Frontostriatal Abnormalities in Adolescents With Bipolar Disorder: Preliminary Observations From Functional MRI
Abstract
OBJECTIVE: This study investigated whether the functional abnormalities in prefrontal systems observed in adult bipolar disorder are manifested in adolescents with this illness. METHOD: Ten adolescents with bipolar disorder and 10 healthy comparison subjects participated in a color-naming Stroop task during event-related functional magnetic resonance imaging. RESULTS: Signal increases in the left putamen and thalamus were significantly greater in the bipolar disorder group than in the healthy group. Age correlated positively with signal increases in the bilateral rostroventral prefrontal cortex and the striatum in the healthy group but not in the bipolar disorder group. In the bipolar disorder subjects, depressive symptoms correlated positively with signal increases in the ventral striatum. CONCLUSIONS: These findings suggest the presence of dysfunction in the subcortical portions of the frontostriatal circuits in adolescents with bipolar disorder. The absence of the prefrontal abnormalities that were observed previously in adults and the absence of the age-related increases in prefrontal activity observed in normal comparison subjects suggest that a developmental disturbance in prefrontal function may emerge in bipolar disorder over the course of adolescence.
Converging evidence from neuroimaging and histological studies implicates abnormalities in frontostriatal systems in adults with bipolar disorder (1–5). Few studies, however, have assessed these systems in adolescents with bipolar disorder. Preliminary magnetic resonance spectroscopic and morphometric data suggest the presence of frontal, striatal, and thalamic abnormalities in juvenile bipolar disorder (6, 7). We report the first study, to our knowledge, to use functional magnetic resonance imaging (fMRI) to examine juvenile bipolar disorder. An event-related color-naming Stroop task, identical to that used in a study of adult bipolar disorder (1), was employed to probe frontostriatal function and to permit investigation of potential similarities and differences in adolescents and adults with bipolar disorder.
Method
The study included 10 subjects aged 10–17 years with DSM-IV bipolar I disorder (including six female, one left-handed, and three unmedicated subjects) and 10 healthy comparison subjects aged 11–18 years with neither personal nor first-degree family history of axis I disorders (including six female subjects and one left-handed subject). None of the subjects had a neurological illness, history of head trauma, MRI abnormalities detected by a neuroradiological review performed by one of the authors (R.K.F.), medical illness (except one female bipolar disorder subject with treated hypothyroidism), history of substance or alcohol dependence, or substance or alcohol use within 24 hours of scanning. Diagnoses were established by the consensus of two examiners (A.M., J.K., or H.P.B.) on the basis of clinical interviews and administration of the revised Schedule for Affective Disorders and Schizophrenia for School-Age Children (Present and Lifetime versions) (8). Structured interviews provided family history research diagnostic criteria. The Childhood Depression Rating Scale—Revised (9) was administered immediately before scanning; the bipolar disorder patients’ mean score was 36.2 (SD=21.5, range=18–88).
Comorbid psychiatric diagnoses in bipolar disorder subjects included attention deficit hyperactivity disorder (ADHD) (N=2), oppositional defiant disorder (N=2), posttraumatic stress disorder (N=2), substance or alcohol abuse (N=2), obsessive-compulsive disorder (OCD) (N=1), generalized anxiety disorder (N=1), specific phobia (N=1), and learning disability not otherwise specified (N=1). Family history included at least one first-degree relative with bipolar disorder (N=3), major depressive disorder (N=3), or substance dependence (N=1) or a second-degree relative with bipolar disorder (N=1) or major depressive disorder (N=2). Medications included lithium (N=4), anticonvulsants (N=3), antidepressants (N=3), antipsychotics (N=2), stimulants (N=1), clonidine (N=1), and levothyroxine (N=1). After a complete description of the study, written informed consent was obtained from 18-year-old individuals and from guardians of individuals under age 18 years, who also provided written assent.
The Stroop activation paradigm, echoplanar imaging acquisition, and image processing were performed according to methods described previously (1). Composite maps were used to compare the mean signal change (incongruent trials versus congruent trials) between the bipolar disorder and healthy groups at each pixel (significance threshold: p<0.005; cluster minimum: 20 adjacent pixels). Exploratory analyses investigated the effects of age and Childhood Depression Rating Scale—Revised scores on regional activation (correlation coefficient: r>0.70; cluster minimum: 20 adjacent pixels).
Results
The patient and comparison groups were similar in age (bipolar disorder group: mean=13.6 years, SD=2.8; comparison group: mean=14.6, SD=2.8; p=0.43); Stroop response accuracy, measured as the percentage of errors on incongruent stimuli (bipolar disorder group: mean=5.0, SD=11.3; comparison group: mean=16.7, SD=22.2; p=0.16); response times, measured by subtracting congruent response time from incongruent response time (bipolar disorder group: mean=203.7 msec, SD=43.5; comparison group: mean=202.6, SD=91.1; p=0.98); head displacement, measured by the maximum in x, y, or z planes across trials (bipolar disorder group: mean=1.1 mm, SD=0.5; comparison group: mean=0.9 mm, SD=0.4; p=0.31); and rotation (pitch, roll, or yaw) (bipolar disorder group: mean=1.2 degrees, SD=0.8; comparison group: mean=1.1 degrees, SD=0.6; p=0.85). All comparisons were made by means of t tests with unequal variance.
The healthy and bipolar disorder groups demonstrated distributed patterns of activation in regions previously reported to be associated with the Stroop task in adults, including the dorsal anterior cingulate; dorsolateral prefrontal, inferior prefrontal, and posterior association cortices; thalamus; and striatum (1). The signal was increased in the bipolar disorder group, compared with the healthy group, in regions of the left putamen and inferior thalamus (Figure 1). In the bipolar disorder group, Childhood Depression Rating Scale—Revised depression scores correlated positively with signal increases in the ventral striatum (Figure 1). In both groups, age correlated positively with signal increases in the dorsal frontal and posterior association cortices, but only the healthy group demonstrated bilateral age-related increases in signal in the rostroventral prefrontal cortex and striatum (Figure 1).
Discussion
During performance of an event-related fMRI color-naming Stroop task, increased signal was detected in the left putamen and thalamus of adolescents with bipolar disorder, compared with healthy adolescents. These regions are components of the frontostriatal circuits in which functional and anatomical disturbances have been implicated in the pathophysiology of bipolar disorder (1–7). Increased signal in the ventral striatum was associated with more severe depressive symptoms in the bipolar disorder group. Deficient activation in the rostroventral prefrontal cortex, which was reported previously for adults with bipolar disorder (1, 2), was not detected in these adolescents with bipolar disorder. An association of age with increasing task-related signal changes was detected in this prefrontal region in the healthy comparison adolescents but not in the bipolar disorder group, suggesting that a progressive divergence in the activity of this region over adolescence may contribute to the larger group differences in its activity that were detected in adulthood.
Abnormalities in the striatum and thalamus have been reported in imaging studies of other disorders, such as ADHD, OCD, and Tourette’s disorder, in which the pathophysiologies are believed to involve disturbances in circuits subserving motivation and regulatory self-control (10, 11). These conditions share some diagnostic features with bipolar disorder in children, which we speculate may be attributable to a common dysfunction in subcortical circuits. The adult bipolar disorder phenotype, moreover, may be difficult to recognize until late adolescence, when a progressive, age-related divergence in the maturation of prefrontal portions of the frontostriatal circuits (12) produces a greater differentiation of executive functioning between these diagnostic groups.
These preliminary results are limited by the small number of subjects in this study. Alternative explanations for the absence of prefrontal abnormalities detected previously in studies of adults with bipolar disorder include the possibilities that the frontal abnormalities in adults are the consequence of repeated disease episodes, disease chronicity, or greater medication exposure. Patterns of functional abnormalities in adolescents and adults with bipolar disorder could also represent two distinct pathophysiological subtypes in bipolar disorder. Potential confounding factors in this study include medication influence on task performance or signal change, as well as the presence of comorbid disorders.
This study demonstrates the feasibility of investigating juvenile bipolar disorder by using fMRI methods. The findings suggest that the presence of frontostriatal abnormalities may be common to adolescents and adults with bipolar disorder. Future investigation of developmental disturbances in frontostriatal circuits may contribute to our understanding of the pathophysiology and progression of bipolar disorder across the lifespan.
Received Aug. 8, 2002; revision received Dec. 27, 2002; accepted Jan. 6, 2003. From the Departments of Psychiatry and Diagnostic Radiology and the Yale Child Study Center, Yale University School of Medicine, New Haven, Conn.; the Mood and Anxiety Research Program, NIMH, Bethesda, Md.; and the Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York. Address reprint requests to Dr. Blumberg, Department of Psychiatry 116A, VA Connecticut Healthcare System, 950 Campbell Ave., West Haven, CT 06516; [email protected] (e-mail). Supported by grants from the Stanley Medical Research Institute, the National Alliance for Research on Schizophrenia and Depression, and the Ethel F. Donaghue Women’s Investigator Program at Yale (Dr. Blumberg); from the Charles A. Dana Foundation and the Suzanne Crosby Murphy Endowment at Columbia University College of Physicians and Surgeons (Dr. Peterson); from the Department of Veterans Affairs (Dr. Blumberg and Dr. Kaufman); and from the NIMH Mental Health Clinical Research Center program (Dr. Blumberg, Dr. Charney, and Dr. Kaufman); NIMH grants MH-01232 and MH-59139 (Dr. Peterson) and MH-01792 (Dr. Martin); and grant AA-00261-01 from the National Institute on Alcohol Abuse and Alcoholism (Dr. Kaufman). The authors thank Kathleen Colonese for expert care in coordinating the research, Nianjun Liu and Ralitza Gueorguieva for statistical consultation, Heather Douglas-Palumberi and Mindy Crouse-Artus for assistance in working with the adolescent research subjects, Hedy Sarofin and Terry Hickey for technical assistance, and the research subjects and their families for their participation.
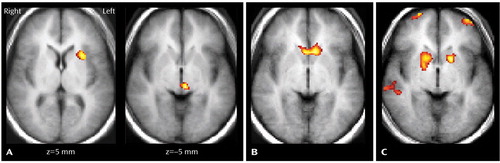
Figure 1. Mean Regional Activation During a Color-Naming Stroop Test in Adolescents With Bipolar Disorder (N=10) and Healthy Comparison Subjects (N=10)a
aPanel A shows increased fMRI signal in the left putamen and inferior thalamus in adolescent bipolar disorder subjects, relative to healthy comparison subjects. Panel B shows areas in the ventral striatum in which increased fMRI signal was correlated positively with Childhood Depression Rating Scale—Revised ratings in bipolar disorder subjects. Panel C shows areas in the rostroventral prefrontal cortex and striatum in which increased fMRI signal was correlated positively with age in healthy comparison subjects.
1. Blumberg HP, Leung HC, Skudlarski P, Lacadie C, Fredericks C, Harris B, Charney D, Krystal J, Gore JC, Peterson BS: A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry 2003; 60:599-607Google Scholar
2. Rubinsztein JS, Fletcher PC, Rogers RD, Ho LW, Aigbirhio FI, Paykel ES, Robbins TW, Sahakian BJ: Decision-making in mania: a PET study. Brain 2001; 124:2550-2563Crossref, Medline, Google Scholar
3. Blumberg HP, Stern E, Martinez D, Ricketts S, de Asis J, White T, Epstein J, McBride PA, Eidelberg D, Kocsis JH, Silbersweig DA: Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry 2000; 48:1045-1052Crossref, Medline, Google Scholar
4. Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME: Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386:824-827Crossref, Medline, Google Scholar
5. Rajkowska G: Cell pathology in mood disorders. Semin Clin Neuropsychiatry 2002; 7:281-292Crossref, Medline, Google Scholar
6. Castillo M, Kwock L, Courvoisie H, Hooper SR: Proton MR spectroscopy in children with bipolar affective disorder: preliminary observations. AJNR Am J Neuroradiol 2000; 21:832-838Medline, Google Scholar
7. Dasari M, Friedman L, Jesberger J, Stuve TA, Findling RL, Swales TP, Schulz SC: A magnetic resonance imaging study of thalamic area in adolescent patients with either schizophrenia or bipolar disorder as compared to healthy controls. Psychiatry Res 1999; 91:155-162Crossref, Medline, Google Scholar
8. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980-988Crossref, Medline, Google Scholar
9. Freeman LN, Mokros H, Poznanski EO: Violent events reported by normal urban school-aged children: characteristics and depression correlates. J Am Acad Child Adolesc Psychiatry 1993; 32:419-423Crossref, Medline, Google Scholar
10. Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC: A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry 1998; 55:326-333Crossref, Medline, Google Scholar
11. Peterson BS, Leckman JF, Tucker D, Scahill L, Staib L, Zhang H, King R, Cohen DJ, Gore JC, Lombroso P: Preliminary findings of antistreptococcal antibody titers and basal ganglia volumes in tic, obsessive-compulsive, and attention deficit/hyperactivity disorders. Arch Gen Psychiatry 2000; 57:364-372Crossref, Medline, Google Scholar
12. Bourgeois JP, Goldman-Rakic PS, Rakic P: Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex 1994; 4:78-96Crossref, Medline, Google Scholar



