Do Urbanicity and Familial Liability Coparticipate in Causing Psychosis?
Abstract
OBJECTIVE: The urban environment and familial liability are risk factors for psychotic illness, but it is not known whether a biological synergism exists between these two proxy causes. METHOD: The amount of biological synergism between familial liability (defined as a family history of delusions and/or hallucinations necessitating psychiatric treatment) and a five-level rating of population density of place of residence was estimated from the additive statistical interaction in a general population risk set of 5,550 individuals. RESULTS: Both the level of urbanicity (adjusted summary odds ratio=1.57, 95% CI=1.30–1.89) and familial liability (adjusted odds ratio=4.59, 95% CI=2.41–8.74) increased the risk for psychotic disorder, independently of each other. However, the effect of urbanicity on the additive scale was much larger for individuals with evidence of familial liability (risk difference=2.58%) than in those without familial liability (risk difference=0.40%). An estimated 60%–70% of the individuals exposed to both urbanicity and familial liability had developed psychotic disorder because of the synergistic action of the two proxy causes. CONCLUSIONS: Given that familial clustering of psychosis is thought to reflect the effect of shared genes, the findings support a mechanism of gene-environment interaction in the causation of psychosis.
Urban birth and upbringing are associated with later risk for schizophrenia (1–4). A plausible explanation for this finding is that one or more environmental risk factors for later psychotic outcomes that operate early in life are prevalent in urban areas (5). If urbanicity represents an as yet unknown environmental risk factor for schizophrenia, the question arises as to what degree this risk interacts with personal vulnerability factors, in particular familial liability to psychosis, which is thought to largely represent the influence of shared genes rather than shared environment (6, 7). Biological synergism between genetic liability and environmental risk is thought to be common in multifactorial disorders such as schizophrenia (8–11). However, recent progress in the study of interactions indicates that the most frequently used statistical models of interaction are not suitable for identifying biological synergism. For example, the commonly used statistical models in which genes and environment multiply each other’s effects (multiplicative models) assume that individuals who are exposed to both the genetic and the environmental factors cannot have contracted the illness because of the effect of genes alone or of the environment alone (12). It has been shown that the true degree to which two causes coparticipate in producing an outcome can be estimated from (but is not the same as) the additive statistical interaction (see reference 12). This method was recently applied to risk for schizophrenia to show synergy between traumatic head injury and familial liability (13). In the current study, we wished to investigate to what degree urbanicity and familial liability for psychosis coparticipate in producing psychosis outcomes, using recently specified models to examine biological synergism between two causes.
Method
Subjects
The Netherlands Mental Health Survey and Incidence Study is a prospective study with three measurement points (time 1, time 2, and time 3) over a period of 3 years (14, 15). The current report is based on the lifetime prevalence of psychosis assessed at time 1 (N=7,076 responders) and on first-degree family history data assessed at time 2 (N=5,618 responders). A multistage, stratified, random sampling procedure was used to identify a total of 7,076 individuals (response rate: 69.7%) who provided written informed consent in conformity with the local ethics committee guidelines. Nearly 44% of the nonresponders agreed to complete a postal questionnaire, including a General Health Questionnaire (16), and were found to have the same mean General Health Questionnaire score as the responders (mean score=1.19 for the responders and 1.16 for the nonresponders). Nonresponse was not associated with the level of urbanicity (14, 15).
Instruments
Subjects were interviewed at home. The Composite International Diagnostic Interview (CIDI) version 1.1 (17) was used, yielding DSM-III-R diagnoses. The CIDI was designed for use by trained interviewers who are not clinicians and has been found to have high interrater reliability (18) and high test-retest reliability (19). Ninety interviewers experienced in systematic data collection collected the data, after having received a 3-day training course in recruiting and interviewing, followed by a 4-day course at the World Health Organization CIDI training center in Amsterdam. Extensive monitoring and quality checks took place throughout the data collection period (15).
Psychosis Ratings
Lifetime ratings from the 17 CIDI core psychosis sections on delusions (13 items) and hallucinations (four items) were used (items G1–G13, G15, G16, G20, G21). These items concern classic psychotic symptoms involving, for example, persecution, thought interference, auditory hallucinations, and passivity phenomena. These items can be rated in six ways: 1=no symptom, 2=symptom is present but not clinically relevant (the person is not bothered by it and not seeking help for it), 3=symptom is a result of ingestion of drugs, 4=symptom is a result of somatic disease, 5=true psychiatric symptom, 6=symptom may not really be a symptom because there appears to be some plausible explanation for it. Because psychotic symptoms are difficult to diagnose in a structured interview (20), clinical reinterviews were conducted over the telephone by an experienced trainee psychiatrist for all individuals who had at least one rating of 5 or 6. Questions from the Structured Clinical Interview for DSM-III-R (SCID), an instrument with proven reliability and validity in the diagnosis of schizophrenia (21), were used in the clinical reinterviews. CIDI ratings were corrected on the basis of these reinterviews.
In the baseline sample of 7,076 responders, the prevalences of the possible CIDI ratings for the 17 psychosis items were: N=915 (12.9%) for any rating of 2, N=39 (0.6%) for any rating of 3 or 4, N=295 (4.2%) for any rating of 5, and N=285 (4.0%) for any rating of 6. Validation of the contrasts implied by these ratings has been presented previously (4, 22).
The Netherlands Mental Health Survey and Incidence Study lifetime DSM-III-R diagnoses of psychotic disorder are based on the data from the clinical reinterviews. Psychotic disorder outcome was defined as any DSM-III-R affective or nonaffective psychotic diagnosis.
Level of Urbanicity
Five levels of urbanicity were defined, following the standard classification of the Dutch Central Bureau of Statistics for the level of urbanization of places of residence. The classification is based on a measure of residential density consisting of the number of addresses per km2 within a circle with a radius of 1 km from a given place of residence as the center of the circle. The mean residential density of all the addresses within a geographical area constitutes the level of urbanicity of that area. Levels 1–5 in the classification designate <500, 500–999, 1000–1499, 1500–2499, and ≥2500 addresses per km2, respectively.
Risk Set
The risk set consisted of 5,550 individuals who 1) had valid CIDI psychosis ratings at baseline and 2) had valid family history data at time 2. The risk set included 2,571 men (46.3%). The mean age of the entire risk set was 41.0 years (SD=11.9). Of the 5,550 individuals, 78 (1.4%) had a DSM-III-R diagnosis of affective or nonaffective psychosis, 211 (3.8%) had at least one CIDI symptom rating of 5, and 694 (12.5%) had at least one CIDI symptom rating of 2.
Family History of Psychosis
At the time 2 interview, subjects were asked whether each first-degree relative had ever had delusions or hallucinations. In addition, subjects were asked if any first-degree relative or half-sibling had ever received treatment from a psychiatrist or had ever been admitted to a psychiatric hospital for a mental health problem. The risk set included 310 probands (5.6%) who indicated that a first-degree relative had ever had delusions or hallucinations, 201 (3.6% of the risk set) of whom indicated that the relative with delusions or hallucinations had received psychiatric treatment. These two groups were designated, respectively, those with a family history of psychosis broadly defined and those with a history of psychosis narrowly defined. A total of 804 probands (14.5%) reported a family history of psychiatric treatment for conditions other than delusions or hallucinations.
The validity of these measures was examined by using logistic regression. A family history of psychosis broadly defined was strongly associated with a family history of treatment by a psychiatrist or admission to a psychiatric hospital (odds ratio=16.88, 95% CI=12.89–22.10) and family history of suicide (odds ratio=7.99, 95% CI=5.09–12.56). A family history of treatment for delusions or hallucinations (family history of psychosis narrowly defined) was strongly associated with any DSM-III-R psychotic disorder in the probands (odds ratio=5.08, 95% CI=2.70–9.56), and the association remained when adjusted for the effect of a family history of any psychiatric treatment (odds ratio=2.89, 95% CI=1.37–6.09). Family history of treatment for delusions or hallucinations (family history of psychosis narrowly defined) was weakly associated with any DSM-III-R disorder (psychotic or nonpsychotic) in the probands (odds ratio=1.70, 95% CI=1.28–2.25), and the association did not remain after adjustment for a family history of any psychiatric treatment (odds ratio=1.13, 95% CI=0.83–1.54).
Data Analyses
The lifetime prevalences of psychotic disorder in the probands, family history of psychosis broadly defined, and family history of psychosis narrowly defined were examined in relation to the level of urbanicity of the place of residence, with the result adjusted for the a priori selected possible confounding effects of age in years; sex; level of education (four levels); and country of birth of the proband, the proband’s mother, and the proband’s father (coded Dutch-born, foreign-born, or data missing). In addition, to assess whether any effect of urbanicity on psychotic disorder in the probands could be explained by urban drift of parents with vulnerability to psychosis, the analysis adjusted for a history of delusions or hallucinations in the mother or the father as reported by the proband. To assess whether any effect of urbanicity on psychosis in the relatives could be explained by a reporting bias in probands with psychotic disorder, analyses excluding data from probands with a DSM-III-R psychotic disorder were also conducted.
In line with recent advances in the conceptualization of interaction, we calculated the statistical additive interaction and used the result to estimate the amount of biological synergism between urbanicity and family history in the population. This was done by using the calculations developed by Darroch (12). For these analyses, a dichotomized measure of urbanicity was used (levels 1, 2, and 3 were coded as 0, and levels 4 and 5 were coded as 1). To calculate the statistical interaction under an additive model, the BINREG procedure in STATA (23), which fits generalized linear models for the binomial family estimating risk differences (24, 25), was used to model interactions between urbanicity and family history in the risk set. The statistical significance of the interactions was assessed by using the Wald test (26). To examine the specificity of any interaction between family history of psychosis and urbanicity, we also examined the interaction between urbanicity and family history of a nonpsychotic condition.
Sensitivity Analysis
Of the total of 479 individuals who were eligible for a clinical reinterview over the telephone at baseline, 226 (47.2%) were actually interviewed. To examine whether the results were affected by the incomplete clinical reinterview rate, we conducted a sensitivity analysis excluding the individuals who were eligible for clinical reinterview but who were not contacted, thus leaving only those who had been rated by clinicians.
Results
Urbanicity, Family History, and Psychosis
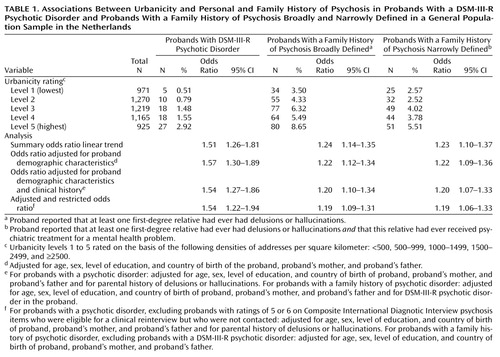
The five-level urbanicity rating was strongly associated with all psychotic outcomes in both the probands and the relatives. A DSM-III-R diagnosis of psychotic disorder in the proband, a family history of psychosis broadly defined, and a family history of psychosis narrowly defined were all more common in progressively more urbanized areas (Table 1). Adjustment for age, sex, level of education, and the countries of birth of the proband and the proband’s parents did not reduce the associations (Table 1). Additional adjustment for parental history of psychosis did not reduce the association between urbanicity and proband psychosis outcomes, and additional adjustment for proband psychotic disorder did not reduce the association between urbanicity and relatives’ psychosis outcomes (Table 1). The association between psychotic disorder in the proband and urbanicity remained unchanged if individuals with CIDI psychosis ratings who had been eligible but were not contacted for clinical reinterview were excluded from the analysis, and the association between relatives’ psychosis outcomes and urbanicity remained unchanged if probands with psychotic disorder were excluded (Table 1).
A family history of delusions and/or hallucinations in first-degree relatives was strongly associated with psychotic disorder in the probands (family history of psychosis broadly defined: odds ratio=5.73, 95% CI=3.37–9.74; family history of psychosis narrowly defined: odds ratio=5.08, 95% CI=2.70–9.56). These associations remained after adjustment for age, sex, level of education, country of birth of the proband and the proband’s parents, and urbanicity (family history of psychosis broadly defined: odds ratio=5.26, 95% CI=3.06–9.04; family history of psychosis narrowly defined: odds ratio=4.59, 95% CI=2.41–8.74).
Interaction Between Urbanicity and Family History
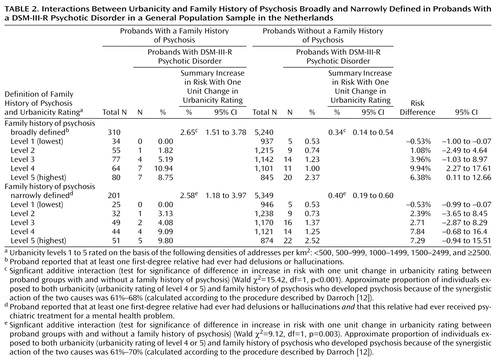
There was a significant positive interaction on the additive scale between urbanicity and family history in their effects on psychotic disorder in the proband (Table 2) (family history of psychosis broadly defined: χ2=15.42, df=1, p<0.001; family history of psychosis narrowly defined: χ2=9.12, df=1, p=0.003). This interaction remained after adjustment for a family history of treatment for any psychiatric disorder (family history of psychosis broadly defined: model did not converge; family history of psychosis narrowly defined: χ2=9.65, df=1, p<0.002). The interaction also remained if individuals with CIDI psychosis ratings who had been eligible for clinical reinterview but were not contacted were excluded from the analysis (our sensitivity analysis) (family history of psychosis broadly defined: χ2=11.58, df=1, p<0.001; family history of psychosis narrowly defined: χ2=5.99, df=1, p<0.02). No such interaction was present for family history of treatment for nonpsychotic disorder (χ2=0.38, df=1, p=0.54).
Biological Synergism
The risk of psychotic disorder in the group exposed to neither urbanicity (the dichotomized measure of urbanicity as described in the Method section) nor family history of psychosis broadly defined was 0.85% (28 of 3,294). The risk of psychotic disorder in the group exposed to urbanicity alone was 1.59% (31 of 1,946), the risk in those exposed only to family history of psychosis broadly defined was 3.01% (5/166), and the risk in those exposed to both urbanicity and family history of psychosis broadly defined was 9.72% (14/144). Filling in these risks in the formulas provided by Darroch (12) revealed that synergism was between 0.0588 and 0.0662, which represents respectively 61% and 68% of the risk in those exposed to both urbanicity and family history (0.0588/0.0972=61% and 0.0662/0.0972=68%) (a spreadsheet to help in calculating synergism is available upon request). Thus, an estimated 60%–70% of the individuals exposed to both urbanicity and family history had developed psychotic disorder because of the synergistic action of the two proxy causes (Table 2).
Discussion
The risk-increasing effect of urbanicity on the occurrence of psychotic disorder was greater in those with higher levels of familial liability for psychosis, independent of familial liability for other psychiatric morbidity. Between 60% and 70% of the psychosis outcome in probands exposed to both familial liability and urbanicity was attributable to the synergistic action of these two factors.
Can Differential Misclassification Explain the Findings?
As psychotic illness in the probands was more common in urban areas, a bias leading to a spurious increase in family history in urban areas could have been introduced if probands with psychotic illness were more likely to report similar symptoms in their relatives than were probands without psychotic illness. However, the association between psychosis outcomes in the relatives and urbanicity remained unchanged when the probands with psychotic disorder were excluded from the analysis. A bias leading to a spurious interaction between family history and urbanicity could have been introduced if 1) probands with psychotic illness in urban areas were more likely to report similar symptoms in their relatives than were probands with psychotic illness in nonurban areas and 2) the same mechanism of differential reporting did not operate in probands without psychotic illness. This is highly implausible. Bias could also have been introduced by differential rating of psychosis due to incomplete clinical reinterview rates at baseline. However, excluding the individuals who were eligible for clinical reinterview but who had not been contacted, thus leaving only those who had been rated by clinicians, did not affect the results. Another potential source of bias is that lifetime rates of psychotic disorder were examined in relation to current urban residence. Thus, one explanation for the findings is that symptomatic probands, or their symptomatic parents, could have “drifted” to urban areas. However, in a previous study in the Netherlands we found a high degree of lifetime stability of urban exposure in the probands (5). In another study the association between urbanicity and psychosis in the probands was not reduced after adjustment for psychosis in the parents (27).
Can Nondifferential Misclassification Explain the Findings?
Our rating of family history is subject to misclassification. However, reports of family history of psychotic disorders are more reliable than those of family history of other psychiatric conditions (28). Furthermore, the validity is suggested by several observations. First, the overall prevalence of a family history of treated delusions and hallucinations was 3.6%, which is very similar to the prevalence of psychotic symptoms associated with treatment seeking in the probands (the prevalence of a CIDI psychotic symptom rating of 5 was 3.8%). Second, the validity of the family history rating was suggested by 1) the strong association with psychiatric treatment and 2) the strong association with suicide. Discriminant validity was suggested by the fact that a family history of psychosis predicted psychosis in the probands independent of a family history of treatment for any psychiatric disorder but did not predict any DSM-III-R disorder independent of a family history of treatment for any psychiatric disorder. Third, our ratings of family history considered much broader phenomena than the DSM-III-R diagnosis of schizophrenia. However, we consider the broader definition of psychosis an advantage rather than a disadvantage, given the fact that genetic liability for schizophrenia is also expressed in milder “schizotypic” phenotypes; thus, our broader measure is likely to produce fewer false negative results than a family history of stringently defined psychotic disorder (29).
Synergism Between Urbanicity and Familial Liability
Familial clustering can be due to both environmental and genetic effects. However, studies teasing apart environmental and genetic factors have indicated that familial clustering of psychosis reflects the effects of shared genes rather than shared environment (6). These previous analyses suggest that our findings can be interpreted in terms of a gene-environment interaction rather than an environment-environment interaction. One previous study examined the interaction between familial liability, as a proxy measure of genetic risk, and urbanicity, but the comparison involved statistical multiplicative interaction rather than biological synergism. Had we used a multiplicative model, we would not have found evidence of interaction (post hoc analysis, family history of psychosis broadly defined: χ2=0.48, df=1, p=0.49; post hoc analysis, family history of psychosis narrowly defined: χ2=0.33 df=1, p=0.57). Our results therefore suggest that an environmental risk factor in the urban environment serves as a powerful potentiator of genetic risk for psychosis.
It has been suggested that the high rates of psychotic illness in urban environments are the result of the influence of environmental factors that operate long before the onset of schizophrenia (5, 30). This theory implies that biological synergism between genetic and environmental risk as identified in this study occurs during development. The validity of a possible developmental mechanism involving gene-environment interaction is supported by data from a Finnish adoption cohort of children at high genetic risk for schizophrenia (31). That study showed that adverse experiences in childhood and adolescence may be crucial in determining the transition from psychosis genotype to phenotype. A possible developmental mechanism whereby social factors in the urban environment may interact with genetic liability for adult psychosis consists of the effects of the wider social environment, such as the neighborhood environment, on child and adolescent development (32). Differences in the level of deprivation and social isolation in the neighborhood environment in urban areas have been shown to be associated with variation in a range of mental health outcomes from problem behavior in children (33) to incidence of schizophrenia (34). Social environments with a high level of deprivation and a low level of social capital (35) may constitute the environments that interact with genetic liability to increase the risk for psychotic illness.
 |
 |
Received Nov. 1, 2001; revisions received March 20 and Aug. 6, 2002; accepted Aug. 14, 2002. From the Department of Psychiatry and Neuropsychology, azM/Mondriaan/Riagg/RIBW/Vijverdal Academic Centre, EURON, Maastricht University; the Division of Psychological Medicine, Institute of Psychiatry, London; the Research and Documentation Centre, Ministry of Justice, The Hague; and the Netherlands Institute of Mental Health and Addiction (Trimbos Institute), Utrecht. Address reprint requests to Prof. van Os, Department of Psychiatry and Neuropsychology, Maastricht University, P.O. Box 616 (DRT 10), 6200 MD Maastricht, the Netherlands; [email protected] (e-mail).
1. Lewis G, David A, Andreasson S, Allebeck P: Schizophrenia and city life. Lancet 1992; 340:137-140Crossref, Medline, Google Scholar
2. Marcelis M, Navarro-Mateu F, Murray R, Selten JP, Van Os J: Urbanization and psychosis: a study of 1942-1978 birth cohorts in the Netherlands. Psychol Med 1998; 28:871-879Crossref, Medline, Google Scholar
3. Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, Andersen PK, Melbye M: Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med 1999; 340:603-608Crossref, Medline, Google Scholar
4. Van Os J, Hanssen M, Bijl RV, Vollebergh W: Prevalence of psychotic disorder and community level of psychotic symptoms: an urban-rural comparison. Arch Gen Psychiatry 2001; 58:663-668Crossref, Medline, Google Scholar
5. Marcelis M, Takei N, Van Os J: Urbanization and risk for schizophrenia: does the effect operate before or around the time of illness onset? Psychol Med 1999; 29:1197-1203Crossref, Medline, Google Scholar
6. Kety SS, Wender PH, Jacobsen B, Ingraham LJ, Jansson L, Faber B, Kinney DK: Mental illness in the biological and adoptive relatives of schizophrenic adoptees: replication of the Copenhagen Study in the rest of Denmark. Arch Gen Psychiatry 1994; 51:442-455Crossref, Medline, Google Scholar
7. Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, Venturi P, Jones LA, Lewis SW, Sham PC, Gottesman II, Farmer AE, McGuffin P, Reveley AM, Murray RM: Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch Gen Psychiatry 1999; 56:162-168Crossref, Medline, Google Scholar
8. Cannon TD, Mednick SA, Parnas J, Schulsinger F, Praestholm J, Vestergaard A: Developmental brain abnormalities in the offspring of schizophrenic mothers, I: contributions of genetic and perinatal factors. Arch Gen Psychiatry 1993; 50:551-564Crossref, Medline, Google Scholar
9. Van Os J, Marcelis M: The ecogenetics of schizophrenia: a review. Schizophr Res 1998; 32:127-135Crossref, Medline, Google Scholar
10. Malaspina D, Sohler NL, Susser ES: Interaction of genes and prenatal exposures in schizophrenia, in Prenatal Exposures in Schizophrenia. Edited by Susser ES, Brown AS, Gorman JM. Washington, DC, American Psychiatric Press, 1999, pp 35-59Google Scholar
11. Tienari P, Sorri A, Lahti I, Naarala M, Wahlberg KE, Pohjola J, Moring J: Interaction of genetic and psychosocial factors in schizophrenia. Acta Psychiatr Scand Suppl 1985; 319:19-30Crossref, Medline, Google Scholar
12. Darroch J: Biologic synergism and parallelism. Am J Epidemiol 1997; 145:661-668Crossref, Medline, Google Scholar
13. Corcoran C, Malaspina D: Traumatic brain injury and schizophrenia. Int J Ment Health 2001; 30:17-33Crossref, Google Scholar
14. Bijl RV, Ravelli A, Van Zessen G: Prevalence of psychiatric disorder in the general population: results from the Netherlands mental health survey and incidence study. Soc Psychiatry Psychiatr Epidemiol 1998; 33:587-596Crossref, Medline, Google Scholar
15. Bijl RV, Van Zessen G, Ravelli A, De Rijk C, Langendoen Y: The Netherlands mental health survey and incidence study (NEMESIS): objectives and design. Soc Psychiatry Psychiatr Epidemiol 1998; 33:581-587Crossref, Medline, Google Scholar
16. Goldberg DP, Williams P: The User’s Guide to the General Health Questionnaire. Slough, UK, National Foundation for Educational Research-Nelson, 1988Google Scholar
17. Smeets RMW, Dingemans PMAJ: Composite International Diagnostic Interview (CIDI) Version 1.1. Geneva, World Health Organization, 1993Google Scholar
18. Wittchen H-U, Robins LN, Cottler L, Sartorius N, Burke JD, Regier D: Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI): the Multicentre WHO/ADAMHA Field Trials. Br J Psychiatry 1991; 159:645-653; correction, 1992; 160:136Google Scholar
19. Wittchen H-U: Reliability and validity studies of the WHO Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res 1994; 28:57-84Crossref, Medline, Google Scholar
20. Cooper SA, Collacott RA: Relapse of depression in people with Down’s syndrome. Br J Developmental Disabilities 1994; 40(78, part 1):32-37Google Scholar
21. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624-629Crossref, Medline, Google Scholar
22. Van Os J, Hanssen M, Bijl RV, Ravelli A: Strauss (1969) revisited: a psychosis continuum in the general population? Schizophr Res 2000; 45:11-20Crossref, Medline, Google Scholar
23. Stata Reference Manual: Release 7. College Station, Tex, Stata Corp, 2001Google Scholar
24. Hardin JW, Cleves MA: Generalized linear models: extensions to the binomial family: seb29. Stata Technical Bulletin 1999; 50:21-25Google Scholar
25. Wacholder S: Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol 1986; 123:174-184Crossref, Medline, Google Scholar
26. Clayton D, Hills M: Wald Tests, in Statistical Models in Epidemiology. Edited by Clayton D, Hills M. Oxford, Oxford Science Publications, 1993, pp 101-102Google Scholar
27. Van Os J, Hanssen M, De Graaf R, Vollebergh W: Does the urban environment independently increase the risk for both negative and positive features of psychosis? Soc Psychiatry Psychiatr Epidemiol 2002; 37:460-464Crossref, Medline, Google Scholar
28. Andreasen NC, Endicott J, Spitzer RL, Winokur G: The family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry 1977; 34:1229-1235Crossref, Medline, Google Scholar
29. What causes schizophrenia? (editorial). Nat Neurosci 1999; 2:295Crossref, Medline, Google Scholar
30. Pedersen CB, Mortensen PB: Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry 2001; 58:1039-1046Crossref, Medline, Google Scholar
31. Wahlberg K-E, Wynne LC, Oja H, Keskitalo P, Pykäläinen L, Lahti I, Moring J, Naarala M, Sorri A, Seitamaa M, Läksy K, Kolassa J, Tienari P: Gene-environment interaction in vulnerability to schizophrenia: findings from the Finnish Adoptive Family Study of Schizophrenia. Am J Psychiatry 1997; 154:355-362Link, Google Scholar
32. Leventhal T, Brooks-Gunn J: The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull 2000; 126:309-337Crossref, Medline, Google Scholar
33. Kalff AC, Kroes M, Vles JSH, Hendriksen JGM, Feron FJM, Steyaert J, van Zeben TMCB, Jolles J, Van Os J: Neighbourhood level and individual level SES effects on child problem behaviour: a multilevel analysis. J Epidemiol Community Health 2001; 55:246-250Crossref, Medline, Google Scholar
34. Van Os J, Driessen G, Gunther N, Delespaul P: Neighbourhood variation in incidence of schizophrenia: evidence for person-environment interaction. Br J Psychiatry 2000; 176:243-248Crossref, Medline, Google Scholar
35. Kawachi I, Kennedy BP, Lochner K, Prothrow-Stith D: Social capital, income inequality, and mortality. Am J Public Health 1997; 87:1491-1498Crossref, Medline, Google Scholar



