Association Between a Functional Catechol O-Methyltransferase Gene Polymorphism and Schizophrenia: Meta-Analysis of Case-Control and Family-Based Studies
Abstract
OBJECTIVE: There is strong evidence for a genetic contribution to schizophrenia, but efforts to identify susceptibility genes have been largely unsuccessful because of the low power of individual studies. The authors’ goal was to evaluate the collective evidence for an association between the Val158/108Met polymorphism of the catechol O-methyltransferase (COMT) gene and schizophrenia. METHOD: They performed separate meta-analyses of existing case-control and family-based association studies. RESULTS: Overall, case-control studies showed no indication of an association between either allele and schizophrenia, and family-based studies found modest evidence implicating the Val allele in schizophrenia risk. The pooled analyses of studies from diverse geographical regions may have obscured ethnic differences in patterns of genetic risk for schizophrenia. Stratification of the studies by ethnicity of the subjects yielded evidence for an association with the Val allele in case-control studies of European samples and, especially, in family-based studies of European samples. Case-control and family-based studies of Asian samples produced mixed results and, overall, little evidence for association. CONCLUSIONS: The results of the two types of association studies diverged somewhat, but the evidence from the family-based studies, although based on fewer reports, may be more accurate. The Val allele may be a small but reliable risk factor for schizophrenia for people of European ancestry, but the influence of this polymorphism on risk in Asian populations remains unclear. These results call for more family-based studies to confirm the association between COMT and schizophrenia in European samples and to clarify its contribution to risk in Asian samples. They also suggest that case-control studies should use methods of genomic control to avoid being confounded by population stratification.
The evidence for a genetic contribution to schizophrenia is strong. Family studies show that relatives of individuals with schizophrenia develop the disorder at a rate higher than the population incidence, and the degree of elevation in risk is correlated with the degree of biological relationship to the person with schizophrenia (1). Twin studies (2) found approximately three times higher concordance for schizophrenia among monozygotic twins than among dizygotic twins. Furthermore, adoption studies (3) indicate that the transmission of schizophrenia is predominantly through biological rather than adoptive parents. It is estimated that as much as 60%–70% of the susceptibility to schizophrenia can be attributed to genes (4).
Genes may not account for all of the susceptibility to schizophrenia, but they clearly play a considerable role in the etiology of the illness. Although the disorder is highly heritable, genetic linkage studies have failed to endorse schizophrenia linkage universally with any chromosomal region (5). The difficulty finding genes for schizophrenia suggests that many genes combine to cause the disorder and that each confers only a small degree of risk (6). Because evidence implicates dopamine system dysfunction in the pathogenesis of this disorder, the search for susceptibility genes has included those which code for all five known dopamine receptor subtypes (7–10), the dopamine transporter (11), and several enzymes involved in the synthesis and degradation of dopamine, such as tyrosine hydroxylase, aromatic l-amino acid decarboxylase, and monoamine oxidase (MAO) A and MAOB (12–14). None of these genes has reliably emerged as a risk factor for schizophrenia.
Because it codes for one of the major enzymes catalyzing the metabolism of dopamine, the gene for catechol O-methyltransferase (COMT) (EC 2.1.1.6 according to the International Union of Biochemistry and Molecular Biology enzyme nomenclature) has been examined numerous times for an association with schizophrenia, but with equivocal findings. This ambiguity parallels that of genetic linkage analyses in the vicinity of the COMT gene on chromosome 22q11.21. Some, but not all, studies (15, 16) have found evidence of linkage with schizophrenia in this region. However, a meta-analysis of genome-wide linkage scans (17) identified chromosome 22q as one of three loci (along with chromosomes 8p and 13q) that had the highest likelihood of harboring schizophrenia-risk genes, which highlights the promise of COMT as a candidate gene for this disorder.
The most commonly examined locus within the COMT gene is a functional polymorphism at codon 158 of the membrane-bound form of the enzyme (codon 108 of the soluble form) in which the wild-type guanine nucleotide is substituted with adenine. This nucleotide transition results in the substitution of methionine (Met) for valine (Val) in the COMT protein, producing an enzyme with approximately four times less physiological activity and introducing an NlaIII restriction site (18). This polymorphism also correlates with performance on the Wisconsin Card Sorting Test, which measures executive cognition and working memory (19, 20), and with prefrontal cortical activity during performance of the 2-back working memory task (19).
Because the conflicting results from association studies have obscured COMT’s true role in schizophrenia, we performed the meta-analysis reported on here to determine if the failure to identify an association consistently is attributable to the low power of individual studies to detect a small effect, etiological heterogeneity, or random error in the absence of a true effect.
Method
Literature Search
To identify studies eligible for meta-analysis, we surveyed MEDLINE citations for January 1966 through August 2002 using the National Library of Medicine’s PubMed online search engine with “schizophrenia” and “COMT” as keywords. The retrieved abstracts were read to identify studies examining the allelic association between a polymorphism within the COMT gene and schizophrenia. Studies of this type were then read in their entirety to assess their appropriateness for inclusion in the meta-analysis. All references cited in these studies were also reviewed to identify additional works not indexed by MEDLINE.
Inclusion Criteria
Only those studies examining the COMT Val158/108Met polymorphism were included in the meta-analysis. (One study [21] examined the Val158/108Met polymorphism but failed to provide the necessary data for each allele. Thus, the allele frequencies in the patient and control groups were extrapolated from data provided for the PmlI polymorphism, which, in this sample, was in total linkage disequilibrium with the NlaIII restriction site.) Furthermore, studies had to meet all of the following criteria: 1) be published in a peer-reviewed journal, 2) be written in English, 3) present original data, and 4) provide enough data to calculate an effect size.
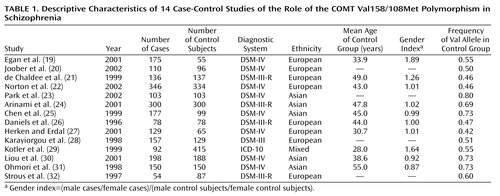
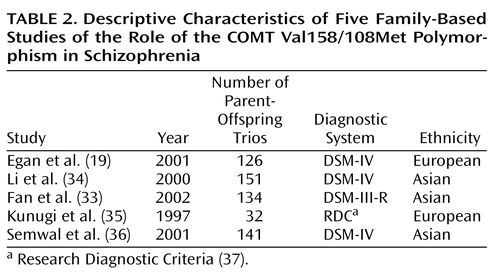
The application of these criteria yielded 18 studies eligible for meta-analysis, of which 13 used case-control designs (20–32) and four used family-based strategies (33–36). The remaining study (19) employed both methods in a single sample; therefore, the groups of case-control and family-based studies are not entirely independent. The 14 case-control studies are described in Table 1, and the five family-based studies are described in Table 2.
Coding of Study Characteristics
To delineate potential moderating influences of different sample characteristics on the size of the effects obtained in the case-control studies under consideration, each study was coded on the following variables: 1) the ethnicity of the sample, 2) the mean age of the control group, and 3) a gender index (calculated as [male cases/female cases]/[male control subjects/female control subjects]). Because only five family-based studies were available, the power to detect significant moderators of the effects obtained in these studies was severely constrained; therefore, only sample ethnicity was examined as a moderator in these studies.
Statistics
Data from each case-control study were used to construct a two-by-two table in which subjects were classified by diagnostic category (case or control) and allele (Met or Val). Data from each family-based study were used to construct a two-by-two haplotype-based relative risk table in which parental alleles were classified by type (Met or Val) and transmission status (transmitted or not transmitted from a heterozygous parent to the offspring with schizophrenia). The strength of association in these two-by-two tables was summarized by using the odds ratio, in which Val was randomly assigned as the risk allele and an odds ratio greater than 1.0 indicated a positive association between this allele and schizophrenia.
For case-control studies, the odds ratio estimated the relative risk, which represents the greater probability of observing the Val allele in cases than in control subjects. For family-based studies, the odds ratio estimated the haplotype relative risk, which represents the greater probability of the offspring with schizophrenia receiving the Val allele relative to the Met allele. Knapp et al. (38) showed that when no recombination occurs between the marker and the disease gene, haplotype relative risk=relative risk. Otherwise, when the marker and disease gene show a positive association, relative risk≥haplotype relative risk.
Case-control studies and family-based studies were analyzed separately by random effects meta-analyses. For each meta-analysis, odds ratios were pooled according to the methods of DerSimonian and Laird (39), and 95% confidence intervals (CIs) were constructed by using Woolf’s method (40). The significance of the pooled odds ratio was determined by the z test, and the heterogeneity of the group of odds ratios was assessed by using a chi-square test of goodness of fit. The influence of individual studies on the pooled odds ratio was determined by sequentially removing each study and recalculating the pooled odds ratio and 95% CI.
Publication bias within groups of homogeneous odds ratios was assessed by the method of Egger et al. (41), in which the standard normal deviate of the odds ratio (z) is regressed on the precision of the odds ratio (the inverse of the standard error of the odds ratio). Since the inverse of the standard error of the odds ratio increases with sample size, the regression of z on the precision of the odds ratio should run through the origin in the absence of bias (i.e., small samples with low precision have large standard errors and small standard normal deviates, whereas large samples with high precision have small standard errors and large standard normal deviates). The slope (b) of the regression line indicates the size and direction of association and, in the presence of bias, the intercept of the regression (a) will be significantly greater than 0, as determined by the t test.
The moderating influences of sample ethnicity, age of the control group, and gender index on the odds ratio derived from each study were assessed by multiple regression. The type I error rate was set at 0.05. All statistical analyses were conducted by using Stata 7.0 (Stata Corp., College Station, Tex.).
Results
Case-Control Studies
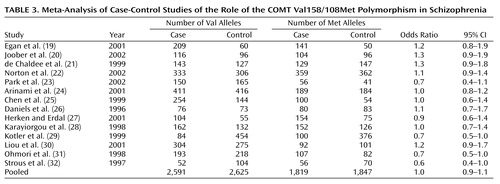
Of the 14 case-control association studies of the COMT Val158/108Met polymorphism and schizophrenia, only two found a statistically significant difference in allele frequencies between patients and control subjects. Both of these studies (29, 31) found an excess of the Met allele in schizophrenic patients. The odds ratio and 95% CI for each case-control study are shown in Table 3. The pooled odds ratio derived from 2,205 cases and 2,236 control subjects was not significant (odds ratio=1.0, z=0.57, p=0.57). Sequential omission of individual case-control studies produced pooled odds ratios ranging from 0.9 to 1.0 with 95% CIs that always encompassed 1.0, indicating that the pooled odds ratio was not unduly influenced by any single study. However, significant heterogeneity was observed among this group of odds ratios (χ2=22.98, df=13, p=0.04), suggesting the presence of some moderating variable.
Neither the age of the control group (z=–0.07, p=0.94) nor the gender index of the sample (z=0.33, p=0.75) significantly influenced the obtained odds ratios, but these odds ratios were significantly related to the ethnicity of the sample (z=2.38, p=0.02). However, stratification of the group of odds ratios by sample ethnicity (Asian or European) yielded only slightly different estimates of the association of this COMT polymorphism with schizophrenia in the two ethnic groups. The pooled odds ratio derived from five Asian studies was 0.9 (95% CI=0.7–1.1), and the pooled odds ratio from eight European studies was 1.1, but the association was not significant in either the Asian (z=1.04, p=0.30) or European (z=1.10, p=0.27) samples.
There was no evidence of publication bias within either the Asian samples (a=–2.65, t=–0.88, df=4, p=0.45) or the European samples (a=–1.10, t=–0.80, df=7, p=0.45), as the 95% CIs of the regressions of z on the precision of the odds ratio encompassed the origin for each group of studies (Figure 1).
Two different genotype-wise analyses were also conducted, including a logistic regression predicting additive risk for schizophrenia with each additional Val allele (Met/Met versus Met/Val versus Val/Val) and a comparison of risk between homozygotes (Met/Met versus Val/Val). The results of these analyses were nearly identical to those of the allele-wise analysis (data available on request).
Family-Based Studies
Of the five family-based association studies, two found significant evidence for differential transmission of parental COMT Val158/108Met alleles to affected offspring. However, in contrast to the two significant case-control studies, these two family-based studies (19, 34) supported Val as the risk allele for schizophrenia. The odds ratio and 95% CI for each of the family-based studies are shown in Table 4. The pooled odds ratio derived from 584 parent-offspring trios was 1.5, which was not significant (z=1.51, p=0.13); however, as in the case-control studies, there was significant heterogeneity among this group of odds ratios (χ2=16.49, df=4, p=0.002). This heterogeneity may have been due to ethnic differences in association (z=1.20, p=0.23), but the power to detect significant moderation of odds ratios by ethnicity (beta=0.03) was severely constrained by the low number of Asian (N=3) and European (N=2) samples.
Based on this trend and the significant ethnic heterogeneity observed in the case-control studies, this group of studies was also stratified by sample ethnicity, which did reveal a pattern of ethnic differences in association. The three family-based studies of Asian patients revealed little evidence for association with either allele (odds ratio=1.2, 95% CI=0.6–2.3, z=0.52, p=0.61). In contrast, the two samples of European patients produced strong evidence for association of schizophrenia with the Val allele (odds ratio=2.2, 95% CI=1.4–3.4, z=3.35, p=0.001).
There was no evidence of publication bias within the group of three Asian samples (a=–94.96, t=–1.25, df=2, p=0.43) (Figure 2), although the power to detect potential bias in this data set (beta=0.23) was again limited by the low number of included studies. Similarly, the limited number of European samples precluded a statistical assessment of publication bias within this group of studies, but the plot of z on the precision of the odds ratio (Figure 2) demonstrated the pattern expected for a group of studies that produced a significant association in the absence of bias (i.e., the study that was larger and thus had greater precision showed a larger standardized effect size).
Discussion
The pooled sample of 14 case-control studies had more than 90% power to detect a small but significant association (odds ratio=1.2) between COMT Val158/108Met alleles and schizophrenia but failed to identify such a relationship. However, rather than reflecting a lack of association, this may have been due to the statistically significant heterogeneity among studies. Overrepresentation of violent patients with schizophrenia in the sample did not appear to skew the results, as one such study (29) found an odds ratio of 0.7 while the other (30) found an odds ratio of 1.2.
The inclusion of schizophrenia spectrum conditions (e.g., schizoaffective disorder or schizotypal personality disorder) in a broader definition of the schizophrenia phenotype also failed to systematically affect the results, as one such study (19) yielded an odds ratio of 1.2 while the other (23) produced an odds ratio of 0.7. Other study characteristics, such as the gender composition of the sample or the age of the control group, did not account for this heterogeneity either. The only factor that reliably influenced the odds ratio obtained from each study was the ethnicity of the sample: the pooled odds ratio from Asian samples was 0.9, slightly lower than the pooled odds ratio of 1.1 from European samples.
In isolation from the findings of the five family-based association studies, several possible conclusions can be drawn from these observations. First, COMT may be a susceptibility gene for schizophrenia, but an overall association between the gene and the disorder may have been obscured by etiological heterogeneity whereby different COMT alleles confer risk to different ethnic groups. The present findings provide limited support for such an interpretation; however, it is important to note that neither allele was significantly associated with risk in either ethnic group. It is also possible that allelic variants of the COMT gene confer risk for schizophrenia to only a restricted segment of the population if at all, or that the COMT genotype is a universal risk factor of such small magnitude that its attributable fraction in the population is negligible.
The five family-based studies help to clarify the nature of the relationship between the COMT Val158/108Met polymorphism and schizophrenia. Like the group of case-control studies, the family-based studies were not a homogeneous group, and this heterogeneity may have been attributable to a different pattern of genetic risk among Asian and European samples. The only two family-based studies of European samples provided nearly identical odds ratios that strongly implicated the Val allele in schizophrenia. This finding, in combination with the trend observed in the case-control studies, suggests that the Val allele may be a small but reliable risk factor for schizophrenia among those of European descent. In contrast, the findings from family-based studies of Asian samples were more variable as a whole and did not detect a significant association of schizophrenia with the Val allele. Thus, the family-based studies reinforced the possibility that the Val allele of this COMT polymorphism confers a different degree of risk to these two ethnic groups.
The variability of the evidence from Asian samples prevents firm conclusions about the role of this polymorphism in schizophrenia susceptibility in this population, and even though the Val allele was implicated more strongly in schizophrenia risk among European samples, this association seems tenuous at best. This relationship is further complicated by the possibility that the causative risk gene that produced this evidence for association is not COMT itself but a nearby gene that is in linkage disequilibrium with it. Clearly, more family-based studies of this putative association are sorely needed and will prove extremely informative. Determining the true nature of the relationship between this polymorphism of COMT and schizophrenia also may be facilitated by the application of emerging genomic-control association methods. The use of genotypes at randomly selected markers throughout the genome to control for population substructure in a case-control design provides a more powerful alternative to family-based association testing that nonetheless remains robust to false positive or false negative associations (42).
There is some division in the conclusions to be drawn from case-control and family-based studies of the association between the COMT Val158/108Met polymorphism and schizophrenia. The former set of studies implicated the Val allele as a very weak risk factor in European samples (odds ratio=1.1), while the family-based studies place the effect of Val in European samples much higher (odds ratio=2.2). There is no simple resolution to this discrepancy, but the most conservative conclusion would be that, without additional evidence, we simply cannot determine if the COMT gene is implicated in schizophrenia. As new studies emerge, the present findings can be updated, and, eventually, more reliable estimates of this association in both ethnic groups may be obtained.
If, however, the present findings are already accurate estimates of the true effects to be obtained from each type of study and if the results from case-control and family-based studies do not converge upon the addition of more samples, then other possibilities must be considered. One alternative interpretation might be that case-control and family-based studies ascertain different populations of schizophrenic patients, and that this COMT polymorphism has a stronger contribution to risk in patients who become involved in family-based studies. The most obvious difference between the two groups of sampled patients is that those eligible for a family-based study must have two parents who are both willing and able to participate in the research. The availability of both parents could reflect a number of differences between patients enrolled in the two types of studies, such as a higher level of familial involvement with the patient and a better social support system or less severe pathology.
In the light of these discrepancies, there is reason to believe that the findings from the group of family-based studies (i.e., European samples, Val odds ratio=2.2) may be more accurate than the findings from the group of case-control studies (i.e., European samples, Val odds ratio=1.1). For example, the family-based study design is less susceptible than the case-control design to inferential errors based on artifacts like population stratification. As can be seen from Table 1, the frequency of the Val allele varies widely across studies, and especially across studies of different ethnic groups. In fact, allele frequency in control groups was highly correlated with ethnicity in this group of studies (r=0.93, p=0.000005), and a significantly higher Val frequency was observed in Asian control samples (0.73) than in European control samples (0.49). These estimates are quite similar to allele frequencies previously reported for each of these populations (43). Thus, despite the efforts to match cases and control subjects in population-based studies, even slight differences in ethnicity (and, consequently, allele frequency) between cases and control subjects can mask the contribution of a gene to the risk for a complex disease and invalidate estimates of association (44).
In addition, the odds ratios derived from the two family-based studies of European samples were strikingly similar in their direction and magnitude, which further suggests they are detecting a significant, albeit small, association. Since a formal analysis of publication bias was not possible in the small group of family-based studies of European samples, it remains possible that evidence against the family-based association of Val with schizophrenia exists but is unpublished. Thus, it may be that a positively biased sample of the family-based studies was obtained and that the inclusion of only a few more negative studies would close the gap between the pooled outcome of case-control studies and the family-based studies of European samples. This possibility is reasonable given the well-known bias against the publication of negative findings in scientific journals (45) and the reluctance of investigators to publish such findings.
The Val allele of this COMT polymorphism may increase susceptibility to schizophrenia, but it is important to recognize that this gene variant may also influence susceptibility to other psychiatric conditions. For example, this polymorphism is also associated with the rate of cycling in bipolar disorder (46, 47). The Val allele may give rise to multiple physiological abnormalities that separately contribute to both of these illnesses, or it may produce a single deficit that is common to both disorders. It is not yet clear if the observed effects of the Val158/108Met genotype on executive cognitive functioning and prefrontal cortical activation during the performance of a working memory task (19, 20) support the former or latter conclusion, since the specificity of these deficits for schizophrenia is unresolved (48). However, COMT resides on chromosome 22q, which is one of only two regions (along with chromosome 13q) that showed significant genome-wide linkage for both schizophrenia and bipolar disorder in a recent meta-analysis (17), further supporting a common effect of this polymorphism in both disorders. Although firm conclusions must await a large, definitive study, the present findings, along with the biological relevance of the Val allele, suggest that it may be considered a risk factor for schizophrenia, especially in those of European descent.
 |
 |
 |
 |
Received April 18, 2002; revision received Aug. 29, 2002; accepted Sept. 6, 2002. From the Department of Psychiatry, Harvard Medical School at Massachusetts Mental Health Center and Massachusetts General Hospital; the Harvard Institute of Psychiatric Epidemiology and Genetics, Boston; the Psychiatry Service, Brockton-West Roxbury Veterans Affairs Medical Center, Brockton, Mass.; and the Department of Epidemiology, Harvard School of Public Health, Boston. Address reprint requests to Dr. Tsuang, Massachusetts Mental Health Center, 74 Fenwood Rd., Boston, MA 02115; [email protected] (e-mail). Supported in part by NIMH grants MH-59624 and MH-60485 (Dr. Tsuang).
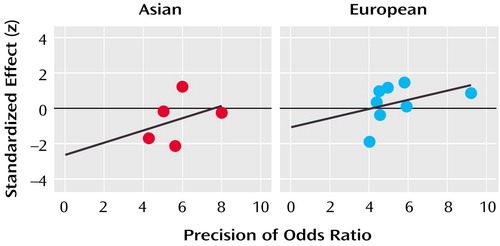
Figure 1. Egger’s Publication Bias Plots for Case-Control Studies of the Role of the COMT Val158/108Met Polymorphism in Schizophrenia in Asian and European Samplesa
aFigure shows the standardized effect of the 95% confidence interval (CI) of the regression of z on the precision of the odds ratio (the inverse of the standard error of the odds ratio). For Asian studies, 95% CI=7 to –12; for European studies, 95% CI=2.2 to –4.4.
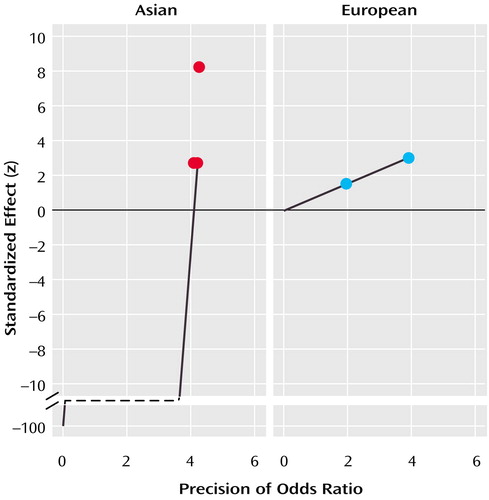
Figure 2. Egger’s Publication Bias Plots for Family-Based Studies of the Role of the COMT Val158/108Met Polymorphism in Schizophrenia in Asian and European Samplesa
aFigure shows the standardized effect of the 95% confidence interval (CI) of the regression of z on the precision of the odds ratio (the inverse of the standard error of the odds ratio). For Asian studies, 95% CI=900 to –1001.
1. Gottesman II: Schizophrenia Genesis: The Origins of Madness. New York, WH Freeman, 1991Google Scholar
2. Kendler KS: Overview: a current perspective on twin studies of schizophrenia. Am J Psychiatry 1983; 140:1413-1425Link, Google Scholar
3. Kety SS, Rosenthal D, Wender PH, Schulsinger F: The types and prevalence of mental illness in the biological and adoptive families of adopted schizophrenics. J Psychiatr Res 1968; 1(suppl):345-362Google Scholar
4. McGue M, Gottesman II, Rao DC: The transmission of schizophrenia under a multifactorial threshold model. Am J Hum Genet 1983; 35:1161-1178Medline, Google Scholar
5. Pulver AE: Search for schizophrenia susceptibility genes. Biol Psychiatry 2000; 47:221-230Crossref, Medline, Google Scholar
6. Faraone SV, Tsuang D, Tsuang MT: Genetics of Mental Disorders: A Guide for Students, Clinicians, and Researchers. New York, Guilford, 1999Google Scholar
7. Spurlock G, Williams J, McGuffin P, Aschauer HN, Lenzinger E, Fuchs K, Sieghart WC, Meszaros K, Fathi N, Laurent C, Mallet J, Macciardi F, Pedrini S, Gill M, Hawi Z, Gibson S, Jazin EE, Yang HT, Adolfsson R, Pato CN, Dourado AM, Owen MJ: European Multicentre Association Study of Schizophrenia: a study of the DRD2 Ser311Cys and DRD3 Ser9Gly polymorphisms. Am J Med Genet 1998; 81:24-28Crossref, Medline, Google Scholar
8. Sobell JL, Lind TJ, Sigurdson DC, Zald DH, Snitz BE, Grove WM, Heston LL, Sommer SS: The D5 dopamine receptor gene in schizophrenia: identification of a nonsense change and multiple missense changes but lack of association with disease. Hum Mol Genet 1995; 4:507-514Crossref, Medline, Google Scholar
9. Macciardi F, Verga M, Kennedy JL, Petronis A, Bersani G, Pancheri P, Smeraldi E: An association study between schizophrenia and the dopamine receptor genes DRD3 and DRD4 using haplotype relative risk. Hum Hered 1994; 44:328-336Crossref, Medline, Google Scholar
10. Kojima H, Ohmori O, Shinkai T, Terao T, Suzuki T, Abe K: Dopamine D1 receptor gene polymorphism and schizophrenia in Japan. Am J Med Genet 1999; 88:116-119Crossref, Medline, Google Scholar
11. Daniels J, Williams J, Asherson P, McGuffin P, Owen M: No association between schizophrenia and polymorphisms within the genes for debrisoquine 4-hydroxylase (CYP2D6) and the dopamine transporter (DAT). Am J Med Genet 1995; 60:85-87Crossref, Medline, Google Scholar
12. Coron B, Campion D, Thibaut F, Dollfus S, Preterre P, Langlois S, Vasse T, Moreau V, Martin C, Charbonnier F, Laurent C, Mallet J, Petit M, Frebourg T: Association study between schizophrenia and monoamine oxidase A and B DNA polymorphisms. Psychiatry Res 1996; 62:221-226Crossref, Medline, Google Scholar
13. Speight G, Turic D, Austin J, Hoogendoorn B, Cardno AG, Jones L, Murphy KC, Sanders R, McCarthy G, Jones I, McCandless F, McGuffin P, Craddock N, Owen MJ, Buckland P, O’Donovan MC: Comparative sequencing and association studies of aromatic l-amino acid decarboxylase in schizophrenia and bipolar disorder. Mol Psychiatry 2000; 5:327-331Crossref, Medline, Google Scholar
14. Kunugi H, Kawada Y, Hattori M, Ueki A, Otsuka M, Nanko S: Association study of structural mutations of the tyrosine hydroxylase gene with schizophrenia and Parkinson’s disease. Am J Med Genet 1998; 81:131-133Crossref, Medline, Google Scholar
15. Polymeropoulos MH, Coon H, Byerley W, Gershon ES, Crow TJ, Rubenstein J, Hoff M, Holik J, Smith AM, Shields G, Bass NJ, Poulter M, Lofthouse R, Vita A, Morganti C, Merril CR, DeLisi LE: Search for a schizophrenia susceptibility locus on human chromosome 22. Am J Med Genet 1994; 54:93-99Crossref, Medline, Google Scholar
16. Pulver AE, Karayiorgou M, Wolyneic P, Lasseter VK, Kasch L, Nestadt G, Antonarakis S, Housman D, Kazazian HH, Meyers D, Ott J, Lamacz M, Liang K-Y, Hanfelt J, Ullrich G, DeMarchi N, Ramu E, McHugh PR, Adler L, Thomas M, Carpenter WT, Manschreck T, Gordon CT, Kimberland M, Babb R, Puck J, Childs B: Sequential strategy to identify a susceptibility gene for schizophrenia: report of potential linkage on chromosome 22q12-q13.1, part 1. Am J Med Genet 1994; 54:36-43Crossref, Medline, Google Scholar
17. Badner JA, Gershon ES: Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry 2002; 7:405-411Crossref, Medline, Google Scholar
18. Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J: Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995; 34:4202-4210Crossref, Medline, Google Scholar
19. Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR: Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 2001; 98:6917-6922Crossref, Medline, Google Scholar
20. Joober R, Gauthier J, Lal S, Bloom D, Lalonde P, Rouleau G, Benkelfat C, Labelle A: Catechol-O-methyltransferase Val-108/158-Met gene variants associated with performance on the Wisconsin Card Sorting Test. Arch Gen Psychiatry 2002; 59:662-663Crossref, Medline, Google Scholar
21. de Chaldee M, Laurent C, Thibaut F, Martinez M, Samolyk D, Petit M, Campion D, Mallet J: Linkage disequilibrium on the COMT gene in French schizophrenics and controls. Am J Med Genet 1999; 88:452-457Crossref, Medline, Google Scholar
22. Norton N, Kirov G, Zammit S, Jones G, Jones S, Owen R, Krawczak M, Williams NM, O’Donovan MC, Owen MJ: Schizophrenia and functional polymorphisms in the MAOA and COMT genes: no evidence for association or epistasis. Am J Med Genet 2002; 114:491-496Crossref, Medline, Google Scholar
23. Park TW, Yoon KS, Kim JH, Park WY, Hirvonen A, Kang D: Functional catechol-O-methyltransferase gene polymorphism and susceptibility to schizophrenia. Eur Neuropsychopharmacol 2002; 12:299-303Crossref, Medline, Google Scholar
24. Arinami T, Ohtsuki T, Takase K, Shimizu H, Yoshikawa T, Horigome H, Nakayama J, Toru M: Screening for 22q11 deletions in a schizophrenia population. Schizophr Res 2001; 52:167-170Crossref, Medline, Google Scholar
25. Chen C-H, Lee Y-R, Chung M-Y, Wei F-C, Koong F-J, Shaw C-K, Yeh J-I, Hsiao K-J: Systematic mutation analysis of the catechol O-methyltransferase gene as a candidate gene for schizophrenia. Am J Psychiatry 1999; 156:1273-1275Abstract, Google Scholar
26. Daniels JK, Williams NM, Williams J, Jones LA, Cardno AG, Murphy KC, Spurlock G, Riley B, Scambler P, Asherson P, McGuffin P, Owen MJ: No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol O-methyltransferase activity. Am J Psychiatry 1996; 153:268-270Link, Google Scholar
27. Herken H, Erdal ME: Catechol-O-methyltransferase gene polymorphism in schizophrenia: evidence for association between symptomatology and prognosis. Psychiatr Genet 2001; 11:105-109Crossref, Medline, Google Scholar
28. Karayiorgou M, Gogos JA, Galke BL, Wolyniec PS, Nestadt G, Antonarakis SE, Kazazian HH, Housman DE, Pulver AE: Identification of sequence variants and analysis of the role of the catechol-O-methyl-transferase gene in schizophrenia susceptibility. Biol Psychiatry 1998; 43:425-431Crossref, Medline, Google Scholar
29. Kotler M, Barak P, Cohen H, Averbuch IE, Grinshpoon A, Gritsenko I, Nemanov L, Ebstein RP: Homicidal behavior in schizophrenia associated with a genetic polymorphism determining low catechol O-methyltransferase (COMT) activity. Am J Med Genet 1999; 88:628-633Crossref, Medline, Google Scholar
30. Liou YJ, Tsai SJ, Hong CJ, Wang YC, Lai IC: Association analysis of a functional catechol-O-methyltransferase gene polymorphism in schizophrenic patients in Taiwan. Neuropsychobiology 2001; 43:11-14Crossref, Medline, Google Scholar
31. Ohmori O, Shinkai T, Kojima H, Terao T, Suzuki T, Mita T, Abe K: Association study of a functional catechol-O-methyltransferase gene polymorphism in Japanese schizophrenics. Neurosci Lett 1998; 243:109-112Crossref, Medline, Google Scholar
32. Strous RD, Bark N, Woerner M, Lachman HM: Lack of association of a functional catechol-O-methyltransferase gene polymorphism in schizophrenia. Biol Psychiatry 1997; 41:493-495Crossref, Medline, Google Scholar
33. Fan JB, Chen WY, Tang JX, Li S, Gu NF, Feng GY, Breen G, St Clair D, He L: Family-based association studies of COMT gene polymorphisms and schizophrenia in the Chinese population. Mol Psychiatry 2002; 7:446-447Crossref, Medline, Google Scholar
34. Li T, Ball D, Zhao J, Murray RM, Liu X, Sham PC, Collier DA: Family-based linkage disequilibrium mapping using SNP marker haplotypes: application to a potential locus for schizophrenia at chromosome 22q11. Mol Psychiatry 2000; 5:77-84Crossref, Medline, Google Scholar
35. Kunugi H, Vallada HP, Sham PC, Hoda F, Arranz MJ, Li T, Nanko S, Murray RM, McGuffin P, Owen M, Gill M, Collier DA: Catechol-O-methyltransferase polymorphisms and schizophrenia: a transmission disequilibrium study in multiply affected families. Psychiatr Genet 1997; 7:97-101Crossref, Medline, Google Scholar
36. Semwal P, Prasad S, Bhatia T, Deshpande SN, Wood J, Nimgaonkar VL, Thelma BK: Family-based association studies of monoaminergic gene polymorphisms among North Indians with schizophrenia. Mol Psychiatry 2001; 6:220-224Crossref, Medline, Google Scholar
37. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
38. Knapp M, Seuchter SA, Baur MP: The haplotype-relative-risk (HRR) method for analysis of association in nuclear families. Am J Hum Genet 1993; 52:1085-1093Medline, Google Scholar
39. DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177-188Crossref, Medline, Google Scholar
40. Woolf B: On estimating the relation between blood group and disease. Ann Eugen 1955; 19:251-253Crossref, Google Scholar
41. Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997; 315:629-634Crossref, Medline, Google Scholar
42. Bacanu SA, Devlin B, Roeder K: The power of genomic control. Am J Hum Genet 2000; 66:1933-1944Crossref, Medline, Google Scholar
43. Palmatier MA, Kang AM, Kidd KK: Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry 1999; 46:557-567Crossref, Medline, Google Scholar
44. Deng HW: Population admixture may appear to mask, change or reverse genetic effects of genes underlying complex traits. Genetics 2001; 159:1319-1323Medline, Google Scholar
45. Begg CB, Berlin JA: Publication bias and dissemination of clinical research. J Natl Cancer Inst 1989; 81:107-115Crossref, Medline, Google Scholar
46. Kirov G, Murphy KC, Arranz MJ, Jones I, McCandles F, Kunugi H, Murray RM, McGuffin P, Collier DA, Owen MJ, Craddock N: Low activity allele of catechol-O-methyltransferase gene associated with rapid cycling bipolar disorder. Mol Psychiatry 1998; 3:342-345Crossref, Medline, Google Scholar
47. Papolos DF, Veit S, Faedda GL, Saito T, Lachman HM: Ultra-ultra rapid cycling bipolar disorder is associated with the low activity catecholamine-O-methyltransferase allele. Mol Psychiatry 1998; 3:346-349Crossref, Medline, Google Scholar
48. Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT: A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr Res 2002; 53:31-44Crossref, Medline, Google Scholar



