Association of Exaggerated HPA Axis Response to the Initial Injection of Interferon-Alpha With Development of Depression During Interferon-Alpha Therapy
Abstract
OBJECTIVE: The authors assessed the relationship between the hypothalamic-pituitary-adrenal (HPA) axis response to interferon-alpha (IFN-α) and the development of major depression during IFN-α treatment. METHOD: Adrenocorticotropic hormone (ACTH), cortisol, and interleukin-6 (IL-6) plasma concentrations were measured in 14 patients with malignant melanoma at regular intervals during the first 12 weeks of IFN-α therapy, both immediately before and 1, 2, and 3 hours after IFN-α administration. Symptom criteria for major depression were also evaluated at each visit. RESULTS: ACTH and cortisol responses but not IL-6 responses to the initial administration of IFN-α were significantly higher in the seven patients who subsequently developed symptom criteria for major depression than in those who did not. No differences in hormonal or cytokine responses were found between these two groups during chronic IFN-α administration. CONCLUSIONS: The HPA axis response to the acute administration of IFN-α reveals a vulnerability to IFN-α-induced depression, possibly due to sensitization of corticotropin-releasing factor pathways.
Interferon-alpha (IFN-α) is used to treat infectious diseases and cancers. Despite its clinical efficacy, IFN-α frequently causes a behavioral syndrome that has overlapping features with major depression (1).
Although the precise mechanism of IFN-α-induced depression remains obscure, attention has focused on corticotropin-releasing factor (CRF), a neuropeptide implicated in the pathophysiology of mood disorders. In vivo and in vitro administration of IFN-α increases CRF production and release (2). Moreover, CRF administered to laboratory animals leads to behavioral changes that overlap with IFN-α-induced symptoms (3). Consistent with activation of hypothalamic CRF pathways, acute administration of IFN-α robustly activates the hypothalamic-pituitary-adrenal (HPA) axis, inducing marked increases in plasma adrenocorticotropic hormone (ACTH) and cortisol (4). Finally, IFN-α-induced depressive-like behavior in rodents can be abolished by pretreatment with a CRF receptor antagonist (5).
In view of interrelationships among CRF, the HPA axis, and IFN-α, we sought to determine whether interindividual differences in HPA axis responsiveness to IFN-α, either acutely or during chronic treatment, were predictive of development of major depression during IFN-α therapy.
Method
Fourteen patients with malignant melanoma drawn from a subset of placebo-treated patients described elsewhere (1) were enrolled in the current study. Exclusion criteria included a DSM-IV diagnosis of schizophrenia, bipolar disorder, or substance abuse; Mini-Mental State Examination score ≤24; or uncontrolled medical disease. All patients provided informed consent. The study was approved by the Emory University Institutional Review Board.
IFN-α (Schering-Plough, Kenilworth, N.J.) was administered at 20 million units per meter2 (MU/m2) of body surface area intravenously 5 days/week for 4 weeks followed by 10 MU/m2 subcutaneously 3 days/week. Concomitant medications for fever, pain, sleep, and nausea were allowed. Antidepressants were excluded.
Plasma samples were obtained before and 1, 2, and 3 hours after IFN-α administration at the beginning of weeks 1, 2, 4, 8, and 12 of IFN-α therapy. ACTH and cortisol were measured by radioimmunoassay. Interleukin-6 (IL-6) was assayed by ELISA; we used both a regular sensitivity assay (detection limit=3.12 pg/ml) and a higher sensitivity assay for samples obtained before IFN-α administration (detection limit=0.3 pg/ml). Inter- and intraassay coefficients of variation were reliably ≤10%.
At each visit, symptom criteria for DSM-IV major depression were evaluated. In addition, the Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale, and Neurotoxicity Rating Scale were administered as described elsewhere (1). According to DSM-IV, a depressive syndrome that develops during IFN-α therapy is referred to as substance (IFN-α)-induced mood disorder.
Biological data for each visit were analyzed by using a two-way analysis of covariance (time by group) that controlled for age. Post hoc comparisons were performed by using the Tukey test. ACTH data at week 1 were log-transformed because of nonhomogeneity of variance (Bartlett test). Sphericity was assessed with the Mauchly test and corrected when necessary (Greenhouse-Geisser adjustment). Individual scores were calculated for specific symptom dimensions, including depressive, anxious, cognitive, neurovegetative (e.g., fatigue, anorexia), and somatic symptoms (e.g., pain, gastrointestinal distress) as described elsewhere (1).
Relationships between biological variables at baseline and symptom dimensions at week 8, which was closest to the mean onset of depression (mean=7 weeks, SD=3.2), were assessed by using nonparametric rank correlation coefficients (Spearman’s correlation [rs]). All probabilities are two-tailed.
Results
Seven patients fulfilled criteria for major depression during the study. No differences were found between depressed and nondepressed patients in gender (two of the depressed patients were women, and five were men; three of the nondepressed patients were women, and four were men) (χ2=0.31, df=1, p=0.58). No significant differences were found between groups in age, although the depressed group was slightly older (depressed group mean age=59.1 years, SD=10.9; nondepressed group mean age=49.4, SD=13.4) (t=1.48, df=12, p=0.16). No patients were clinically depressed before starting IFN-α therapy. However, subjects who subsequently became depressed while taking IFN-α tended to exhibit higher mean baseline Hamilton depression scale scores (mean=6.3, SD=7.4, versus mean=1.7, SD=2.1) (t=1.57, df=12, p=0.14).
ACTH and cortisol responses to the initial injection of IFN-α (i.e., week 1, before any previous IFN-α) were dramatic in both groups (Figure 1). Nevertheless, patients who subsequently met symptom criteria for major depression exhibited significantly higher ACTH and cortisol responses than patients who did not become depressed, especially 3 hours after IFN-α injection (ACTH: F=11.59, df=1, 11, p<0.01; 95% confidence interval [CI]=2.3–3.1 log [pg/ml] for depressed group; 95% CI=1.4–2.2 log [pg/ml] for nondepressed group) (cortisol: F=11.88, df=1, 11, p<0.01; 95% CI=26.3–36.5 μg/dl for depressed group; 95% CI=14.5–24.7 μg/dl for nondepressed group). Of note, there was also a significant correlation between baseline Hamilton depression scale scores and the initial HPA response to IFN-α (ACTH: rs=0.74, N=14, p<0.01; cortisol: rs=0.57, N=14, p<0.05). Moreover, baseline Hamilton depression scale scores were significantly correlated with the intensity of depressive symptoms (as assessed by the Hamilton depression scale) at week 8 in the study group as a whole (rs=0.54, N=14, p<0.05). Accordingly, the statistical analyses of the relationship between the initial HPA axis response to IFN-α and the subsequent development of depression were repeated controlling for initial Hamilton depression scale scores. Similar results were obtained (ACTH: F=6.54, df=1, 10, p<0.05; cortisol: F=14.23, df=1, 10, p<0.01).
Correlations with symptom dimensions revealed that the ACTH and cortisol responses (3-hour response minus baseline) to the initial injection of IFN-α were significantly associated with depressive, anxious, and cognitive symptoms at week 8 of IFN-α therapy (ACTH: rs=0.67, p<0.01, rs=0.70, p<0.01, and rs=0.66, p<0.05, respectively; cortisol: rs=0.53, p<0.05, rs=0.52, p=0.05, and rs=0.66, p<0.01, respectively, all N=14). No such correlations were found with neurovegetative or somatic symptoms (ACTH: rs=0.35, p=0.22, and rs=0.29, p=0.30, respectively; cortisol: rs=0.40, p=0.16, and rs=0.24, p=0.42, respectively).
Both groups exhibited marked elevations in IL-6 at week 1, but no differences between groups were found. Moreover, no differences between groups in ACTH, cortisol, or IL-6 responses were found during chronic IFN-α therapy (weeks 2–12), either before or immediately after IFN-α administration. During chronic treatment, IFN-α administration no longer elicited a significant ACTH or cortisol response, and IL-6 responses were markedly reduced (Figure 1 depicts week 8 as a representative visit during chronic IFN-α therapy).
Discussion
The results indicate that the HPA axis response to the initial injection of IFN-α reveals a vulnerability to IFN-α-induced mood disorder, possibly related to sensitized CRF pathways. This vulnerability also appears to be associated with subclinical behavioral symptoms before starting IFN-α, as revealed by the correlation between HPA axis responses to IFN-α and Hamilton depression scale scores at baseline.
Without a placebo comparison, it remains possible that the HPA axis hyperresponsiveness to the initial injection of IFN-α seen in patients who developed depression may represent a more generalized (i.e., not immune-specific) hyperresponsiveness to stress independent of IFN-α. The equivalent initial IL-6 responses to IFN-α in both groups support this notion.
Hyperactivity of the HPA axis in response to stress has been identified in other groups of individuals who are at high risk for neuropsychiatric disorders. For example, a group of individuals exposed to early life stress who exhibited a high rate of major depression also exhibited significantly higher pituitary-adrenal responses to a psychosocial laboratory stressor than control subjects (6). These findings are believed to represent sensitization of CNS CRF pathways, on the basis of animal studies indicating an association among early maternal separation, increased hypothalamic CRF mRNA expression, higher ACTH and corticosterone responses to restraint stress, and increased depressive-like behavior in adulthood (7).
HPA axis hyperresponsiveness to the first administration of IFN-α was closely associated with development of mood and cognitive changes but not neurovegetative and somatic symptoms during later stages of IFN-α therapy. Interestingly, mood and cognitive changes during IFN-α therapy were most responsive to treatment with paroxetine (1), suggesting that stress reactivity and CRF may be more relevant to vulnerability to cytokine-induced depression and its treatment with antidepressants than other aspects of sickness such as fatigue, anorexia, and pain (1).
In conclusion, these preliminary findings generate new hypotheses regarding the pathophysiology of mood disorders in the context of immune activation. Nevertheless, because of the small number of subjects in the present study, these findings warrant replication in a larger group of patients undergoing IFN-α therapy.
Received May 16, 2002; revisions received Aug. 29 and Dec. 26, 2002; accepted Dec. 26, 2002. From the Department of Psychiatry and Behavioral Sciences and the Winship Cancer Institute, Department of Hematology and Oncology, Emory University School of Medicine. Address reprint requests to Dr. Miller, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, 1639 Pierce Dr., Suite 4000, Atlanta, GA 30322; [email protected] (e-mail). Supported in part by NIMH grant MH-00680 (Dr. Miller) and by grants from the Centers for Disease Control, Schering-Plough Pharmaceuticals, and Glaxo-SmithKline.
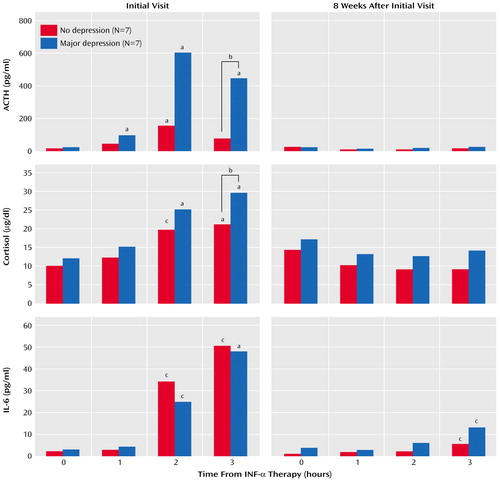
Figure 1. Adrenocorticotropic Hormone (ACTH), Cortisol, and Interleukin-6 (IL-6) Responses to Interferon-Alpha (INF-α) at Week 1 (Initial Visit) and Week 8 in Patients Who Did or Did Not Develop Major Depression During INF-α Therapy
aSignificantly different from 0 hours (p<0.01, Tukey’s test).
bSignificant difference between groups (p<0.01, simple main effects).
cSignificantly different from 0 hours (p<0.05, Tukey’s test).
1. Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH: Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 2002; 26:643-652Crossref, Medline, Google Scholar
2. Raber J, Koob GF, Bloom FE: Interferon-alpha and transforming growth factor-beta 1 regulate corticotropin-releasing factor release from the amygdala: comparison with the hypothalamic response. Neurochem Int 1997; 30:455-463Crossref, Medline, Google Scholar
3. Owens MJ, Nemeroff CB: The role of corticotropin-releasing-factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found Symp 1993; 172:296-308Medline, Google Scholar
4. Gisslinger H, Svoboda T, Clodi M, Gilly B, Ludwig H, Havelec L, Luger A: Interferon-alpha stimulates the hypothalamic-pituitary-adrenal axis in vivo and in vitro. Neuroendocrinology 1993; 57:489-495Crossref, Medline, Google Scholar
5. Yamano M, Yuki H, Yasuda S, Miyata K: Corticotropin-releasing hormone receptors mediate consensus interferon-alpha YM643-induced depression-like behavior in mice. J Pharmacol Exp Ther 2000; 292:181-187Medline, Google Scholar
6. Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB: Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000; 284:592-597Crossref, Medline, Google Scholar
7. Plotsky PM, Meaney MJ: Early, postnatal experience alters hypothalamic corticotropin-releasing-factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res 1993; 18:195-200Crossref, Medline, Google Scholar



