parietal and superior temporal lobules, and brainstem and bilaterally in the inferior frontal gyri, insula, parahippocampal region, caudate, thalamus, lingual gyri, and cerebellum.
Direct comparison of the acute psychosis group and the group in remission revealed greater activation in the acute psychosis group in the left middle frontal, superior temporal, and inferior parietal cortices and bilaterally in the anterior cingulate, inferior frontal cortices, and insula. There were no regions that were more activated in the group in remission. All contrasts were covaried for subject performance (Figure 2 and Table 3).
Activation with increasing task demand
The effect of task demand was first examined by comparing results for the easy and difficult conditions in each group while covarying for the number of erroneous responses. In the healthy comparison subjects, increasing task demand (difficult condition versus easy condition) was associated with greater activation in the left anterior cingulate (Brodmann’s area 32; Talairach and Tournoux coordinates: x=–8, y=22, z=39; cluster size=128 voxels) (45).
In the acute psychosis group, increasing task demand was associated with greater activation in the left anterior insula (x=–30, y=8, z=1; cluster size=52 voxels), the left posterior insula (x=–30, y=–9, z=–1; cluster size=92 voxels), and the left putamen (x=–29, y=–22, z=6; cluster size=16 voxels). These differences reflected an activation that was evident only during the difficult condition. Conversely, the easy condition was associated with greater activation of the right medial temporal cortex (cluster size=348 voxels), extending from the parahippocampal gyrus (x=23, y=–10, z=–16) to the hippocampus (x=37, y=–55, z=–1); lingual gyrus (x=–2, y=–78, z=–3, cluster size=320 voxels); left caudate (extending from z=19, y=17, z=1 to x=12, y=7, z=16, cluster size=128 voxels); right medial prefrontal cortex (extending from Brodmann’s area 10, x=12, y=53, z=24 to Brodmann’s area 8, x=8, y=44, z=40, cluster size=80 voxels); and right postcentral gyrus (x=55, y=–10, z=48, cluster size=16 voxels). These differences reflected activations that were evident only during the easy condition.
In the group in remission, there were no significant differences in activation between the easy and difficult conditions, although the finding of greater activation of the anterior cingulate cortex with the difficult condition approached significance.
Interaction between the effects of psychotic state and task demand
There were only a limited number of significant findings when the differences in activation between the difficult and easy conditions in each patient group were compared with those in the healthy group. The group in remission had a smaller difference in activation in the occipital cortex between the difficult and easy conditions, compared with the healthy volunteers. No significant differences were found between the acute psychosis group and the healthy volunteers. However, when the two patient groups were compared, the acute psychosis group showed greater differences in activation in the right middle frontal gyrus and the left anterior cingulate gyrus, as well as in the left inferior parietal lobule, a region that extended from the left postcentral gyrus to the supramarginal gyrus, and in the precuneus (Figure 3). In all of these regions, the differences reflected an increase in activation with increased task demand in the acute psychosis group and an absence of difference between the difficult and easy conditions in the group in remission. The group in remission showed a greater difference than the acute psychosis group in only one region, which spanned the right medial temporal and inferior temporal cortices (Figure 3). Overall these findings reflected greater activation in the group in remission with increased demand but significantly reduced activation with increased demand in the acute psychosis group.
Discussion
Verbal fluency is a classic neuropsychological test of language production in which subjects generate and articulate a word in response to a cue (37, 38). Studies of verbal fluency with fMRI have revealed a consistent set of activated regions (21, 25, 54–58), with the most frequently reported sites of frontal activation being the left inferior frontal gyrus (25, 54–58), left middle frontal gyrus (dorsolateral prefrontal cortex) (21, 25, 54, 56, 57), and anterior cingulate gyrus (54, 58). The healthy comparison group in our study recruited all of these regions.
To assess task performance online we used a paradigm that involved overt speech. Image acquisition during fMRI is associated with an acoustic noise that can make it difficult for subjects and investigators to hear overt verbal responses (59), and overt articulation can be associated with head movement (60). We therefore employed a “clustered” acquisition sequence that incorporated brief periods of silence (61, 62), which allowed subjects to speak and hear their responses in the absence of noise and reduced the risk of articulation-related movement artefacts (45). Online measurement of behavioral responses indicated that all three groups were able to adequately perform the letter verbal fluency paradigm, with no significant between-group differences in the proportion of errors produced. We also covaried for task performance when comparing activation between groups.
The schizophrenia group as a whole showed impaired activation, relative to the comparison group, in the inferior frontal and anterior cingulate gyri bilaterally and in the right but not the left middle frontal gyrus. These findings are broadly consistent with those reported in the literature, and the absence of differences in the left middle frontal gyrus is compatible with data from previous studies of verbal fluency that used a paced presentation of stimuli in which task performance was equivalent in the patient and comparison groups (24, 33, 36, 42, 43). We predicted that abnormalities of prefrontal activation would be more evident in patients who were actively psychotic than in patients who were in remission. However, the group in remission showed a more marked attenuation of prefrontal activation, relative to the comparison subjects, than did the acute psychosis group, and direct comparison of the patient groups revealed that there was significantly greater activation in the inferior frontal and dorsolateral prefrontal, insular, and anterior cingulate cortices bilaterally in the actively psychotic patients. Thus, although the patients in remission engaged these regions less than did the healthy volunteers, in the actively psychotic patients, less activation, relative to that in the comparison subjects, was evident in the anterior cingulate cortex, but not in lateral prefrontal areas. These observations may seem counterintuitive in the light of evidence that prefrontal function in schizophrenia is more impaired in patients with positive psychotic symptoms than those in remission (44, 63, 64). However, compromised prefrontal function in schizophrenia may also be manifested as increased activation during cognitive processing. For example, Sommer et al. (36) found a correlation between the severity of auditory hallucinations in schizophrenia and right prefrontal activation during verbal fluency. Moreover, Callicott et al. (31) reported greater prefrontal activation in patients with schizophrenia than in comparison subjects during a verbal working memory task, and we have found that healthy volunteers with positive psychotic symptoms induced by ketamine show greater engagement of prefrontal areas during verbal fluency than when performing the task while taking placebo (65). These observations are consistent with the notion that in patients with impaired prefrontal capacity, additional activation may be needed to maintain normal task performance (31).
As predicted, abnormalities of prefrontal activation became more marked with increased task demand in patients with positive symptoms. However, comparison of the acute psychosis group and the group in remission revealed that rather than leading to a further reduction of activation, increasing demand in the symptomatic patients was associated with greater engagement of the right prefrontal and anterior cingulate cortex. The findings cannot be easily attributed to thought disorder or other disorganization symptoms, as neither group had significant measures on these symptoms. Although we cannot exclude the possibility that we may have detected subtle degrees of formal thought disorder if we had used detailed instruments for this purpose, we think this outcome is unlikely. None of the patients had any previous history of formal thought disorder, and patients with formal thought disorder usually have a record of this symptom across the course of illness. Instead, these observations suggest that the effect of increasing the demands of verbal fluency in schizophrenia depends on the level of positive psychotic symptoms at the time of scanning. If actively psychotic patients need to engage the prefrontal cortex to a greater extent than those in remission to attain a given level of performance, this greater extent of activation may be particularly evident when the task becomes more demanding. In our study, patients were able to maintain adequate task performance, even when the demands were increased. However, had performance substantially deteriorated, the effects of task demand on activation may have been quite different. Thus, data from other studies indicate that attenuated engagement of the prefrontal cortex in schizophrenia is evident only when the task load has been increased to the extent that patients can no longer perform the task (28, 30). Alternatively, we cannot exclude the possibility that the remitted patients differed from the psychotic group on neuropsychological performance, as formal measurements were not performed, and that this difference may have been related to their hypofrontality.
A further speculative explanation involves an influence of dopaminergic neurotransmission on cerebral blood flow and the blood-oxygen-level-dependent response that is measured in fMRI. Acute psychosis in schizophrenia, but not schizophrenia in remission, is associated with increased phasic dopamine release, a “hyperdopaminergic” state (66–69). The severity of central dopaminergic dysfunction appears to be correlated with abnormal prefrontal activation during the Wisconsin Card Sorting Test in schizophrenia (70–74). Experimental administration of dopaminergic agents modulates prefrontal activation during cognitive tasks (75, 76), and their effects on activation during verbal fluency differ between patients with schizophrenia and comparison subjects (42). Differential activation in prefrontal regions in patients with active psychotic symptoms, relative to patients in remission, may thus be related to a state-related perturbation of central dopaminergic activity, which might also underlie their positive symptoms. This possibility could be further investigated by directly comparing the effects of dopaminergic agents on prefrontal activation in actively psychotic patients and patients in remission.
One limitation of the present study was that all the subjects with schizophrenia were taking antipsychotic medication. Treatment with antipsychotic medications has been associated with increased metabolism in the basal ganglia (77–82). Their effect on activity in the prefrontal cortex is less clear, although Honey et al. (83) reported that patients treated with risperidone showed greater activation during a verbal working memory task than when they were treated with typical antipsychotics. However, a differential effect of atypical versus typical antipsychotic drugs is unlikely to account for the differences between the patient groups in our study, as the proportions of patients who received atypical and typical antipsychotics in each group were comparable.
Overall our data suggest that while schizophrenia is associated with impaired prefrontal function, how this impairment is manifest in functional imaging studies of cognitive tasks can vary with the severity of psychotic symptoms at the time of scanning and the level of task difficulty. When patients can carry out a task as well as comparison subjects, the presence of active psychosis and increasing task demand may necessitate engagement of prefrontal areas in order to maintain behavioral performance. On the other hand, if the task is such that patients can no longer carry it out, the engagement of the prefrontal cortex may be attenuated.
 |
 |
 |
Received Dec. 2, 2003; revision received April 22, 2004; accepted May 14, 2004. From the Institute of Psychiatry; and the Brain Mapping Unit, Department of Psychiatry, University of Cambridge, Cambridge, U.K. Address correspondence and reprint requests to Dr. Fu, Division of Psychological Medicine, Institute of Psychiatry, 103 Denmark Hill, London SE5 8AF UK; [email protected] (e-mail). Supported by a Wellcome Trust Travelling Fellowship. The authors thank the study participants and the radiographers at the South London and Maudsley Trust MRI Unit for their assistance.
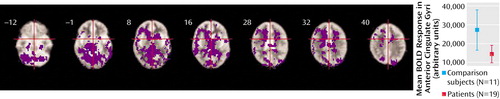
Figure 1. Prefrontal Cortical Activation During a Verbal Fluency Task in Patients With Schizophrenia, Relative to Healthy Comparison Subjectsa
aImages are transverse sections at levels indicated by the distance in millimeters relative to the intercommissural plane. The right side of the brain is shown on the left side of each section. Patients showed less activation (areas colored purple) than comparison subjects in the left inferior frontal cortex (Brodmann’s area 44) (Talairach and Tournoux coordinates in millimeters: x=–38, y=10, z=20; cluster size=8 voxels) and bilateral anterior cingulate gyri (Brodmann’s area 32) (x=–24, y=20, z=32; cluster size=128 voxels; and x=9, y=26, z=32; cluster size=56 voxels), as well as in the temporal and parietal cortices and subcortical regions. Significance testing was at voxel-wise p<0.001 and spatial extent threshold p<0.005, such that the estimated number of false positive clusters across the entire volume was <1. The graph shows mean changes in blood-oxygen-level-dependent (BOLD) response in the anterior cingulate gyri for the two groups. Plots for other regions are similar.
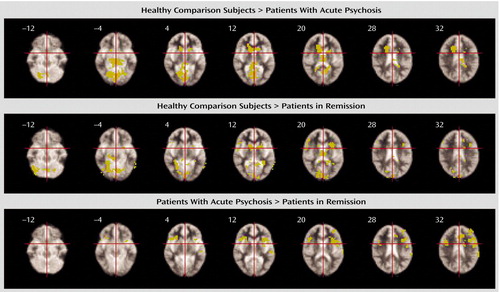
Figure 2. Effects of Psychotic State on Prefrontal Cortical Activation During a Verbal Fluency Task in Schizophrenia Patients With Acute Psychosis and Schizophrenia Patients in Remission, Relative to Healthy Comparison Subjectsa
aRegions of activation in the healthy comparison group, relative to the acute psychosis group and to the group in remission, are shown in the top and middle rows, respectively. Both patient groups showed less activation than the comparison subjects in an extensive set of cortical and subcortical areas. In direct comparison of the patient groups (bottom row), the acute psychosis group showed greater activation than the group in remission in the anterior cingulate, inferior frontal and dorsolateral prefrontal cortices, and insula; there were no regions of greater activation in the group in remission, compared to the group with acute psychosis. Significance testing was at voxel-wise p<0.01 and spatial extent threshold p<0.05 such that the estimated number of false positive clusters across the entire volume was <1 for each comparison.
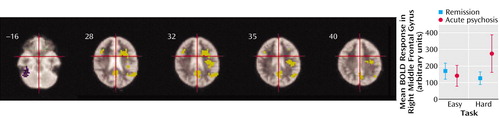
Figure 3. Effects on Prefrontal Cortical Activation of the Interaction Between Increased Task Demand During a Verbal Fluency Task and Psychotic State in Schizophrenia Patients With Acute Psychosis and Schizophrenia Patients in Remission, Relative to Healthy Comparison Subjectsa
aIn the acute psychosis group, but not the group in remission, increased task demand was associated with activation in the right prefrontal cortex, left anterior cingulate gyrus, precuneus, angular gyrus, and a left temporoparietal region (areas colored yellow). Conversely, a region in the right inferior temporal cortex (colored in purple) showed activation with increased task difficulty in the group in remission, but less activation with increased task difficulty in the acute psychosis group. The interaction in the right middle frontal gyrus (Brodmann’s area 9; Talairach and Tournoux coordinates in millimeters: x=41, y=15, z=31; cluster size=68 voxels) between task demand and psychotic state is shown in the plot of change in blood-oxygen-level-dependent (BOLD) response for the easy and difficult task conditions for each group.
1. Ingvar DH, Franzen G: Distribution of cerebral activity in chronic schizophrenia. Lancet 1974; 2:1484–1486Crossref, Medline, Google Scholar
2. Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ: Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 1999; 18:32–42Crossref, Medline, Google Scholar
3. Weinberger DR, Berman KF: Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci 1996; 351:1495–1503Crossref, Medline, Google Scholar
4. Fu CHY, McGuire PK: Functional neuroimaging in psychiatry. Philos Trans R Soc Lond B Biol Sci 1999; 354:1359–1370Crossref, Medline, Google Scholar
5. Kurachi M, Kobayashi K, Matsubara R, Hiramatsu H, Yamaguchi N, Matsuda H, Maeda T, Hisada K: Regional cerebral blood flow in schizophrenic disorders. Eur Neurol 1985; 24:176–181Crossref, Medline, Google Scholar
6. Berman KF, Zec RF, Weinberger DR: Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, II: role of neuroleptic treatment, attention, and mental effort. Arch Gen Psychiatry 1986; 43:126–135Crossref, Medline, Google Scholar
7. Chabrol H, Guell A, Bes A, Moron P: Cerebral blood flow in schizophrenic adolescents (letter). Am J Psychiatry 1986; 143:130Link, Google Scholar
8. Weinberger DR, Berman KF, Zec RF: Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional cerebral blood flow evidence. Arch Gen Psychiatry 1986; 43:114–124Crossref, Medline, Google Scholar
9. Geraud G, Arne-Bes MC, Guell A, Bess A: Reversibility of hemodynamic hypofrontality in schizophrenia. J Cereb Blood Flow Metab 1987; 7:9–12Crossref, Medline, Google Scholar
10. Mathew RJ, Wilson WH, Tant SR, Robinson L, Prakash R: Abnormal resting regional cerebral blood flow patterns and their correlates in schizophrenia. Arch Gen Psychiatry 1988; 45:542–549Crossref, Medline, Google Scholar
11. Gur RE, Skolnick BE, Gur RC, Caroff S, Rieger W, Obrist WD, Younkin D, Reivich M: Brain function in psychiatric disorders, II: regional cerebral blood flow in medicated unipolar depressives. Arch Gen Psychiatry 1984; 41:695–699Crossref, Medline, Google Scholar
12. Baxter LR Jr, Phelps ME, Mazziotta JC, Schwartz JM, Gerner RH, Selin CE, Sumida RM: Cerebral metabolic rates for glucose in mood disorders: studies with positron emission tomography and fluorodeoxyglucose F 18. Arch Gen Psychiatry 1985; 42:441–447Crossref, Medline, Google Scholar
13. Kling AS, Metter EJ, Riege WH, Kuhl DE: Comparison of PET measurement of local brain glucose metabolism and CAT measurement of brain atrophy in chronic schizophrenia and depression. Am J Psychiatry 1986; 143:175–180Link, Google Scholar
14. Szechtman H, Nahmias C, Garnett ES, Firnau G, Brown GM, Kaplan RD, Cleghorn JM: Effect of neuroleptics on altered cerebral glucose metabolism in schizophrenia. Arch Gen Psychiatry 1988; 45:523–532Crossref, Medline, Google Scholar
15. Silfverskiold P, Risberg J: Regional cerebral blood flow in depression and mania. Arch Gen Psychiatry 1989; 46:253–259Crossref, Medline, Google Scholar
16. Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME: A functional anatomical study of unipolar depression. J Neurosci 1992; 12:3628–3641Crossref, Medline, Google Scholar
17. Berman KF, Doran AR, Pickar D, Weinberger DR: Is the mechanism of prefrontal hypofunction in depression the same as in schizophrenia? Br J Psychiatry 1993; 162:183–192Crossref, Medline, Google Scholar
18. Andreasen NC, Rezai K, Alliger R, Swayze VW II, Flaum M, Kirchner P, Cohen G, O’Leary DS: Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with Xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943–958Crossref, Medline, Google Scholar
19. Ebmeier KP, Blackwood DH, Murray C, Souza V, Walker M, Dougall N, Moffoot AP, O’Carroll RE, Goodwin GM: Single-photon emission computed tomography with 99mTc-exametazime in unmedicated schizophrenic patients. Biol Psychiatry 1993; 33:487–495Crossref, Medline, Google Scholar
20. Gur RC, Gur RE: Hypofrontality in schizophrenia: RIP. Lancet 1995; 345:1383–1384Crossref, Medline, Google Scholar
21. Yurgelun-Todd DA, Waternaux CM, Cohen BM, Gruber SA, English CD, Renshaw PF: Functional magnetic resonance imaging of schizophrenic patients during word production. Am J Psychiatry 1996; 153:200–205Link, Google Scholar
22. Curtis VA, Bullmore ET, Brammer MJ, Wright IC, Williams SCR, Morris RG, Sharma TS, Murray RM, McGuire PK: Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 1998; 155:1056–1063Link, Google Scholar
23. Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Recalling word lists reveals “cognitive dysmetria” in schizophrenia: a positron emission tomography study. Am J Psychiatry 1999; 156:386–392Abstract, Google Scholar
24. Frith CD, Friston KJ, Herold S, Silbersweig D, Fletcher P, Cahill C, Dolan RJ, Frackowiak RSJ, Liddle PF: Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry 1995; 167:343–349Crossref, Medline, Google Scholar
25. Curtis VA, Bullmore ET, Morris RG, Brammer MJ, Williams SCR, Simmons A, Sharma T, Murray RM, McGuire PK: Attenuated frontal activation in schizophrenia may be task dependent. Schizophr Res 1999; 37:35–44Crossref, Medline, Google Scholar
26. Stevens AA, Goldman-Rakic PS, Gore JC, Fulbright RK, Wexler BE: Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Arch Gen Psychiatry 1998; 55:1097–1103Crossref, Medline, Google Scholar
27. Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S: Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry 1999; 45:1128–1137Crossref, Medline, Google Scholar
28. Fletcher PC, McKenna PJ, Frith CD, Grasby PM, Friston KJ, Dolan RJ: Brain activations in schizophrenia during a graded memory task studied with functional neuroimaging. Arch Gen Psychiatry 1998; 55:1001–1008Crossref, Medline, Google Scholar
29. Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD: Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry 1998; 155:1285–1287Link, Google Scholar
30. Carter CS, MacDonald AW III, Ross LL, Stenger VA: Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry 2001; 158:1423–1428Link, Google Scholar
31. Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR: Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078–1092Crossref, Medline, Google Scholar
32. Perlstein WM, Carter CS, Noll DC, Cohen JD: Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry 2001; 158:1105–1113Link, Google Scholar
33. Spence SA, Liddle PF, Stefan MD, Hellewell JSE, Sharma T, Friston KJ, Hirsch SR, Frith CD, Murray RM, Deakin JFW, Grasby PM: Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Br J Psychiatry 2000; 176:52–60Crossref, Medline, Google Scholar
34. McGuire PK, Quested DJ, Spence SA, Murray RM, Frith CD, Liddle PF: Pathophysiology of “positive” thought disorder in schizophrenia. Br J Psychiatry 1998; 173:231–235Crossref, Medline, Google Scholar
35. Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK: Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 2000; 57:1033–1038Crossref, Medline, Google Scholar
36. Sommer IEC, Ramsey NF, Kahn RS: Language lateralization in schizophrenia, an fMRI study. Schizophr Res 2001; 52:57–67Crossref, Medline, Google Scholar
37. Benton AL, Hamsher KD: Multilingual Aphasia Examination. New York, Oxford University Press, 1994Google Scholar
38. Lezak MD: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995, pp 544–546Google Scholar
39. Indefrey P, Levelt WJM: The neural correlates of language production, in The New Cognitive Neurosciences, 2nd ed. Edited by Gazzaniga MS. Cambridge, Mass, MIT Press, 2000, pp 845–865Google Scholar
40. Lewis SW, Ford RA, Syed GM, Reveley AM, Toone BK: A controlled study of 99mTc-HMPAO single-photon emission imaging in chronic schizophrenia. Psychol Med 1992; 22:27–35Crossref, Medline, Google Scholar
41. Artiges E, Martinot JL, Verdys M, Attar-Levy D, Mazoyer B, Tzourio N, Giraud MJ, Paillere-Martinot ML: Altered hemispheric functional dominance during word generation in negative schizophrenia. Schizophr Bull 2000; 26:709–721Crossref, Medline, Google Scholar
42. Fletcher PC, Frith CD, Grasby PM, Friston KJ, Dolan RJ: Local and distributed effects of apomorphine on fronto-temporal function in acute unmedicated schizophrenia. J Neurosci 1996; 16:7055–7062Crossref, Medline, Google Scholar
43. Dye SM, Spence SA, Bench CJ, Hirsch SR, Stefan MD, Sharma T, Grasby PM: No evidence for left superior temporal dysfunction in asymptomatic schizophrenia and bipolar disorder: PET study of verbal fluency. Br J Psychiatry 1999; 175:367–374Crossref, Medline, Google Scholar
44. Spence SA, Hirsch SR, Brooks DJ, Grasby PM: Prefrontal cortex activity in people with schizophrenia and control subjects: evidence from positron emission tomography for remission of “hypofrontality” with recovery from acute schizophrenia. Br J Psychiatry 1998; 172:316–323Crossref, Medline, Google Scholar
45. Fu CHY, Morgan K, Suckling J, Williams SCR, Andrew C, Vythelingum GN, McGuire PK: An fMRI study of overt letter verbal fluency using a clustered acquisition sequence: greater anterior cingulate activation with increased task demand. Neuroimage 2002; 17:871–879Crossref, Medline, Google Scholar
46. Annett MA: A classification of hand preference by association analysis. Br J Psychol 1970; 61:303–321Crossref, Medline, Google Scholar
47. Nelson HE: National Adult Reading Test (NART): Test Manual. Windsor, UK, National Foundation for Educational Research, 1983Google Scholar
48. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
49. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
50. Addington D, Addington J, Schissel B: A depression rating for schizophrenics. Schizophr Res 1990; 3:247–251Crossref, Medline, Google Scholar
51. Bullmore ET, Long C, Suckling J, Fadili J, Calvert GA, Zelaya F, Carpenter TA, Brammer MJ: Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp 2001; 12:61–78Crossref, Medline, Google Scholar
52. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
53. Brammer MJ, Bullmore ET, Simmons A, Williams SC, Grasby PM, Howard RJ, Woodruff PW, Rabe-Hesketh S: Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn Reson Imaging 1997; 15:763–770Crossref, Medline, Google Scholar
54. Yetkin FZ, Hammeke TA, Swanson SJ, Morris GL, Mueller WM, McAuliffe TL, Haughton VM: A comparison of functional MR activation patterns during silent and audible language tasks. AJNR Am J Neuroradiol 1995; 16:1087–1092Medline, Google Scholar
55. Friedman L, Kenny JT, Wise AL, Wu D, Stuve TA, Miller DA, Jesberger JA, Lewin JS: Brain activation during silent word generation evaluated with functional MRI. Brain Lang 1998; 64:231–256Crossref, Medline, Google Scholar
56. Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD: Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry 1998; 64:492–498Crossref, Medline, Google Scholar
57. Hutchinson M, Schiffer W, Joseffer S, Liu A, Schlosser R, Dikshit S, Goldberg E, Brodie JD: Task-specific deactivation patterns in functional magnetic resonance imaging. Magn Reson Imaging 1999; 17:1427–1436Crossref, Medline, Google Scholar
58. Lurito JT, Kareken DA, Lowe MJ, Chen SHA, Mathews VP: Comparison of rhyming and word generation with fMRI. Hum Brain Mapp 2000; 10:99–106Crossref, Medline, Google Scholar
59. Amaro E Jr, Williams SCR, Shergill SS, Fu CHY, MacSweeney M, Pichionni M, Brammer MJ, McGuire PK: Acoustic noise and functional magnetic resonance imaging: current strategies and future prospects. J Magn Reson Imaging 2002; 16:497–510Crossref, Medline, Google Scholar
60. Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SCR, Sharma T, McGuire PK: Methods for diagnosis and treatment of stimulus correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp 1999; 7:38–48Crossref, Medline, Google Scholar
61. Eden GF, Joseph JE, Brown HE, Brown CP, Zeffiro TA: Utilizing hemodynamic delay and dispersion to detect fMRI signal change without auditory interference: the behavior interleaved gradients technique. Magn Reson Med 1999; 41:13–20Crossref, Medline, Google Scholar
62. Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, Bowtell RW: “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp 1999; 7:213–223Crossref, Medline, Google Scholar
63. Rice CD, Done DJ, Manly T, McKenna PJ: Schizophrenic patients with symptoms show more impairment than those without symptoms on an ecologically valid test of executive function (abstract). Schizophr Res 2002; 53(3 suppl):133Google Scholar
64. Tso IF, Chan RCK, Chen EYH, Dunn ELW, Chen RYL, Chan WF, Miao YK, Yeung WS, Wong CK, Tang WN: Longitudinal profiles of neurocognitive function in first-episode psychosis (abstract). Schizophr Res 2002; 53(3 suppl):121Google Scholar
65. Fu CHY, Abel K, Allin M, Vythelingum N, Costafreda S, Williams SCR, McGuire PK: “K” is for ketamine: an fMRI study of the neural correlates of a ketamine-induced psychotic state on word generation (abstract). Schizophr Res 2003; 60(1 suppl):78Google Scholar
66. Early TS, Posner MI, Reiman EM, Raichle ME: Hyperactivity of the left striato-pallidal projection, part I: lower level theory. Psychiatr Dev 1989; 7:85–108Medline, Google Scholar
67. Grace AA: Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 1991; 41:1–24Crossref, Medline, Google Scholar
68. Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R: Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 1999; 46:56–72Crossref, Medline, Google Scholar
69. Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M: Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 2000; 97:8104–8109Crossref, Medline, Google Scholar
70. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
71. Weinberger DR, Berman DF, Illowsky BP: Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia, III: a new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry 1988; 45:609–615Crossref, Medline, Google Scholar
72. Deutch AY: The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm Suppl 1992; 36:61–89Medline, Google Scholar
73. Grace AA: Cortical regulation of subcortical dopamine systems and its possible relevance to schizophrenia. J Neural Transm Gen Sect 1993; 91:111–134Crossref, Medline, Google Scholar
74. Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF: Reduced prefrontal cortical activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci 2002; 5:267–271Crossref, Medline, Google Scholar
75. Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW: Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci 2000; 20:RC65Google Scholar
76. Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, Hyde TM, Weinberger DR: Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann Neurol 2002; 51:156–164Crossref, Medline, Google Scholar
77. Buchsbaum MS, Wu JC, DeLisi LE, Holcomb HH, Hazlett E, Cooper-Langston K, Kessler R: Positron emission tomography studies of basal ganglia and somatosensory cortex neuroleptic drug effects: differences between normal controls and schizophrenic patients. Biol Psychiatry 1987; 22:479–494Crossref, Medline, Google Scholar
78. Buchsbaum MS, Potkin SG, Siegel BV Jr, Lohr J, Katz M, Gottschalk LA, Gulasekaram B, Marshall JF, Lottenberg S, Teng CY, Abel L, Plon L, Bunney WE Jr: Striatal metabolic rate and clinical response to neuroleptics in schizophrenia. Arch Gen Psychiatry 1992; 49:966–974Crossref, Medline, Google Scholar
79. Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA: Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry 1996; 153:41–49Link, Google Scholar
80. Cohen RM, Nordahl TE, Semple WE, Andreason P, Litman RE, Pickar D: The brain metabolic patterns of clozapine- and fluphenazine-treated patients with schizophrenia during a continuous performance task. Arch Gen Psychiatry 1997; 54:481–486Crossref, Medline, Google Scholar
81. Miller DD, Andreasen NC, O’Leary DS, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD: Effect of antipsychotics on regional cerebral blood flow measured with positron emission tomography. Neuropsychopharmacology 1997; 17:230–240; correction, 1998; 18:323–324Google Scholar
82. Scottish Schizophrenia Research Group: Regional cerebral blood flow in first-episode schizophrenia patients before and after antipsychotic drug treatment. Acta Psychiatr Scand 1998; 97:440–449Crossref, Medline, Google Scholar
83. Honey GD, Bullmore ET, Soni W, Varatheesan M, Williams SCR, Sharma T: Differences in frontal cortical activation by a working memory task after substitution of risperidone for typical antipsychotic drugs in patients with schizophrenia. Proc Natl Acad Sci USA 1999; 96:13432–13437Crossref, Medline, Google Scholar



